Chapter 237 Principles of Antiviral Therapy
Antiviral chemotherapy typically involves a delicate interplay between host cellular functions and viral targets of action. Many antiviral agents exert significant host cellular toxicity, a limitation that has hindered antiviral drug development. In spite of this limitation, a number of agents are licensed for use against viruses, particularly herpesviruses, respiratory viruses, and hepatitis viruses. In addition to licensed antivirals and recommended regimens (![]() see Table 237-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com), several studies are actively enrolling children for evaluation of novel antiviral therapeutic approaches. These studies are funded by the National Institutes of Health and administered through the Collaborative Antiviral Study Group (CASG), and up-to-date information is available about active clinical protocols at the CASG web page (http://medicine.uab.edu/Peds/CASG/).
see Table 237-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com), several studies are actively enrolling children for evaluation of novel antiviral therapeutic approaches. These studies are funded by the National Institutes of Health and administered through the Collaborative Antiviral Study Group (CASG), and up-to-date information is available about active clinical protocols at the CASG web page (http://medicine.uab.edu/Peds/CASG/).
Table 237-1 CURRENTLY LICENSED ANTIVIRAL DRUGS*
| ANTIVIRAL | TRADE NAME | MECHANISM OF ACTION |
|---|---|---|
| Acyclovir | Zovirax | Inhibits viral DNA polymerase |
| Adefovir | Hepsera | Nucleotide reverse transcriptase inhibitor |
| Amantadine | Symmetrel | Blocks M2 protein ion channel |
| Cidofovir | Vistide | Inhibits viral DNA polymerase |
| Famciclovir | Famvir | Inhibits viral DNA polymerase |
| Fomivirsen | Vitravene | Phosphorothioate oligonucleotide inhibits viral replication via antisense mechanism |
| Foscarnet | Foscavir | Inhibits viral DNA polymerase and reverse transcriptase at pyrophosphate-binding site |
| Ganciclovir | Cytovene | Inhibits viral DNA polymerase |
| Idoxuridine | Herplex | Inhibits viral DNA polymerase |
| Interferon-α | Intro-A (interferon-α 2b) Roferon-A (interferon-α 2a) Infergen (interferon alfacon-1) |
Produces multiple effector proteins that exert antiviral effects; also directly interacts with immune system components |
| Interferon-α 2b plus ribavirin | Rebetron | Not established |
| Lamivudine | Epivir | Inhibits viral DNA polymerase and reverse transcriptase |
| Oseltamivir | Tamiflu | Neuraminidase inhibitor; interference with de-aggregation and release of viral progeny |
| Pegylated interferon | PEG-Intron (α 2b), Pegasys (α 2a) | Same as interferon |
| Penciclovir | Denavir | Inhibits viral DNA polymerase |
| Ribavirin | Virazole, Rebetol, Copegus | Interference with viral messenger RNA |
| Rimantadine | Flumadine | Blocks M2 protein ion channel |
| Trifluridine | Viroptic | Inhibits viral DNA polymerase |
| Valacyclovir | Valtrex | Same as acyclovir |
| Valganciclovir | Valcyte | Same as ganciclovir |
| Vidarabine | Ara-A | Inhibits viral DNA polymerase (and to lesser extent, cellular DNA polymerase) |
| Zanamivir | Relenza | Neuraminidase inhibitor; interference with de-aggregation and release of viral progeny |
| FDA-APPROVED COMBINATION THERAPIES | ||
| Interferon-α 2b + ribavirin | Rebetron (Intron-A plus Rebetol) | |
| Interferon-α 2a + ribavirin | Roferon-A + ribavirin | |
| Pegylated interferon-α 2b + ribavirin | PEG-Intron + Rebetol | |
| Pegylated interferon-α 2a + ribavirin | Pegasys + Copegus | |
* See Chapter 268 for antiretroviral drugs.
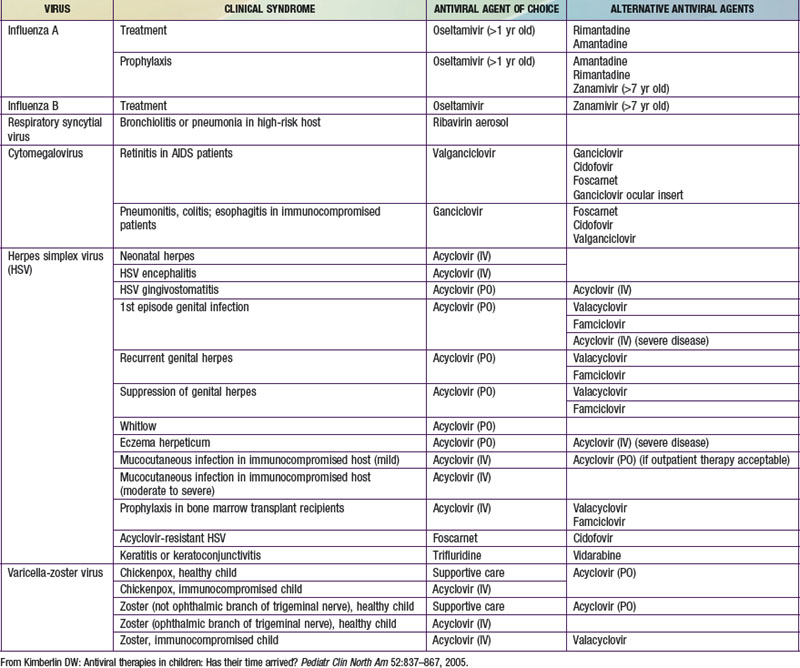
In making the decision to commence antiviral drugs, it is important for the clinician to obtain appropriate diagnostic specimens, which can help clarify the antiviral of choice. The choice of a specific antiviral is based on the recommended agent of choice for a particular clinical condition, pharmacokinetics, toxicities, cost, and the potential for development of resistance (Table 237-2). Intercurrent conditions in the patient, such as renal insufficiency, should also be considered. Clinicians must monitor antiviral therapy closely for adverse events or toxicities, both anticipated and unanticipated.
Antivirals Used for Herpesviruses
Antivirals Used for Respiratory Viral Infections
Oseltamivir and Zanamivir
Oseltamivir is administered as an esterified prodrug that has high oral bioavailability. It is eliminated by tubular secretion, and dosage adjustment is required for patients with renal insufficiency. Gastrointestinal adverse effects, including nausea and vomiting, are occasionally observed. The drug is indicated for both treatment and prophylaxis. The usual adult dosage for treatment of influenza is 75 mg twice daily for 5 days. Treatment should be initiated within 2 days of the appearance of symptoms. Recommended treatment dosages for children vary by weight: 30 mg twice a day for children ≤15 kg, 45 mg twice a day for children weighing 15-23 kg, 60 mg twice a day for those weighing 23-40 kg, and 75 mg twice a day for children ≥40 kg. Dosages for chemoprophylaxis are the same for each weight group, but drug should be administered only once daily rather than twice daily. Although the drug is not approved for children <1 year of age, the Centers for Disease Control and Prevention have issued a recommendation that oseltamivir be used in children in this age group for prophylaxis and treatment of H1N1 (swine) influenza. Dosage recommendations are available for this age group at www.cdc.gov/h1n1flu/recommendations.htm. Oseltamivir has been described to produce neuropsychiatric and psychological side effects in some patients; drug should be discontinued if behavioural or psychiatric side effects are observed.
Antivirals Used for Hepatitis Viruses and Papillomaviruses
Lamivudine
Lamivudine is a reverse transcriptase (RT) inhibitor that is used for management of HIV infection (Chapter 268). Because an RT step is required for replication of hepatitis B virus, it was not surprising that lamivudine was found to have activity against this virus. Lamivudine is useful in the management of hepatitis B infection in children and is available as an oral suspension for pediatric dosing. It is rapidly absorbed following oral administration, with a bioavailability of 80-87%. Lamivudine crosses the placenta and is distributed in breast milk and excreted via the kidneys. A majority of drug is unchanged following oral administration, so dosage adjustment is necessary in the setting of renal insufficiency.
Antiviral Immune Globulins
Immune globulins are useful adjuncts in the management of viral disease. However, they are most useful when administered as prophylaxis against infection and disease in high-risk patients; their value as therapeutic agents in the setting of established infection is less clear. Varicella-zoster immune globulin (human) [VariZIG] is valuable for prophylaxis against VZV in high-risk children, particularly newborns and immunocompromised children (Chapter 245). Cytomegalovirus immune globulin (CMV-IG) is warranted for children at high risk for CMV disease, particularly solid organ and hematopoietic stem cell transplant patients, and can play a role in preventing injury to the infected fetus when administered to the pregnant patient (Chapter 252). Palivizumab, a monoclonal antibody with anti-RSV activity, is effective for preventing severe RSV lower respiratory tract disease in high-risk premature infants and has replaced respiratory syncytial virus immune globulin (Chapter 252); a more-potent monoclonal for RSV, motavizumab, is in clinical trials. Hepatitis B immune globulin (HBIG) is indicated in infants born to hepatitis B surface antigen-positive mothers (Chapter 350).
Andrei G, De Clercq E, Snoeck R. Drug targets in cytomegalovirus infection. Infect Disord Drug Targets. 2009;9:201-222.
Balfour HHJr. Antiviral drugs. N Engl J Med. 1999;340:1255-1268.
Caviness AC, Demmler DJ, Almendarez Y, et al. The prevalence of neonatal herpes simplex virus infection compared with serious bacterial illness in hospitalized neonates. J Pediatr. 2008;153:164-169.
Centers for Disease Control and Prevention. Antiviral agents for the treatment and chemoprophylaxis of influenza. MMWR. 2011;60(1):1-25.
De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115-133.
James SH, Kimberlin DW, Whitley RJ. Antiviral therapy for herpesvirus central nervous system infections: Neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res. 2009;83:207-213.
Kimberlin DW. Antiviral therapies in children: has their time arrived? Pediatr Clin North Am. 2005;52:837-867.
Kimberlin DW. Management of HSV encephalitis in adults and neonates: diagnosis, prognosis and treatment. Herpes. 2007;14:11-16.
Kurbegov AC, Sokol RJ. Hepatitis B therapy in children. Expert Rev Gastroenterol Hepatol. 2009;3:39-49.
Littler E, Oberg B. Achievements and challenges in antiviral drug discovery. Antivir Chem Chemother. 2005;16:155-168.
Long SS. In defense of empiric acyclovir therapy in certain neonates. J Pediatr. 2008;153:157-158.
2011 The Medical Letter: Antiviral drugs for influenza. Med Lett. 2011;53(1355):1-3.
Nigro G, Adler SP, La Torre R, et al. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362.
Nokes JD, Cane PA. New strategies for control of respiratory syncytial virus infection. Curr Opin Infect Dis. 2008;21:639-643.
Oxford JS. Antivirals for the treatment and prevention of epidemic and pandemic influenza. Influenza Other Respi Viruses. 2007;1:27-34.
Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA. 2010;304(8):859-866.
Rungrotmongkol T, Intharathep P, Malaisree M, et al. Susceptibility of antiviral drugs against 2009 influenza A (H1N1) virus. Biochem Biophys Res Commun. 2009;385:390-394.
Sugaya N, Tamura D, Yamazaki M, et al. Comparison of the clinical effectiveness of oseltamivir and zanamivir against influenza virus infection in children. Clin Infect Dis. 2008;47:339-345.
Whitley RJ. The role of oseltamivir in the treatment and prevention of influenza in children. Expert Opin Drug Metab Toxicol. 2007;3:755-767.