Chapter 8 Principles of Chemotherapy
Although the earliest clinical manifestation of cancer is a mass or tumor, in many patients cancer is often not a localized disease at the time of its discovery. In most patients, the disease has already spread to lymph nodes and other distant sites, even though clinical imaging and random biopsies may fail to disclose its presence. For more than a century, thoughtful investigators have sought systemic treatments for cancer, often using as their paradigms the lessons of infectious illnesses and nutritional disease. Vaccines, natural extracts, vitamins, and synthetic chemicals were touted as cures. However, not until the initial trials of alkylating agents by scientists and clinicians at Yale University, first described in 1946, was there proof that a systemically administered agent could cause regression of a human tumor, which in this case was a mediastinal mass in a patient with Hodgkin’s disease.1 The clinical studies of nitrogen mustard, based on observations of the toxicity of mustard gases in World War I, lacked a clear conceptual basis. Although it was known at the time that these compounds were highly reactive with proteins, nucleic acids, and other electron-rich molecules, the specific intracellular target was not identified until more than a decade later, and we are only now beginning to understand the reasons for the selective action of alkylating agents on tumor cells.
This early experience with alkylating agents led a number of bold investigators to undertake an alternative approach, the synthesis of compounds that would act as fraudulent counterparts of natural metabolites known to stimulate cancer cell growth. Among these metabolic targets, vitamins and nucleic acid bases proved to be most vulnerable (Fig. 8-1). In the late 1940s, scientists from American Cyanamid synthesized analogs of folic acid, a vitamin known to stimulate the proliferation of cancer cells in culture and in humans. Sidney Farber tested these analogs and found striking but short-lived responses to aminopterin and to the closely related compound methotrexate in children with acute lymphoblastic leukemia (ALL).2 Prospects for the treatment of cancer with drugs dramatically escalated in the following decade. Hitchings, Elion, and others at Burroughs Wellcome experimented with a series of purine analogs and found antitumor effects in animals, leading to the successful development of 6-thioguanine and 6-mercaptopurine. Soon afterward, corticosteroids were discovered as anti-inflammatory agents and were found to kill malignant lymphocytes in children with leukemia and lymphoma.3
Equally rewarding efforts in the field of natural product chemistry yielded additional new anticancer drugs with unique mechanisms of action. In the course of their search for natural products for the treatment of diabetes, scientists at Eli Lilly discovered the antimitotic and antitumor properties of the vinca alkaloids.4 Shortly thereafter, analysis of fermentation broths led to the discovery of mitomycin C, a novel alkylating agent5; bleomycin, a DNA-cleaving peptide6; and the anthracyclines, which inhibit topoisomerase II,7 an enzyme that relaxes DNA supercoils during transcription and replication. In the 1960s, further efforts in plant chemistry led to the isolation of paclitaxel,8 a drug that inhibits formation of the mitotic spindle, and the camptothecins, which block the action of topoisomerase I.9 However, paclitaxel and the camptothecins did not reach the clinic until 10 to 20 years later.
Models for Chemotherapy
In parallel with the work that led to cytotoxic chemotherapy, Skipper and Perry10 at the Southern Research Institute characterized murine tumor models, notably the L1210 and P388 leukemias, as well as murine solid tumors such as sarcoma 180. Their model systems allowed reproducible, quantitative chemotherapy experiments to be performed in mice.10 They tested various strategies for treatment and established a rational basis for understanding the kinetics of cell kill, the evaluation of drug combinations, and the mechanisms of drug resistance. Important concepts of cancer chemotherapy came from their experiments, which established the theoretical basis for rational combination chemotherapy and the cure of ALL in children. Skipper’s experiments with murine leukemias established the following principles:
These principles profoundly influenced all aspects of clinical chemotherapy, including regimen design, the use of drugs in combination, adjuvant chemotherapy, and high-dose chemotherapy. Relying on these principles, the cure of ALL was accomplished through the efforts of Frei and Freireich at the National Cancer Institute and Holland at Roswell Park, who developed effective combination therapy; Pinkel and colleagues at St. Jude Hospital in Memphis, who identified the central nervous system as a sanctuary for leukemic cells; and the national pediatric cooperative groups, which tested these insights in randomized clinical trials.11 These investigators combined a knowledge of the drugs and the disease to establish principles for leukemia therapy that persist to this day: intensive, marrow-ablative induction therapy, followed by consolidation to further reduce the leukemic cell population; central nervous system prophylaxis with irradiation and methotrexate; and maintenance chemotherapy to eliminate the last logs of tumor cells. In the course of their work, they identified the importance of supportive care measures, including aggressive broad-spectrum antibiotic treatment of febrile episodes in neutropenic patients, platelet support to prevent bleeding due to thrombocytopenia, and management of opportunistic infection. Each of these insights, further refined by the use of new antibiotics and bone marrow colony-stimulating factors, has become a basic component of modern chemotherapy.
Solid Tumor Chemotherapy
The principles enumerated earlier have been applied with greatest success to the treatment of aggressive and rapidly proliferating tumors such as leukemias, lymphomas, and testicular tumors. The development of drug therapy for the more common solid tumors has taken a slower and more tortuous course. Drugs identified in mouse leukemia screening systems have been less effective against most solid tumors; notable exceptions are choriocarcinoma, testicular cancer, and the lymphomas, all of which can be cured with cycles of intensive chemotherapy. However, chemotherapy has had only limited impact on the common solid tumors in humans, particularly in widely metastatic, end-stage disease. Rational synthesis of 5-fluorouracil (5-FU) by Heidelberger and associates12 led to the first modestly effective antimetabolite for colon and breast cancers. Other important clinically effective drugs were identified through random screening efforts and serendipity: doxorubicin and cisplatin in the early 1970s, etoposide and paclitaxel in the late 1980s, and the camptothecin analogs and gemcitabine in the 1990s.13
An important conceptual breakthrough in solid tumor chemotherapy was the proposal to employ drugs in the adjuvant setting after removal of the primary tumor in patients at high risk for relapse. The work of Fisher,14 Bonnadonna, and others established that drugs capable of producing partial responses in advanced disease could cure one third of women with stage II breast cancer, who were otherwise destined to suffer distant recurrence and death. The conceptual basis for this strategy is the increased probability of curing metastatic disease when the tumor burden is small and tumor cells are actively proliferating and are, therefore, more susceptible to cytotoxic drugs. Drugs have a firmly established role in the treatment of lung, breast, and colorectal cancers after or before surgery.
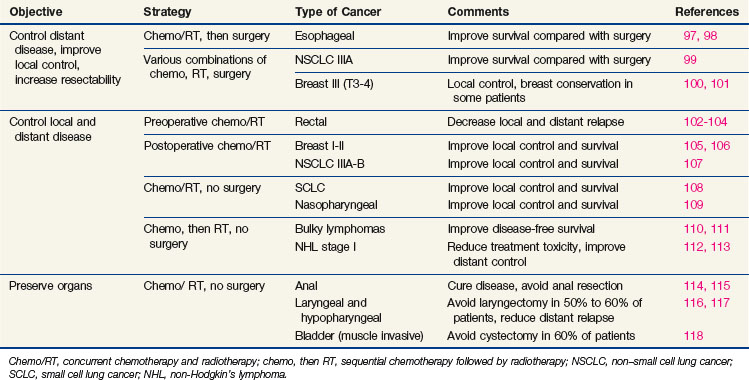
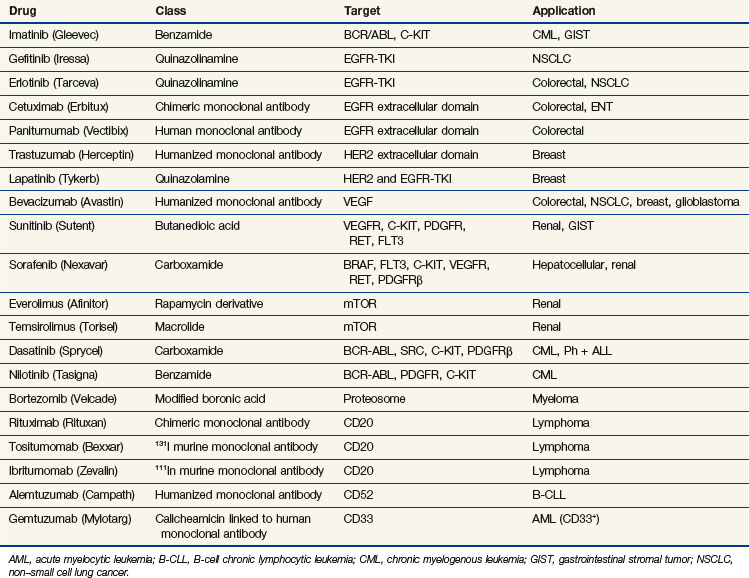
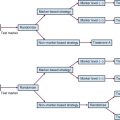
Newer concepts aimed at improving local control of otherwise inoperable tumors have led to so-called neoadjuvant, or induction, therapy.15 In this strategy, drugs are used alone or in combination with radiation therapy, before surgery, in the initial treatment of locally advanced tumors of the breast, head and neck, bladder, rectum, and lung (Table 8-1). This preoperative therapy reduces the size of otherwise unresectable tumors to a point where surgical removal is feasible or, in some cases, unnecessary.
Because drugs have become an integral part of the initial therapy of many patients with cancer, it is essential that the medical oncologist, surgeon, and radiation oncologist understand the principles of chemotherapy and the specific features of the commonly used agents. This chapter explains the conceptual basis for the use of cancer chemotherapeutic drugs and provides detailed information for understanding their actions, toxicities, and late side effects.16
The Basis Of Cancer Chemotherapy: Cancer Cell Biology
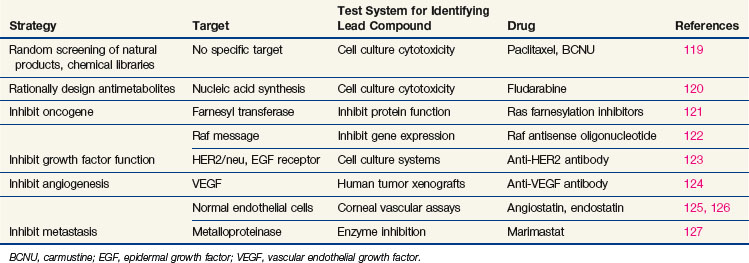
Every phase of chemotherapeutic research, from discovery to clinical application, is based on the concepts of cancer cell biology, and drug research has evolved as knowledge of biology has progressed. The essential properties of the cancer cell, properties that distinguish the cell from its normal counterpart and that form the basis for treatment, are excess proliferation, defective repair of DNA leading to high mutation rates and population diversity, reduced rates of apoptosis (i.e., programmed cell death), invasive capacity, ability to induce nutrient vessels, ability to escape immune surveillance, and ability to metastasize. Each of these properties has been the starting point for drug discovery efforts,17 as illustrated in Table 8-2. Most cancer cells display defects in DNA repair that generate a diversity of subclones, increase their adaptation to diverse and at times adverse environments, and increase the probability of drug resistance. However, with a few notable exceptions, the successful cytotoxic drugs have attacked only the first of these properties, proliferation. The new generation of targeted agents is expanding the horizon for cancer treatment by addressing the full circle of biologic changes in human tumors.
Drugs That Target Dna Synthesis And Mitosis
Although much of the effort in modern cancer drug development is directed at targets that regulate growth signal transmission, most of the successful drugs in current clinical use act directly on the synthesis or integrity of DNA. These drugs inhibit synthesis of DNA or its precursors, block cell division, inhibit necessary changes in DNA topology, or covalently bind to DNA, causing strand breaks or miscoding. All such drugs affect the integrity of DNA, and in the presence of the normal machinery for monitoring DNA integrity, they induce apoptosis. Unfortunately, they also tend to be cytotoxic toward normal cells. The reasons for selectively greater effects of cytotoxic drugs on malignant versus normal cells, as is apparent in the cure of certain malignant tumors, are poorly understood, although the process of malignant transformation may enhance sensitivity to DNA damage by virtue of defects in DNA repair.18 For example, many of the defective genes responsible for inherited cancer syndromes, such as BRCA1 and BRCA2 genes, mismatch repair genes, TP53, and nucleotide excision repair, create sensitivity to agents that inhibit various aspects of DNA repair. Another key process in malignant transformation is the loss of cell cycle controls that allow DNA repair and prevent apoptosis.
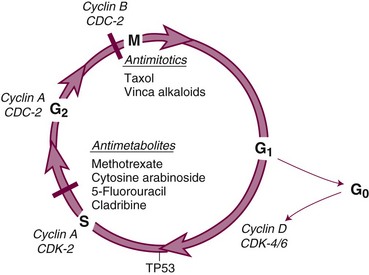
The cell cycle and its primary controls are shown in Figure 8-3. Antimetabolites such as methotrexate, 5-FU, cytosine arabinoside, gemcitabine, and the purine antagonists require cells to be actively proliferating to kill them; therefore, they are cell cycle dependent. Many of these agents, including cytosine arabinoside, fludarabine phosphate, and cladribine, must be incorporated into DNA to be cytotoxic. Alternately, agents such as etoposide and doxorubicin produce irreparable DNA strand breaks at any stage of the cell cycle, although these breaks become lethal only as the cell enters DNA synthesis. Still others, such as alkylating agents and platinum compounds, bind covalently to DNA and produce strand cross-links and strand breaks. Their toxicity seems less dependent on the stage of the cell cycle; some agents of this class, such as the nitrosoureas, are equally capable of killing nondividing and dividing cells. Antimitotic agents, constituting a separate class of drugs, block the formation or dissociation of the mitotic spindle through their effects on microtubules and thereby prevent separation of chromosomes to the daughter cells. Drugs of this class are therefore most effective against cells that enter the mitotic phase of the cell cycle.
Once it has been damaged through its encounter with a cytotoxic drug, the cancer cell has several options, and its eventual viability depends on which pathway it takes. If the normal monitors for genomic integrity (including most prominently the product of the TP53 gene) are intact, the cell may halt further progression in the cell cycle while its DNA is repaired. If the damage is sufficiently severe, intact TP53 may initiate apoptosis. If, however, the TP53 function is absent, cell cycle progression may continue despite drug-induced DNA damage, and the cell may prove viable. In most experimental settings, lack of wild-type TP53 is associated with drug and radiation resistance, but with certain drugs (e.g., paclitaxel), loss of the G1-S checkpoint function does not interfere with response.19 Paradoxically, certain DNA repair defects, such as those commonly found in mismatch repair (colon cancer) or excision repair (ovarian cancer), may be associated with resistance to platinum analogs, antimetabolites, and alkylating agents, perhaps by preventing recognition of DNA adducts and failing to initiate apoptosis.
Drugs That Target the Controls of Cell Proliferation and Cell Death
In the past few years, drug discovery efforts have targeted specific pathways and mutations that promote proliferation (see Table 8-2). Among these mutations, a number of pharmaceutical companies have focused attention on dominant mutations, such as the activation of RAS in pancreatic, colon, and bladder cancer or the activation of cell surface growth factor pathways such as the epidermal growth factor receptor (EGFR) and other tyrosine kinase receptors in epithelial tumors. Their choice of targets (activating mutations or oncogenes) was largely driven by the difficulty of developing a product or strategy to replace a missing suppressor gene product such as TP53 or CDKN2A (previously designated p16).
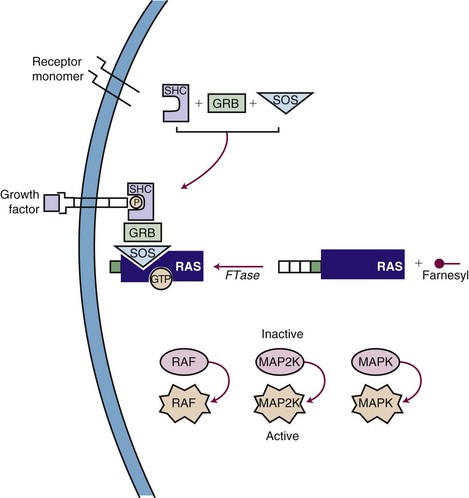
Inhibiting the function of a dominant gene product is a classic problem in drug discovery. The first efforts at targeted inhibition of a signaling pathway led to development of inhibitors of the KRAS protein, a signal transducer that links growth factor receptors to downstream molecules. KRAS is mutated in 80% to 90% of pancreatic cancers and provides the proliferative drive in a number of human cancers.22 The RAS protein is activated by a series of enzymatic steps that attach a lipid tail, a farnesyl group, to its near-terminal cysteine; methylate the cysteine; and cleave off the three terminal amino acids (Fig. 8-4). In this case, drug design seemed to be a relatively straightforward task in which a key enzymatic process or receptor is identified and a potent and specific inhibitor is developed. Several companies focused on the farnesylation step in RAS processing as a target for inactivating RAS and have found that inhibitors of farnesylation cause regression of experimental tumors. However, these drugs failed in clinical trials because the inhibitors lacked specificity for farnesylation of RAS, and alternative pathways for lipid modification of the gene product exist in cells.
A second pathway of great interest in modern cancer drug development is the RB pathway, which provides a brake on cell proliferation. Mutations may affect any one of the several genes and their proteins (e.g., CDKN2A, CDK4, CCND [cyclin D], RB1) that regulate phosphorylation and inactivation of the RB protein and free tumor cells from controls. These mutations have been found in a variety of human tumors and seem to be an essential step in converting tumors to a highly malignant phenotype. Several pharmaceutical firms have identified candidate inhibitors of cyclin-dependent kinases (CDKs), including CDK4 and CDK9, although most such compounds lack exquisite specificity for their target. One such compound, flavopiridol, an inhibitor of CDK9, has shown promising activity in chronic lymphocytic leukemia, particularly in patients with high-risk features and refractory disease.23 The most promising approach to inhibiting regulatory pathways of cell proliferation has resulted from targeting receptor tyrosine kinases. These receptors are constitutively activated by mutations (EGFR in non–small cell lung cancer [NSCLC], BRAF in melanoma and other solid tumors), amplifications (HER2/neu in breast cancer), and activating translocations (EML-4-ALK in NSCLC, BCR-ABL in chronic myelogenous leukemia [CML]). The net effect is to create a cell under constant proliferative stimulus and addicted to the aberrant signal; it dies when the signal is interrupted. These examples have led to a search for additional targets, exploration of both small molecules and antibodies, and a search for therapeutic agents. Some of the agents, particularly the monoclonal antibodies, have proven synergistic with chemotherapy.
Epidermal growth factor receptor (EGFR) inhibitors constitute another class of agents that have proved useful in NSCLC. EGFR is overexpressed in many solid tumors. It plays a critical role in cell cycle progression, proliferation, and survival. Its effects are mediated through RAS. Activation of EGFR is also associated with production of vascular endothelial growth factor (VEGF), leading to angiogenesis and facilitating metastasis. Although the U.S. Food and Drug Administration (FDA) approved the small-molecule tyrosine kinase inhibitor gefitinib as a single agent for the third-line treatment of NSCLC in 2003, it later limited availability of gefitinib to patients continuing to derive benefit. This change occurred in response to new trials that failed to demonstrate a survival benefit in unselected patients with NSCLC24,25 (Table 8-3). Another oral EGFR tyrosine kinase inhibitor, erlotinib, demonstrated an improvement in overall and progression-free survival rates compared with placebo, in patients with NSCLC who had failed at least one prior chemotherapy.26 Surprisingly, however, when gefitinib or erlotinib was added to standard chemotherapy in the first-line management of patients with advanced NSCLC, there was no improvement in response or survival rates.27,28–30
Subsequent trials have revealed that this new class of drugs is highly effective for the small subset of NSCLC patients with tumors that harbor activating mutations in EGFR. Although 40% to 80% of NSCLCs overexpress EGFR, only about 10% to 19% of patients with chemotherapy-refractory NSCLC respond to this therapy.31,32,33 Responses are more frequent in women, nonsmokers, and patients with bronchoalveolar carcinoma or adenocarcinoma with bronchoalveolar features.34 Mutations caused by in-frame deletions or amino acid substitutions clustered around the ATP-binding pocket of the tyrosine kinase domain of EGFR strongly predict the response to gefitinib, suggesting a gain of function and addiction to EGFR signaling and increased sensitivity to gefitinib.35,36 Patients with such mutations of EGFR have an approximately 70% chance of responding to erlotinib or gefitinib.37
In 2004, the FDA approved cetuximab, a chimeric IgG1 monoclonal antibody targeting the extracellular portion of the EGFR, for the treatment of irinotecan-refractory colorectal cancer. The addition of irinotecan to cetuximab in this population results in nearly doubled response rates, suggesting reversal of resistance.38 Panitumumab is another commercially available monoclonal antibody targeting the EGFR, which resulted in an improvement in progression-free survival rates compared with placebo alone in chemotherapy-refractory patients.39 Cetuximab alone results in an improvement in survival rates compared with placebo in chemotherapy-refractory patients.40 Several studies have demonstrated a superior response in patients who develop the typical acneiform rash associated with these agents. More importantly, several trials have proven that patients who have activating mutations of KRAS or BRAF derive no benefit from these antibodies, and, in fact, their inclusion may have a negative effect on response.41,42 In studies evaluating the combination of bevacizumab and chemotherapy, the addition of anti-EGFR antibodies resulted in a deleterious response, most strikingly in KRAS-mutant tumors.43,44 Cetuximab has also proved to be an active agent in the management of squamous cell carcinoma of the head and neck, with or without irradiation.45,46 Despite the activity in combination with irradiation in head and neck cancer, cetuximab combined with chemotherapy and irradiation may have a negative impact on the pathologic complete response in rectal cancer.47 Hypotheses for this include the up-regulation of cyclin-dependent kinase p27, G1 cell cycle arrest induced by cetuximab, and the redundancy of the EGFR pathway.
Another tyrosine kinase inhibitor, imatinib, was designed to target the BCR/ABL mutation that causes chronic myelogenous leukemia. Imatinib produces long-term disease control, with normalization of both blood counts and cytogenetics, in the chronic phases of the disease.48 Newer inhibitors, norlotinib and dasatinib, kill chronic myelogenous leukemia cells with mutations that confer resistance to imatinib and provide alternative therapy for drug-resistant patients. Imatinib was also found to inhibit the KIT (CD117) receptor, and it has proved to be a powerful agent in the management of gastrointestinal stromal tumors. Overexpression of KIT is not the sole predictor of response to imatinib, because this agent has been shown to be ineffective in small cell lung cancer, a tumor that overexpresses KIT. Identification of specific activating mutations within the KIT gene predicts for response in patients with gastrointestinal stromal tumors49 (see Table 8-3). The multitargeted tyrosine kinase inhibitor sunitinib has demonstrated benefit in imatinib-refractory patients, even in those with mutations that are typically associated with a low response rate to imatinib.50
Other regulatory pathways have attracted the interest of drug discovery programs. The phosphatidylinositol-3-kinase (PI3K) pathway activates proliferation and down-regulates the activity of the intrinsic pathway of apoptosis. Inhibitors of various components of this pathway, including PI3K, AKT, mammalian target of rapamycin (mTOR), and IGF-1R, display antitumor activity. There are multiple agents in development and several that target the PI3K-AKT-mTOR pathway, have been approved. The drugs furthest along in the development process are the mTOR inhibitors. Temsirolimus (CCI779) is a synthetic rapamycin ester that received FDA approval for previously untreated patients with high-risk advanced renal cell carcinoma. In a randomized trial of temsirolimus, interferon, or the combination of both agents, progression-free survival rates favored temsirolimus over interferon (3.8 months vs. 1.9 months; p <.001), as did improvement in overall survival rates (10.9 months for temsirolimus, 7.3 months for interferon, and 8.4 months for the combination regimen).51 Everolimus (RAD001) is another mTOR inhibitor that is FDA approved for the treatment of renal cell carcinoma in patients refractory to prior targeted therapies; everolimus improved progression-free survival rates compared with placebo (4 months vs. 1.9 months; hazard ratio [HR], 0.3; p <.0001).52 Everolimus has also demonstrated activity in carcinoid and pancreatic neuroendocrine tumors.53,54 Development of agents targeting PI3K and AKT has been more challenging, and these agents remain under development. Anti-IGF1-R antibodies have notable activity against Ewing’s sarcoma and are progressing through clinical evaluation.
Activating translocations of the anaplastic large-cell kinase gene (ALK) have been found in about 5% of a Western population of patients with NSCLC.55 In a phase I/II trial of the MET-ALK inhibitor PF-02341066, patients possessing the ALK fusion protein exhibited a surprising 53% clinical response rate, with an additional 20% of patients having stable disease for 3 months or more.56 This agent will be evaluated in a phase III trial in patients with NSCLC possessing the ALK fusion protein.
In 2003, the FDA approved bortezomib, a proteosome inhibitor, for patients who have progressed while receiving prior therapy for multiple myeloma. Although the exact mechanism of action for this drug is unclear, it acts in part by down-regulating nuclear factor kappa B (NFκB), a potent survival pathway for cancer cells.57 It is currently approved as part of first-line therapy for multiple myeloma in combination with melphalan and prednisone and as second-line therapy as a single agent for mantle cell lymphoma. It is also being tested in other hematologic malignancies and in solid tumors, either as a single agent or in combination with cytotoxic chemotherapy and radiation therapy.
Newest Areas of Drug Discovery: Angiogenesis and Metastasis; Epigenetics
Although most drug discovery efforts are aimed at pathways and targets that govern the balance of cell proliferation and cell death, other unique aspects of cancer biology have attracted notice, particularly the processes of angiogenesis and metastasis. Work has delineated the molecular mechanisms that allow tumor epithelial cells to metastasize. These cells are able to digest their basement membrane, move through interstitial spaces into capillaries, penetrate vessel walls, and adhere to the extracellular matrix at a distant site. Key to tissue penetration is the elaboration by tumors of metalloproteinases that digest collagen and elastin. Inhibitors of matrix metalloproteinases have entered clinical trials as antimetastatic and antiangiogenic agents, but as a class, the antimetastatic drugs have been disappointing. The process of tumor angiogenesis has been traced to tumor cell secretion of angiogenic factors such as basic fibroblast growth factor and vascular endothelial growth factor (VEGF). Specific inhibitors of this process, such as anti-VEGF antibody (bevicizumab) (see Table 8-3) and anti-VEGFR small molecules (sunitinib and sorafenib), are now commercially available.58
In 2004, the humanized monoclonal antibody bevacizumab was approved by the FDA for the first-line treatment of metastatic colorectal cancer. Compared with chemotherapy alone, the addition of bevacizumab improved the response rate, progression-free survival rate, and overall survival rate.59 Bevacizumab has demonstrated activity as a single agent in renal cell carcinoma, but in many solid tumors, it has little or no effect when used alone. A study of neoadjuvant 5-FU combined with radiation therapy for T3 rectal cancer demonstrated a decrease in tumor perfusion, vascular volume, microvascular density, interstitial fluid pressure, and the number of viable circulating endothelial cells and progenitor cells after a single administration of bevacizumab.60,61 It is anticipated that a reduction in interstitial pressure can improve oxygenation and delivery of the chemotherapy to the tumor, enhancing the radiosensitization properties of the chemotherapy. Bevacizumab is currently approved by the FDA for combination with 5-FU-based chemotherapy in first- and second-line treatment of metastatic colorectal cancer, for combination with interferon for renal cell carcinoma, for second-line treatment for glioblastoma multiforme, for metastatic HER2-negative breast cancer, and for combination with chemotherapy for metastatic NSCLC.
Other antibodies target the extracellular portion of the VEGF receptors and small molecules inhibit the intracellular tyrosine kinase functions of these receptors. Some of these compounds have multiple targets, affecting more than one tyrosine kinase. One such example is sunitinib (SU11248), a multitargeted tyrosine kinase inhibitor. Sunitinib inhibits KDR (formerly designated VEGFR or VEGFR2), c-KIT, and platelet-derived growth factor receptor (PDGFR). In a phase III trial of sunitinib versus interferon-alfa in patients with metastatic renal cell carcinoma, sunitinib produced a superior progression-free survival rate, leading to its approval by the FDA.62 Sunitinib also improved time to tumor progression in patients with gastrointestinal stromal tumors (GIST) who failed imatinib therapy, resulting in its approval by the FDA.50 Sunitinib is currently being studied in many solid tumors, including hepatocellular carcinoma and colorectal cancer. Another drug designed to target angiogenesis is sorafenib (BAY 43-9006), an inhibitor of RAF kinase and KDR. In a phase III trial of sorafenib versus placebo in patients with Child A cirrhosis and unresectable hepatocellular carcinoma, sorafenib resulted in an improvement in overall survival rates.63 Sorafenib also improved survival rates when compared with placebo in patients with metastatic clear cell carcinoma of the kidney.64
Epigenetics is a rapidly expanding area of research that examines the control of gene expression by histones and by DNA methylation. Histones wrap around DNA and, in their unmodified state, block access by polymerases and transcription factors. Methylation, acetylation, or phosphorylation of histones decompacts chromatin, increases gene expression, and can induce differentiation and apoptosis in tumor cells. Histone deacetylase inhibitors (HDACs) are a new class of antitumor drugs that can induce cell cycle arrest and differentiation. They may cause hyperacetylation of nonhistone proteins as well, including TP53, HSP90, Raf, Akt, ERB2, and BCR-ABL. Through their effects on histones or key oncoproteins they exert antitumor activity.65 They may also serve as radiation sensitizers.66 Two new agents of this class, vorinostat and romedepsin, are FDA approved for patients with cutaneous T cell lymphoma. Their most common side effects are diarrhea, fatigue, nausea, and loss of appetite, but they may also prolong the cardiac QT interval and may induce arrhythmias. They are currently being studied in multiple solid tumors, including NSCLC.
Poly(ADP-ribose) polymerase (PARP) is a nuclear enzyme that signals the presence of DNA damage by catalyzing the addition of ADP-ribose units to DNA, histones, and other DNA repair enzymes by facilitating repair of DNA strand breaks.67 Increased activity of this enzyme may confer resistance to DNA-damaging agents such as topoisomerase inhibitors, platinum analogs, alkylators, and radiation. Patients with BRCA mutations, who have mutations in DNA repair enzymes, may be more sensitive to the effects of PARP inhibition. In a phase II trial, patients with triple-negative breast cancer were randomized to receive gemcitabine and carboplatin with or without the PARP1 inhibitor BSI-201. The addition of BSI-201 resulted in an improvement in response rates (48% vs. 16%, p = .002), progression-free survival rates (6.9 months vs. 3.3 months, p <.001), and overall survival rates (9.2 months vs. 5.7 months, p = .0005).68 A phase III trial of the same design is currently under way. Promising results have also been forthcoming from PARP inhibitor trials in ovarian cancer. Combination therapy trials with DNA damaging agents such as cisplatin and temozolomide are planned.
Thalidomide and its analogs have defied categorization with respect to their antitumor activity. Thalidomide was initially developed as a sedative, but it was taken off the market in the early 1960s when it was found to cause congenital birth defects. These congenital defects led investigators to postulate that the drug interfered with angiogenesis. It has long been used in the treatment of erythema nodosum leprosum. Its role in angiogenesis was confirmed in a rabbit cornea micropocket assay. The exact mechanisms of action remain unclear. Thalidomide and its analogs have other biologic actions; they strongly modulate inflammatory cytokines such as tumor necrosis factor–alpha (TNF-α), gamma-interferon, interleukin-12, cyclooxygenase-2, and, possibly, NFκB. They also inhibit interleukin-6 and the stimulating effects of insulin-like growth factor-1. Thalidomide is an important secondary therapy for multiple myeloma, and it is being investigated in several other malignant diseases. A potentially more effective and less toxic thalidomide analog, lenalidomide, has been approved for myeloma. It produces impressive hematologic remissions in the 5q- forms of myelodysplasia, although the mechanism of its antitumor activity is not clear.69
Arsenic trioxide is another rediscovered drug and is approved for the management of acute promyelocytic leukemia. Similar to thalidomide, the exact mechanisms of action responsible for its efficacy are unclear. It appears to be antiproliferative and antiangiogenic. Its antiproliferative effects are mediated through the inactivation of cyclins and cyclin-dependent kinases.70 Exposure of human xenograft models has been shown to induce apoptosis of endothelial cells in new blood vessels. Arsenic trioxide has been shown to inhibit production of VEGF in a leukemic cell line.71
Pharmacokinetics and Pharmacodynamics
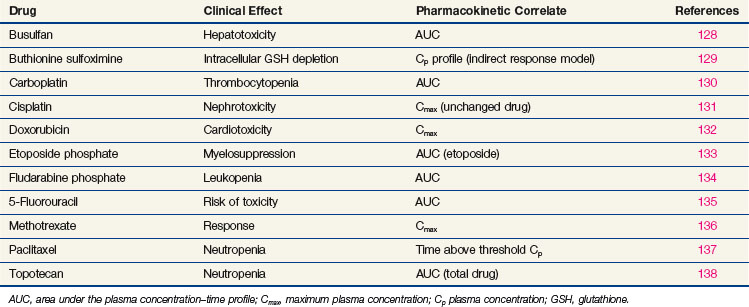
In the treatment of cancer patients, the ultimate effectiveness of therapy is determined by three factors: the inherent sensitivity of the tumor, the ability to deliver drug to its site of action in therapeutic concentrations, and the limitations of host toxicity. It is generally not possible, except in hematologic malignancies, to measure drug concentrations in tumor, although new imaging techniques such as positron emission tomography and nuclear magnetic resonance imaging do allow noninvasive tracking of the distribution and transformation of some drugs in humans. The pharmacokinetic profile of drug in plasma, or change in drug concentration over time, represents the closest correlate of tumor exposure accessible to measurement in the usual clinical setting. In the initial clinical trials of new agents, pharmacokinetic studies are critical for determining whether cytotoxic drug concentrations are achieved and for adjusting the route and schedule of drug administration to achieve an optimal profile, as suggested by preclinical models. In phase II and III trials, pharmacokinetics can yield important additional information on the effects of age, gender, and organ dysfunction on drug clearance. Pharmacokinetic studies can reveal the existence of drug interactions. Correlations between pharmacokinetic and pharmacodynamic endpoints, such as organ toxicity and therapeutic outcome, are more difficult to establish, but some important insights into these relationships have been identified for several anticancer drugs (Table 8-4).
For antimetabolites, most natural product drugs, and platinum compounds, pharmacodynamic endpoints such as antitumor effects and toxicity to bone marrow and intestinal epithelium correlate best with the area under the plasma concentration time curve (see Fig. 8-2). For other drugs, such as paclitaxel, the duration of time above a threshold plasma concentration (0.05 to 0.1 µM) correlates best with toxicity to marrow, and there is preliminary evidence that duration above a threshold level also correlates with clinical effectiveness72 (see Fig. 8-2). Only for alkylating agents, which display a relatively simple relationship between peak plasma concentration and toxicity, has the duration of systemic exposure had little correlation with the pharmacologic effect. In this instance, dose rather than drug concentration over time correlates best with toxicity and tumor cell kill.
The Special Problem of Drug Sanctuaries
Most chemotherapy agents achieve very low concentrations in the brain when administered systemically because the blood-brain barrier, a compact alignment of microvascular endothelium, limits the passage of large molecules and hydrophilic agents. A disruption in the barrier by metastases or primary brain tumors may allow for successful penetration into the central nervous system. The activity of the agent on primary brain tumors and metastases not only depends on penetration of the blood-brain barrier but also on the sensitivity of the underlying malignant tumor to chemotherapy. Although radiotherapy is a more effective means of treating many types of brain metastases, systemic chemotherapy with temozolomide, a highly lipophilic alkylating agent, extends the survival time of malignant glioma patients when it is used in conjunction with irradiation. Other drugs clearly reach intracranial tumors.73–75 EGFR inhibitors have demonstrated activity against brain metastases due to NSCLC.76 In fact, for brain metastases due to choriocarcinoma, combination chemotherapy that includes methotrexate is the preferred mode of treatment.77 Similarly, primary central nervous system lymphomas are effectively treated with high-dose methotrexate instead of radiotherapy.78 The VEGF inhibitor bevacizumab demonstrated activity in patients with glioblastoma multiforme (GBM) who progressed following temozolomide and radiotherapy,79 and has been approved for this clinical use by the FDA. The pan-VEGF receptor tyrosine kinase inhibitor cediranib dramatically decreases peritumoral edema in these patients.80 Therefore, despite the blood-brain barrier, there is precedent for treating intracranial tumors with chemotherapy.
Determinants of Drug Clearance
Anticancer drugs are cleared from the body by one mechanism or a combination of several mechanisms, including renal excretion, hepatic metabolism, chemical decomposition, or metabolic alteration at extrahepatic sites (Table 8-5). Considerable variation is often found among individuals in their ability to clear anticancer drugs, which may necessitate dose adjustment for patients presenting with renal or hepatic dysfunction. Other factors, such as gender, age, serum albumin concentration, lean body mass, nutritional status, and performance status, can influence the pharmacokinetic behavior and pharmacologic effect of a drug, but their quantitative impact on drug disposition is difficult to predict. Inherited defects (polymorphisms) in metabolic capability may lead to severe unexpected toxicity for drugs such as 5-FU (i.e., dihydropyrimidine dehydrogenase deficiency)81 and 6-mercaptopurine (i.e., thiopurine methyltransferase deficiency).82 Genetic variation in the UDP*–glucuronosyltransferase 1A1 gene (UGT1A gene), which is associated with Gilbert’s syndrome, results in severe toxicity, primarily neutropenia, in patients receiving irinotecan because of the inability to conjugate and clear the active compound, SN-38.83
| Primary Clearance Mechanism | Drug | Dose Modification for Organ Dysfunction |
|---|---|---|
| Hepatic metabolism CYP450-mediated oxidation | Busulfan | Y |
| Chlorambucil | N | |
| Cyclophosphamide* | N | |
| Ifosfamide* | N | |
| Paclitaxel | Y | |
| Thiotepa | N | |
| Vinca alkaloids | Y | |
| Reduction | Mitomycin C* | N |
| Conjugation | Etoposide | N |
| 6-Mercaptopurine | Y† | |
| Soluble enzymes | Ara-C | N |
| 5-Fluorouracil | Y‡ | |
| Gemcitabine | N | |
| Nonenzymatic hydrolysis | Camptothecins | N |
| BCNU* | N | |
| Mechlorethamine | N | |
| Melphalan | N | |
| Biliary excretion | Doxorubicin | P |
| Irinotecan | Y | |
| Vinca alkaloids | Y | |
| Renal excretion | Bleomycin | Y |
| Carboplatin | Y | |
| Deoxycoformycin | Y | |
| Etoposide | Y | |
| Fludarabine | Y | |
| Hydroxyurea | Y | |
| Methotrexate | Y | |
| Topotecan | Y |
Y, yes; N, no; P, probably.
* Enzymatic or spontaneous chemical reactions required for drug activation.
† Methyltransferase deficiency.
‡ Dihydropyrimidine dehydrogenase deficiency.
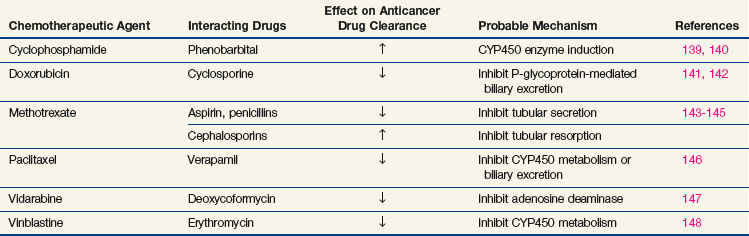
When drugs are used in combination, the potential for interactions that enhance or inhibit antitumor effects becomes an important consideration. Synergistic effects have been convincingly demonstrated for a number of combinations in experimental systems, but clinical results are difficult to interpret. The success of drug combinations against acute leukemia, lymphomas, testicular cancer, and breast cancer (in the adjuvant setting), when single drugs have only temporary effects, probably represents clinical synergy. In other circumstances, drug action may be enhanced by the concurrent administration of a second agent that increases drug binding to its target, such as leucovorin (5-formyl tetrahydrofolate) enhancement of 5-FU binding to thymidylate synthase. Purely pharmacokinetic interactions of cancer drugs with antibiotics, anti-inflammatory agents, and psychotropic drugs may lead to unexpected complications or a lack of drug effect; the more important examples are shown in Table 8-6.
TABLE 8-6 Drug Resistance Mechanisms in Cancer Chemotherapy
| Molecular Mechanism | Example | Drugs Affected |
|---|---|---|
| Decrease in cellular uptake | Deletion of folate transporter | Methotrexate |
| Deletion of nucleoside transporter | Ara-C | |
| Increase in cellular efflux | Increased multidrug resistance (CD116) expression or gene amplification | Vinca alkaloids |
| Anthracyclines | ||
| Paclitaxel | ||
| Etoposide | ||
| Topotecan | ||
| Alteration of target protein | Mutant dihydrofolate reductase | Methotrexate |
| Mutant topoisomerase I | Topotecan | |
| Mutant tubulin | Vinca alkaloids | |
| Paclitaxel | ||
| Altered BCR-ABL | Imatinib | |
| Deletion of target protein | Deletion of topoisomerase I | Topotecan |
| Deletion of topoisomerase II | Anthracyclines | |
| Etoposide | ||
| Decreased activation of enzyme | Deoxycytidine kinase | Ara-C |
| Decreased folylpolyglutamate synthetase | Fludaribine | |
| Cladribine | ||
| Methotrexate | ||
| Increased detoxification | Increased glutathione or glutathione transferase | Alkylating agents |
| Enhanced DNA repair | Increased nucleotide excision repair | Platinum compounds |
| Nitrosoureas | ||
| Increased O-6-alkyl-guanine alkyl transferase | Procarbazine | |
| Temozolamide | ||
| Defective recognition of DNA adducts | Mismatch repair defect | Platinum compounds |
| Constitutive expression of downstream protein | Mutant KRAS, BRAF | Cetuximab |
| Panitumumab | ||
| Alteration of apoptosis pathways | Mutant TP53, BCL2 | Alkylating agentsAntimetabolites |
From Chabner BA, Longo DL editors: Cancer chemotherapy and biotherapy: principles and practice, ed 2, Philadelphia, 1996, Lippincott-Raven.
Predicting Tumor Response
Pharmacogenomics may enable the clinician to determine which drugs will be poorly tolerated by an individual and may allow more rational selection of a particular antineoplastic agent in the individual patient. For example, a high level of thymidylate synthase, in some tumors the result of a polymorphism in tandem repeats in the gene’s promoter region, predicts for resistance to 5-FU in patients with colorectal cancer.84 Although it is tempting to rely on such information from the primary tumor, thymidylate synthase levels within metastases of various locations may be very different. Genomic polymorphisms in the DNA repair genes ERCC1 (formerly designated XPD) and XRCC1 may be prognostic factors in patients with NSCLC treated with cisplatin.85 Genomic polymorphisms in ERCC1, GSTP1, and the TS 3′ untranslated region also may be helpful in predicting prognosis in patients with colorectal cancer receiving 5-FU and oxaliplatin.86
Transcriptional silencing may alter gene expression and affect the outcome of chemotherapy. O-6-methylguanine-DNA methyltransferase (MGMT) is an enzyme responsible for DNA repair following alkylating agent chemotherapy. The gene may be silenced in tumors by methylation of its promoter, creating vulnerability of the tumor cell to DNA damage. MGMT gene silencing occurs in about 20% of glioblastomas and is associated with a high response rate to the alkylating agent temozolomide in patients with glioblastoma and neuroendocrine tumors.87,88
Schedules of Drug Administration
Cancer drugs are given in repetitive cycles of administration interrupted by rest periods that facilitate the recovery of normal tissues, particularly bone marrow and gastrointestinal mucosa. With the availability of myeloid stimulatory factors such as granulocyte colony-stimulating factor, it is possible to dramatically shorten the period between cycles. The ability to provide such dose-dense therapy with the aid of growth colony-stimulating factor may result in improved outcome without increase in toxicity.89 The theoretical basis for cyclic chemotherapy was established by the work of Skipper and Schabel, who showed in animal models that for any given drug schedule and dose, a specific fraction of tumor cells is killed. Repeated cycles of treatment are therefore required for curative therapy. Whether it is necessary to eliminate the last tumor cell to cure the disease is still in doubt. Molecular markers for residual disease may continue to show the presence of tumor-related sequences years after remission induction in children with acute leukemia, although in diseases such as breast cancer and other solid tumors, such findings are usually the harbinger of relapse.90
Although peak drug plasma concentration, area under the curve (AUC), and time over a threshold level have been correlated with response and toxicity for selected chemotherapeutic agents, clinical investigators lack pharmacokinetic data for most combination therapy regimens. The endpoint in combination clinical studies is typically to maximize dose intensity, which is the amount of drug administered per unit of time. Clinical data support a strong relationship between dose intensity and response in the treatment of breast, ovarian, and colon tumors.91 Taken to its extreme, high-dose chemotherapy with bone marrow stem cell replacement yields the highest dose intensity and the highest response rates in patients who have otherwise refractory tumors, but at a high dollar cost and at the risk of fatal toxicity. In clinical practice, most oncologists administer full doses of drug, using completely reversible and readily tolerated toxicity as their endpoint.
Drug Resistance
The fractional cell kill hypothesis must be modified to incorporate concepts of drug resistance. In practice, the response to an initial cycle of chemotherapy may be much greater than to subsequent doses, and in many solid tumors, such as breast cancer and lung cancer, tumors may again grow rapidly after an initial response. The explanation for this finding lies in the problem of drug resistance and the ability of cancer drugs to select for drug-resistant cells. Mechanisms of resistance may affect any of the steps necessary for drug action: entry of drug into tumor cells by means of active transporters, enzymatic drug activation, action at a molecular target within cells, and even the processes in the cell that trigger cell death. Although many examples of drug resistance have been characterized in model systems, the understanding of resistance in clinical practice remains incomplete. Some of the more common mechanisms in model systems and the evidence for their presence in human tumors are given in Table 8-6.
With few exceptions, single-agent treatment of cancer produces only temporary responses. This rule applies as well to the targeted agents, where resistance to agents such as EGFR inhibitors develops through mutation in the binding site of the target protein or through activation of an alternative proliferation pathway (cMET). The reason for the development of resistance is that at the time of clinical recognition, tumors are significantly heterogeneous because of their underlying genetic instability. Only the sensitive fraction of cells is killed with each dose of drug. Drug-resistant mutants already exist at the time of initial treatment, as demonstrated for imatinib resistance in CML before therapy, and under the selective pressure of therapy, the resistant tumor cells expand in number. Based on the experience of antibiotic treatment of bacterial infections, it was logical to combine drugs that had different mechanisms of resistance. If the frequency of mutation to resistance is 1 cell of 106, the chances of any cell being simultaneously resistant to two drugs would be the product of these probabilities, or 10−12. Goldie and colleagues92 have argued convincingly that the best strategy for chemotherapy is to employ as many non–cross-resistant agents as early as possible in the treatment regimen. Certain qualifying considerations include the following:
Combination Chemotherapy
These are not hard and fast rules. There are numerous examples of clinical regimens that employ multiple alkylating agents, multiple natural products (despite their shared cross-resistance), or combinations of myelotoxic drugs. Often, these choices are dictated by impressive single-agent activity in the tumor being treated and the uncertainty of knowledge about clinical mechanisms of drug resistance. For example, individual alkylating agents differ in their mechanisms of activation, transport, and adduct formation. Their DNA adducts are repaired by a variety of enzymatic pathways. It is not clear which of these factors contributes to clinical drug resistance (see Table 8-6). Although these agents belong to the same class of drugs, in the absence of more complete knowledge about resistance, it is possible to construct a reasonable argument for combining different alkylating agents.
The development of targeted drugs has greatly expanded opportunities to address resistance to cytotoxic chemotherapy. Drugs such as bevacizumab and trastuzumab have significantly enhanced the effectiveness of cytotoxic chemotherapy of colon and breast cancers, respectively. Trastuzumab blocks a pathway that promotes proliferation and survival, whereas bevacizumab normalizes tumor vasculature and improves access of cytotoxics to poorly perfused tumor cells. The further development of targeted drugs in combination may allow successful inactivation of multiple pathways, as exemplified by the joint use of EGFR and MET inhibitors in NSCLC, a strategy that holds great promise for cancer treatment.95 An obvious requirement for implementing this strategy is the ability to perform molecular profiling of tumor samples on a routine basis, something that is not widely available in current practice.
Clinical Schedules of Drug Administration
10 Skipper HE, Perry S. Kinetics of normal and leukemic leukocyte populations and relevance to chemotherapy. Cancer Res. 1970;30:1883.
11 Reiter A, Schrappe M, Ludwig WD, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84:3122.
14 Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673.
19 Li Y, Benzera R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246.
24 Mok TS, Wu YL, Ohe Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;36:947.
25 Thatcher N, Chang A, Parkin P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small cell lung cancer. Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366:1527.
26 Shephard FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small cell lung cancer. N Engl J Med. 2005;353:122.
27 Herbst RS, Prager D, Hermann R, et al. Tribute. A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small cell lung cancer. J Clin Oncol. 2005;23:5892.
31 Artega CL. Erb-B targeted therapeutic approaches in human cancer. Exp Cell Res. 2003;284:122.
34 Shah NT, Miller VA, et al: Bronchoalveolar histology and smoking history predict response to gefitinib [abstract 2524]. Proceedings of the American Association of Clinical Oncology, Orlando, Florida, 2003.
37 Sequist LV, Lynch TJ. EGFR tyrosine kinase inhibitors in lung cancer. An evolving story. Annu Rev Med. 2008;59:429.
38 Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337.
40 Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040.
41 DiNicolantonio F, Martini M, Molinari F, et al. Wild-type Braf is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705.
42 Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with or without cetuximab in the first line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663.
43 Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2008;27:672.
45 Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous cell carcinoma of the head and neck. N Engl J Med. 2006;354:567.
47 Bertolini F, Chiara S, Bengala C, et al. Neoadjuvant treatment with single agent cetuximab followed by 5-FU, cetuximab, and pelvic radiotherapy. A phase II study in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2009;73:466.
48 Druker B. Perspectives on the development of a molecularly targeted agent. Cancer Cell. 2002;1:31.
49 Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342.
50 Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib. A randomised controlled trial. Lancet. 2006;368:1329.
51 Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271.
52 Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma. A double blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449.
53 Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octeotide LAR in advanced low-intermediate grade neuroendocrine tumors. Results of a phase II study. J Clin Oncol. 2008;26:4311.
55 Rodig S, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the Western population. Clin Cancer Res. 2009;15:5216.
56 Kwak EL, Camidge DR, Clark J, et al: Clinical activity observed in a phase I dose escalation study of an oral c-met and ALK inhibitor, PF-02341066 [abstract 3509]. Proceedings of the American Association of Clinical Oncology, Chicago, 2009.
57 Voorhees PM, Dees EC, O’Neil B, et al. The proteosome as a target for cancer therapy. Clin Cancer Res. 2003;9:6316.
59 Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335.
60 Willett C, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145.
61 Willet CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer. A multidisciplinary phase II study. J Clin Oncol. 2009;27:3020.
62 Motzer RI, Hutson TE, Tomczak TE, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584.
63 Lovett JM, Ricci S, Mazzeferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378.
66 Munshi A, Toshimitsu T, Hobbs ML, et al. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of γ-H2AX foci. Mol Cancer Ther. 2006;5:1967.
67 Ratnam K, Low JA. Current development of clinical inhibitors of poly (ADP-ribose) polymerase in oncology. Clin Cancer Res. 2007;13:1383.
68 O’Shaughnessy J, Osborne C, Pippen J, et al: Efficacy of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP-1) inhibitor, in combination with gemcitabine/carboplatin (G/C) in patients with triple negative breast cancer (TNBC). Results of a randomized phase II trial [abstract 3]. Proceedings of the American Association of Clinical Oncology, Chicago, 2009.
78 Ferreri AJ, Reni M, Villa E. Therapeutic management of primary central nervous system lymphoma. Lessons from prospective trials. Ann Oncol. 2000;11:927.
79 Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733.
80 Batchelor TT, Sorensen AG, di Tomaso E. AZD 2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83.
1 Goodman LS, Wintrobe MM, Dameshek W, et al. Use of methylbis(beta-chloroethyl)amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia. JAMA. 1946;132:126.
2 DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology, ed 4, Philadelphia: J.B. Lippincott, 1993.
3 Elion GB. Biochemistry and pharmacology of purine analogs. Fed Proc. 1967;26:898.
4 Johnson IS. Historical background of Vinca alkaloid research and areas of future interest. Cancer Chemother Rep. 1968;52:455.
5 Wakaki S, Marumo H, Tomioka K. Isolation of new fractions of antitumor mitomycins. Antibiot Chemother. 1958;8:228.
6 Umezawa H, Maeda K, Takeuchi T, et al. New antibiotics, bleomycin A and B. J Antibiot (Tokyo). 1966;19:200.
7 Arcamone F, Cassinelli G, Fantini G, et al. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng. 1969;11:1101.
8 Wani MC, Taylor HL, Wall ME, et al. Plant antitumor agents. VI. The isolation and structure of Taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325.
9 Wall ME, Wani MC, Cook CE, et al. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc. 1966;88:3888.
10 Skipper HE, Perry S. Kinetics of normal and leukemic leukocyte populations and relevance to chemotherapy. Cancer Res. 1970;30:1883.
11 Reiter A, Schrappe M, Ludwig WD, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84:3122.
12 Heidelberger C, Chaudhuari NK, Daneberg P, et al. Fluorinated pyrimidines. A new class of tumor inhibitory compounds. Nature. 1957;179:663.
13 Huang P, Chubb S, Hertel LW, et al. Action of 2′,2′-difluo-rodeoxycytidine on DNA synthesis. Cancer Res. 1991;51:6110.
14 Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673.
15 Series: Multimodality treatment of cancer. Oncology. 1997;11(Suppl 9):9.
16 Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57.
17 Barinaga M. From bench top to bedside. Science. 1997;278:1036.
18 Lowe SW, Ruley HE, Jacks T, et al. P53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957.
19 Li Y, Benzera R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246.
20 Koc ON, Phillips WPJr, Lee K, et al. Role of DNA repair in resistance to drugs that alkylate O6 of guanine. Cancer Treat Res. 1996;87:123.
21 Gorlick R, Goker E, Trippett T, et al. Defective transport is a common mechanism of acquired methotrexate resistance in acute lymphocytic leukemia and is associated with decreased reduced folate carrier expression. Blood. 1997;89:1013.
22 Gibbs JB, Oliff A, Kohl NE. Farnesyltransferase inhibitors. Ras research yields a potential cancer therapeutic. Cell. 1994;77:175.
23 Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399.
24 Mok TS, Wu YL, Ohe Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;36:947.
25 Thatcher N, Chang A, Parkin P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small cell lung cancer. Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366:1527.
26 Shephard FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small cell lung cancer. N Engl J Med. 2005;353:122.
27 Herbst RS, Prager D, Hermann R, et al. Tribute. A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small cell lung cancer. J Clin Oncol. 2005;23:5892.
28 Gatzmeier U, Pluzanska A, Sczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small cell lung cancer. The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545.
29 Giaccone G, Herbst RS, Manegold G, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer. A phase III trial—Intact 1. J Clin Oncol. 2004;22:777.
30 Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer. A phase III trial—Intact 2. J Clin Oncol. 2004;22:785.
31 Artega CL. Erb-B targeted therapeutic approaches in human cancer. Exp Cell Res. 2003;284:122.
32 Kris MG, Natale RB, Herbst RB, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer. A randomized trial. JAMA. 2003;290:2149.
33 Fukuoka M, Yano S, Giaccone G, et al. Randomized multi-institutional phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol. 2003;21:2237.
34 Shah NT, Miller VA, et al: Bronchoalveolar histology and smoking history predict response to gefitinib [abstract 2524]. Proceedings of the American Association of Clinical Oncology, Orlando, Florida, 2003.
35 Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small cell lung cancer to gefitinib. N Engl J Med. 2003;35:2129.
36 Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497.
37 Sequist LV, Lynch TJ. EGFR tyrosine kinase inhibitors in lung cancer. An evolving story. Annu Rev Med. 2008;59:429.
38 Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337.
39 van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658.
40 Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040.
41 DiNicolantonio F, Martini M, Molinari F, et al. Wild-type Braf is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705.
42 Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with or without cetuximab in the first line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663.
43 Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2008;27:672.
44 Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563.
45 Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous cell carcinoma of the head and neck. N Engl J Med. 2006;354:567.
46 Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171.
47 Bertolini F, Chiara S, Bengala C, et al. Neoadjuvant treatment with single agent cetuximab followed by 5-FU, cetuximab, and pelvic radiotherapy. A phase II study in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2009;73:466.
48 Druker B. Perspectives on the development of a molecularly targeted agent. Cancer Cell. 2002;1:31.
49 Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342.
50 Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib. A randomised controlled trial. Lancet. 2006;368:1329.
51 Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271.
52 Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma. A double blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449.
53 Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octeotide LAR in advanced low-intermediate grade neuroendocrine tumors. Results of a phase II study. J Clin Oncol. 2008;26:4311.
54 Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrinetumors after failure of cytotoxic chemotherapy. A phase II trial. J Clin Oncol. 2010;28:69.
55 Rodig S, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the Western population. Clin Cancer Res. 2009;15:5216.
56 Kwak EL, Camidge DR, Clark J, et al: Clinical activity observed in a phase I dose escalation study of an oral c-met and ALK inhibitor, PF-02341066 [abstract 3509]. Proceedings of the American Association of Clinical Oncology, Chicago, 2009.
57 Voorhees PM, Dees EC, O’Neil B, et al. The proteosome as a target for cancer therapy. Clin Cancer Res. 2003;9:6316.
58 Folkman J. Angiogenic therapy. In: Devita VT, Rosenberg SA, Hellman S, editors. Cancer: principles and practice of oncology. ed 5. Philadelphia: Lippincott-Raven; 1997:3075.
59 Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335.
60 Willett C, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145.
61 Willet CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer. A multidisciplinary phase II study. J Clin Oncol. 2009;27:3020.
62 Motzer RI, Hutson TE, Tomczak TE, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584.
63 Lovett JM, Ricci S, Mazzeferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378.
64 Escudier B, Eisen T, Stadler WM, et al. Sorafenib in clear cell renal cell carcinoma. N Engl J Med. 2007;356:125.
65 Kim TY, Bang YJ, Robertson KD. Histone deacetylase inhibitors for cancer therapy. Epigenetics. 2006;1:14.
66 Munshi A, Toshimitsu T, Hobbs ML, et al. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of γ-H2AX foci. Mol Cancer Ther. 2006;5:1967.
67 Ratnam K, Low JA. Current development of clinical inhibitors of poly (ADP-ribose) polymerase in oncology. Clin Cancer Res. 2007;13:1383.
68 O’Shaughnessy J, Osborne C, Pippen J, et al: Efficacy of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP-1) inhibitor, in combination with gemcitabine/carboplatin (G/C) in patients with triple negative breast cancer (TNBC). Results of a randomized phase II trial [abstract 3]. Proceedings of the American Association of Clinical Oncology, Chicago, 2009.
69 Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet. 2004;363:1802.
70 Lew YS, Brown SL, Griffin RJ, et al. Arsenic trioxide causes selective necrosis in solid murine tumors by vascular shutdown. Cancer Res. 1999;59:6033.
71 Roboz GJ, Dias S, Lam G, et al. Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000;96:1525.
72 Wilson WH, Berg S, Bryant G, et al. Paclitaxel in doxorubicin-refractory or mitoxantrone-refractory breast cancer. A phase I/II trial of 96-hour infusion. J Clin Oncol. 1994;12:1621.
73 Moscetti L, Nelli F, Felici A, et al. Up-front chemotherapy and radiation treatment in newly diagnosed non-small cell lung cancer with brain metastases. Survey by Outcome Network for Evaluation of Treatment Reuslts in Oncology. Cancer. 2007;15:274.
74 Chen G, Huynh M, Chen A. Chemotherapy for brain metastases in small-cell lung cancer. Clin Lung Cancer. 2008;9:35.
75 Rosner D, Nemoto T, Lane WW. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer. 1986;58:832.
76 Kim JE, Lee DH, Choi Y. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastases. Lung Cancer. 2009;65:351.
77 Rustin GJ, Newlands ES, Begent RH. Weekly alternating etoposide, methotrexate, and actinomycin/vincristine and cyclophosphamide chemotherapy for the treatment of CNS metastases of choriocarcinoma. J Clin Oncol. 1989;7:900.
78 Ferreri AJ, Reni M, Villa E. Therapeutic management of primary central nervous system lymphoma. Lessons from prospective trials. Ann Oncol. 2000;11:927.
79 Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733.
80 Batchelor TT, Sorensen AG, di Tomaso E. AZD 2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83.
81 Diasio RB, Beavers TL, Carpenter T. Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity. J Clin Invest. 1988;81:47.
82 Lennard L, VanLoon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity. Relationship to thiopurine methyl-transferase genetic polymorphism. Clin Pharmacol Ther. 1989;46:149.
83 Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22:1382.
84 Marsh S, McLeod HL. Cancer pharmacogenetics. Br J Cancer. 2004;90:8.
85 Gurubhagavatula S, Liu G, Park S, et al. XPD and XRCC1 genetic polymorphisms are prognostic factors in advanced non-small-cell lung cancer patients treated with platinum chemotherapy. J Clin Oncol. 2004;22:2594.
86 Stoehlmacher J, Park DJ, Zhang W, et al. A multivariate analysis of genomic polymorphisms. Prediction of clinical outcome to 5-FU/oxaliplatin combination chemotherapy in refractory colorectal cancer. Br J Cancer. 2004;91:344.
87 Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189.
88 Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15:338.
89 Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer. First report of Intergroup C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:2226.
90 Cote RJ, Taylor C, Neville AM. Detection of occult metastases. Cancer J. 1997;8:49.
91 Hryniuk WM, Levine MN, Levin L. Analysis of dose intensity for chemotherapy in early stage (stage II) and advanced breast cancer. Natl Cancer Inst Monogr. 1986;1:87.
92 Goldie JH, Coldman AJ, Gudanskas GA. Rationale for the use of alternating non-cross-resistant chemotherapy. Cancer Treat Rep. 1982;65:439.
93 Sidransky D, Hollstein M. Clinical implications of the p53 gene. Annu Rev Med. 1996;47:285.
94 Gianni L, Munzone E, Capri G, et al. Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer. High antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol. 1995;13:2688.
95 Turke AB, Zejnullahu K, Song Y, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77.
96 Cassidy J, Clarke S, Diaz-Rubio E, et al. Oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006.
97 Al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer. An intergroup study. J Clin Oncol. 1997;15:277.
98 Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462.
99 Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small cell lung cancer. N Engl J Med. 1994;330:153.
100 Piccart MJ, de Valeriola D, Paridaens R, et al. Six-year results of a multimodality treatment strategy for locally advanced breast cancer. Cancer. 1988;62:2501.
101 Scholl SM, Fourquet A, Asselain B, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast-conserving surgery. Preliminary results of a randomized trial—S6. Eur J Cancer. 1994;30A:645.
102 Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709.
103 Tepper JE, O’Connell MJ, Petroni GR, et al. Adjuvant postoperative fluorouracil-modulated chemotherapy combined with pelvic radiation therapy for rectal cancer. Initial results of inter-group 0114. J Clin Oncol. 1997;15:2030.
104 Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731.
105 Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b trial. N Engl J Med. 1997;337:949.
106 Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956.
107 Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small cell lung cancer. Seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88:1210.
108 Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small cell lung cancer. N Engl J Med. 1992;327:1618.
109 Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer. Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310.
110 Fabian CJ, Mansfield CM, Dahlberg S, et al. Low-dose involved field radiation after chemotherapy in advanced Hodgkin disease. A Southwest Oncology Group randomized study. Ann Intern Med. 1994;120:903.
111 Horning SJ, Weller E, Kim KM. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma. Eastern Cooperative Oncology Group Study 1484. J Clin Oncol. 2004;22:3032.
112 Connors JM, Klimo P, Fairey RN, Voss N. Brief chemotherapy and involved field radiation therapy for limited-stage, histologically aggressive lymphoma. Ann Intern Med. 1987;107:25.
113 Longo DL, Glatstein E, Duffey PL, et al. Treatment of localized aggressive lymphomas with combination chemotherapy followed by involved-field radiation therapy. J Clin Oncol. 1989;7:1295.
114 Epidermoid anal cancer. Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049.
115 Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer. Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040.
116 Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324:1685.
117 Lefebvre JL, Chevalier D, Luboinski B, et al. Larynx preservation in pyriform sinus cancer. Preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:890.
118 Kachnic LA, Kaufman DS, Heney NM, et al. Bladder preservation by combined modality therapy for invasive bladder cancer. J Clin Oncol. 1997;15:1022.
119 Wani MC, Taylor HL, Wall ME, et al. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1997;93:2325.
120 Brockman RW, Schabel FMJr, Montgomery JA. Biologic activity of 9-beta-D-arabinofuranosyl-2-fluoroadenine, a metabolically stable analog of 9-beta-D-arabinofuranosyladenine. Biochem Pharmacol. 1977;26:2193.
121 Kohl NE, Mosser SD, deSolms SJ, et al. Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science. 1993;260:1934.
122 Monia BP, Johnston JF, Geiger T, et al. Antitumor activity of a phosphorothioate antisense oligodeoxynucleotide targeted against C-raf kinase. Nat Med. 1996;2:668.
123 Pegram MD, Baly D, Wirth C, et al: Antibody-dependent cell-mediated cytotoxicity in breast cancer patients in phase III clinical trials of a humanized anti-HER2 antibody [abstract 4044]. Proceedings of the American Association for Cancer Research, San Diego, 1997.
124 Yuan F, Chen Y, Dellian M, et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an antivascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci U S A. 1996;93:14765.
125 O’Reilly M, Boehm T, Shing Y, et al. Endostatin. An endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277.
126 O’Reilly M, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med. 1996;2:689.
127 Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260.
128 Vassal G. Pharmacologically guided dose adjustment of busulfan in high-dose chemotherapy regimens. Rationale and pitfalls [review]. Anticancer Res. 1994;14:2363.
129 Gallo JM, Brennan J, Hamilton TC, et al. Time-dependent pharmacodynamic models in cancer chemotherapy. Population pharmacodynamic model for glutathione depletion following modulation by buthionine sulfoximine (BSO) in a phase I trial of melphalan and BSO. Cancer Res. 1995;55:4507.
130 Egorin MJ, Van Echo DA, Olman EA, et al. Prospective validation of a pharmacologically based dosing scheme for the cis-diamminedichloroplatinum(II) analogue diamminecyclobutanedicarboxylatoplatinum. Cancer Res. 1985;45:6502.
131 Nagai N, Kinoshita M, Ogata H, et al. Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol. 1996;39:131.
132 Cummings J, Smyth JF. Pharmacology of adriamycin. The message to the clinician. Eur J Cancer Clin Oncol. 1988;24:579.
133 Kaul S, Srinivas NR, Igwemezie LN, Barbhaiya RH. A pharmacodynamic evaluation of hematologic toxicity observed with etoposide phosphate in the treatment of cancer patients. Semin Oncol. 1996;23:15.
134 Malspeis L, Grever MR, Staubus AE, Young D. Pharmacokinetics of 2-F-ara-A (9-β-D-arabinofuranosyl-2-fluoroadenine) in cancer patients during the phase I clinical investigation of fludarabine phosphate. Semin Oncol. 1990;17:18.
135 van Groeningen CJ, Pinedo HM, Heddes J, et al. Pharmacokinetics of 5-fluorouracil assessed with a sensitive mass spectrometric method in patients on a dose escalation schedule. Cancer Res. 1988;48:6956.
136 Graf N, Winkler K, Betlemovic M, et al. Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin Oncol. 1994;12:1443.
137 Gianni L, Kearns CM, Giani A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol. 1995;13:180.
138 Grochow LB, Rowinsky EK, Johnson R, et al. Pharmacokinetics and pharmacodynamics of topotecan in patients with advanced cancer. Drug Metab Dispos. 1992;20:706.
139 Jao JY, Jusko WJ, Cohen JL. Phenobarbital effects on cyclophosphamide pharmacokinetics in man. Cancer Res. 1972;32:2761.
140 Chen TL, Passos-Coelho JL, Noe DA, et al. Nonlinear pharmacokinetics of cyclophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Cancer Res. 1995;55:810.
141 Rushing DA, Raber SR, Rodvold KA, et al. The effects of cyclosporine on the pharmacokinetics of doxorubicin in patients with small cell lung cancer. Cancer. 1994;74:834.
142 Kivisto KT, Kroemer HK, Eichelbaum M. The role of human cytochrome P450 enzymes in the metabolism of anticancer agents. Implications for drug interactions. Br J Clin Pharmacol. 1995;40:523.
143 Bannwarth B, Pehourcq F, Schaeverbeke T, Dehais J. Clinical pharmacokinetics of low-dose pulse methotrexate in rheumatoid arthritis. Clin Pharmacokinet. 1996;30:194.
144 Ronchera CL, Hernandez T, Peris JE, et al. Pharmacokinetic interaction between high-dose methotrexate and amoxicillin. Ther Drug Monit. 1993;15:375.
145 Iven I, Brasch H. Cephalosporins increase the renal clearance of methotrexate and 7-hydroxymethotrexate in rabbits. Cancer Chemother Pharmacol. 1990;26:139.
146 Berg SL, Tolcher A, O’Shaughnessy JA, et al. Effect of R-verapamil on the pharmacokinetics of paclitaxel in women with breast cancer. J Clin Oncol. 1995;13:2039.
147 Major PP, Agarwal RP, Kufe DW. Clinical pharmacology of arabinofuranosyladenine in combination with deoxycoformycin. Cancer Chemother Pharmacol. 1983;10:125.
148 Tobe SW, Siu LL, Jamal SA, et al. Vinblastine and erythromycin. An unrecognized serious drug interaction. Cancer Chemother Pharmacol. 1995;35:188.
Chabner BA, Ryan DP, Paz-Ares L, et al. Antineoplastic agents. In Handman JG, Limbird LL, editors: Goodman and Gilman’s: the pharmacologic basis of therapeutics, ed 11, New York: McGraw-Hill, 2006.
Chabner BA, Longo DL, editors. Cancer chemotherapy and biotherapy: principles and practice, ed 4, Philadelphia: Lippincott-Raven, 2006.

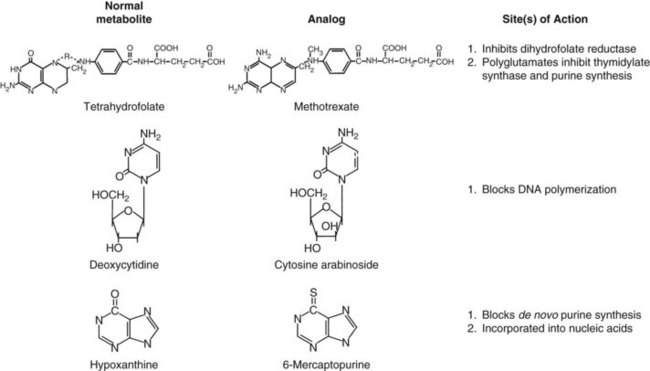
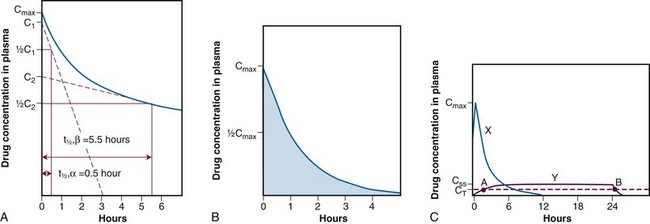
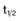 ,α) is the time for C1 to decay to
,α) is the time for C1 to decay to 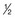 C1. The terminal (β) phase half-life (
C1. The terminal (β) phase half-life (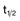 ,β) is the time for C2 to decay to
,β) is the time for C2 to decay to 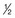 C2. This biphasic behavior results from distribution of the drug among rapidly and slowly perfused regions of the body, as well as its elimination. B, Drug concentration in plasma versus time is plotted on linear axes. The blue shaded area is the area under the curve (AUC); it represents the integral of drug concentration over time. The AUC is a measure of total systemic exposure to the drug. C, Linear plots of drug concentration versus time are illustrated for a rapid intravenous injection (X) and a 24-hour continuous infusion (Y) of the same total dose of drug (the AUCs are equivalent). Notice that the duration of drug concentrations above the threshold for cytotoxicity (CT) is much longer with the continuous infusion (represented as B-A) than with the bolus administration. Conversely, the maximum plasma concentration achieved by bolus administration (Cmax) is much larger than that of the continuous infusion (CSS).
C2. This biphasic behavior results from distribution of the drug among rapidly and slowly perfused regions of the body, as well as its elimination. B, Drug concentration in plasma versus time is plotted on linear axes. The blue shaded area is the area under the curve (AUC); it represents the integral of drug concentration over time. The AUC is a measure of total systemic exposure to the drug. C, Linear plots of drug concentration versus time are illustrated for a rapid intravenous injection (X) and a 24-hour continuous infusion (Y) of the same total dose of drug (the AUCs are equivalent). Notice that the duration of drug concentrations above the threshold for cytotoxicity (CT) is much longer with the continuous infusion (represented as B-A) than with the bolus administration. Conversely, the maximum plasma concentration achieved by bolus administration (Cmax) is much larger than that of the continuous infusion (CSS).