Chapter 173 Principles of Antibacterial Therapy
Several key considerations must be incorporated in decisions about the appropriate empirical use of antibacterial agents in infants and children. It is important to know the age-appropriate differential diagnosis with respect to likely pathogens. This information affects the choice of antimicrobial agent and also the dose, dosing interval, and route of administration (oral vs parenteral). A complete history and physical examination combined with appropriate laboratory and radiographic studies are necessary to identify specific diagnoses, in turn affecting the choice, dosing, and degree of urgency of administration of antimicrobial agents. The vaccination history may reflect reduced risk for some invasive infections, but not necessarily elimination of risk. The risk of serious bacterial infection in pediatric practice is also affected by the child’s immunologic status, which may be compromised by immaturity (neonates), underlying disease, and associated treatments (Chapter 171). Infections in immunocompromised children often result from bacteria that are not considered pathogenic in immunocompetent children. The presence of foreign bodies also increases the risk of bacterial infections (Chapter 172). The likelihood of central nervous system (CNS) involvement must be considered in all pediatric patients, because many of the more common bacteremic infections in childhood, including Haemophilus influenzae type b, pneumococcus, and meningococcus, carry a significant risk for hematogenous spread to the CNS.
Antimicrobial resistance occurs through many modifications of the bacterial genome (Tables 173-1 and 173-2). Mechanisms include enzyme inactivation of the antibiotic, decreased cell membrane permeability to intracellularly active antibiotics, efflux of antibiotics out of the bacteria, protection or alteration of the antibiotic target site, excessive production of the target site, or bypassing the antimicrobial site of action.
Table 173-1 MECHANISMS OF RESISTANCE TO β-LACTAM ANTIBIOTICS
Table 173-2 AMINOGLYCOSIDE-MODIFYING ENZYMES
| ENZYMES | USUAL ANTIBIOTICS MODIFIED | COMMON GENERA |
|---|---|---|
| PHOSPHORYLATION | ||
| APH(2″) | K, T, G | SA, SR |
| APH(3′)-I | K | E, PS, SA, SR |
| APH(3′)-III | K, ±A | E, PS, SA, SR |
| ACETYLATION | ||
| AAC(2′) | G | PR |
| AAC(3)-I | ±T, G | E, PS |
| AAC(3)-III, -IV, OR-V | K, T, G | E, PS |
| AAC(6′) | K, T, A | E, PS, SA |
| ADENYLATION | ||
| ANT(2″) | K, T, GE, PS | |
| ANT(4′) | K, T, A | SA |
A, amikacin; AAC, aminoglycoside acetyltransferase; ANT, aminoglycoside nucleotidyltransferase; APH, aminoglycoside phosphotransferase; E, Enterobacteriaceae; G, gentamicin; K, karamycin; PR, Providencia-Proteus; PS, pseudomonads; SA, staphylococci; SR, streptococci; T, tobramycin.
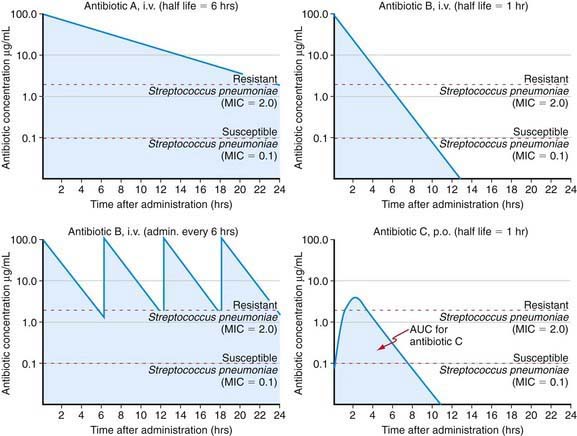
Antibiotic action is related to achieving therapeutic levels at the site of infection. Although measuring the level of antibiotic at the site of infection is not always possible, one may measure the serum level and use it as a surrogate target to achieve the desired effect at the tissue level. Various target serum levels are appropriate for different antibiotic agents and are assessed by the peak and trough serum levels, and the area under the therapeutic drug level curve (Fig. 173-1). These levels are in turn a reflection of the route of administration, drug absorption (IM, PO), volume of distribution, and drug elimination half-life, as well as of drug-drug interactions that might enhance or impede enzymatic inactivation of an antibiotic or result in antimicrobial synergism or antagonism (Fig. 173-2).
Age- and Risk-Specific Use of Antibiotics in Children
Neonates
The causative pathogens of neonatal infections are typically acquired around the time of delivery. Thus, empirical antibiotic selection must take into account the importance of these pathogens in neonates (Chapter 103). Among the causes of neonatal sepsis in infants, group B streptococcus is the most common, although intrapartum antibiotic prophylaxis has greatly decreased the incidence of this infection (Chapter 177). Gram-negative enteric organisms acquired from the maternal birth canal, in particular Escherichia coli, are other common causes of neonatal sepsis. Although rare, Listeria monocytogenes is also an important pathogen, insofar as it is intrinsically resistant to cephalosporin antibiotics, which are often used as empirical therapy in young children. All of these organisms can be associated with meningitis in the neonate; therefore, lumbar puncture should always be considered in the setting of bacteremic infections in this age group, and, if meningitis cannot be excluded, antibiotic management should include agents capable of crossing the blood-brain barrier.
Older Children
Antibiotic choices in toddlers and young children were once driven by the high risk of this age group to invasive disease caused by H. influenzae type b (Chapter 186). With the advent of conjugate vaccines against H. influenzae type b, invasive disease has declined dramatically. It is still appropriate to consider the use of antimicrobials that are active against this pathogen, particularly if meningitis is a consideration. Other particularly important pathogens to be considered in this age group include S. pneumoniae, Neisseria meningitidis, and S. aureus. Antimicrobial resistance is commonly exhibited by S. pneumoniae and S. aureus. Strains of S. pneumoniae that are resistant to penicillin and cephalosporin antibiotics are frequently encountered in clinical practice. Similarly, MRSA is highly prevalent in many regions. Resistance of S. pneumoniae as well as MRSA is due to mutations that confer alterations in penicillin binding proteins, the molecular targets of penicillin and cephalosporin activity (see Table 173-1).
Immunocompromised and Hospitalized Patients
It is important to consider the risks associated with immunocompromising conditions (malignancy, solid organ, or hematopoietic stem cell transplantation) and the risks conferred by conditions leading to prolonged hospitalization (intensive care, trauma, burns). Immunocompromised children are predisposed to develop a wide range of bacterial, viral, fungal, or parasitic infections. Prolonged hospitalization can lead to nosocomial infections, often associated with indwelling lines and catheters and commonly caused by gram-negative enteric organisms. In addition to the usual bacterial pathogens, Pseudomonas aeruginosa and enteric organisms, including E. coli, Klebsiella pneumoniae, Enterobacter, and Serratia, are important considerations as opportunistic pathogens in these settings. Selection of appropriate antimicrobials is challenging because of the diverse causes and scope of antimicrobial resistance exhibited by these organisms. Many strains of enteric organisms have resistance due to extended spectrum β-lactamases (ESBLs) (see Table 173-1). P. aeruginosa encodes proteins that function as efflux pumps to eliminate multiple classes of antimicrobials from the cytoplasm or periplasmic space. In addition to these gram-negative pathogens, infections caused by Enterococcus faecalis and Enterococcus faecium are inherently difficult to treat. These organisms may cause urinary tract infection or infective endocarditis in immunocompetent children and may be responsible for a variety of syndromes in immunocompromised patients, especially in the setting of prolonged intensive care. The emergence of infections caused by vancomycin-resistant Enterococcus (VRE) has further complicated antimicrobial selection in high-risk patients and has necessitated the development of newer antimicrobials that target these highly resistant gram-positive infections. Although experience with many of these newer agents in the management of complex hospitalized pediatric patients is limited, they are important agents to be aware of (described below).
Infections Associated with Medical Devices
A special situation affecting antibiotic use is the presence of an indwelling medical device, such as a venous catheter, ventriculoperitoneal shunt, stent, or other catheter (Chapter 172). In addition to S. aureus, coagulase-negative staphylococci are also a major consideration. Coagulase-negative staphylococci seldom cause serious disease without a risk factor such as an indwelling catheter. Empirical antibiotic regimens must take this risk into consideration. In addition to appropriate antibiotic therapy, removal or replacement of the colonized prosthetic material is commonly required for cure.
Antibiotics Commonly Used in Pediatric Practice (Table 173-3)
| DRUG (TRADE NAMES, FORMULATIONS) | INDICATIONS (MECHANISM OF ACTION) AND DOSING | COMMENTS |
|---|---|---|
| Amikacin sulfate Amikin. Injection: 50 mg/mL, 250 mg/mL. |
Aminoglycoside antibiotic active against gram-negative bacilli, especially Escherichia coli, Klebsiella, Proteus, Enterobacter, Serratia, and Pseudomonas. | Cautions: Anaerobes, Streptococcus (including S. pneumoniae) are resistant. May cause ototoxicity and nephrotoxicity. Monitor renal function. Drug eliminated renally. Administered IV over 30-60 min. |
| Neonates: Postnatal age ≤7 days: 1,200-2,000 g: 7.5 mg/kg q 12-18 hr IV or IM; >2,000 g: 10 mg/kg q 12 hr IV or IM; postnatal age >7 days: 1,200-2,000 g IV or IM: 7.5 mg/kg q 8-12 hr IV or IM; >2,000 g: 10 mg/kg q 8 hr IV or IM. | Drug interactions: May potentiate other ototoxic and nephrotoxic drugs. Target serum concentrations: Peak 25-40 mg/L; trough <10 mg/L. |
|
| Children: 15-25 mg/kg/24 hr divided q 8-12 hr IV or IM. | ||
| Adults: 15 mg/kg 24 hr divided q 8-12 hr IV or IM. | ||
| Amoxicillin Amoxil, Polymox. Capsule: 250, 500 mg. |
Penicillinase-susceptible β-lactam: gram-positive pathogens except Staphylococcus; Salmonella, Shigella, Neisseria, E. coli, and Proteus mirabilis. | Cautions: Rash, diarrhea, abdominal cramping. Drug eliminated renally. Drug interaction: Probenecid. |
| Tablet: chewable: 125, 250 mg. Suspension: 125 mg/5 mL, 250 mg/5 mL. |
Children: 20-50 mg/kg/24 hr divided q 8-12 hr PO. Higher dose of 80-90 mg/kg 24 hr PO for otitis media. | |
| Drops: 50 mg/mL. | Adults: 250-500 mg q 8-12 hr PO. | |
| Uncomplicated gonorrhea: 3 g with 1 g probenecid PO. | ||
| Amoxicillin-clavulanate Augmentin. Tablet: 250, 500, 875 mg. Tablet, chewable: 125, 200, 250, 400 mg. Suspension: 125 mg/5 mL, 200 mg/5 mL, 250 mg/5 mL, 400 mg/5 mL. |
β-Lactam (amoxicillin) and β-lactamase inhibitor (clavulanate) enhances amoxicillin activity against penicillinase-producing bacteria. S. aureus (not methicillin-resistant organism), Streptococcus, Haemophilus influenzae, Moraxella catarrhalis, E. coli, Klebsiella, Bacteroides fragilis. | Cautions: Drug dosed on amoxicillin component. May cause diarrhea, rash. Drug eliminated renally. Drug interaction: Probenecid. Comment: Higher dose may be active against penicillin tolerant/resistant S. pneumoniae. |
| Neonates: 30 mg/kg/24 hr divided q 12 hr PO. | ||
| Children: 20-45 mg/kg 24 hr divided q 8-12 hr PO. Higher dose 80-90 mg/kg/24 hr PO for otitis media. | ||
| Ampicillin Polycillin, Omnipen. Capsule: 250, 500 mg. Suspension: 125 mg/5 mL, 250 mg/5 mL, 500 mg/5 mL. Injection. |
β-Lactam with same spectrum of antibacterial activity as amoxicillin. Neonates: Postnatal age ≤7 days ≤2,000 g: 50 mg/kg/24 hr IV or IM q 12 hr (meningitis: 100 mg/kg/24 hr divided q 12 hr IV or IM); >2,000 g: 75 mg/kg/24 hr divided q 8 hr IV or IM (meningitis: 150 mg/kg/24 hr divided q 8 hr IV or IM). Postnatal age >7 days <1,200 g: 50 mg/kg/24 hr IV or IM q 12 hr (meningitis: 100 mg/kg/24 hr divided q 12 hr IV or IM); 1,200-2,000 g: 75 mg/kg/24 hr divided q 8 hr IV or IM (meningitis: 150 mg/kg/24 hr divided q 8 hr IV or IM); >2,000 g: 100 mg/kg/24 hr divided q 6 hr IV or IM (meningitis: 200 mg/kg/24 hr divided q 6 hr IV or IM). |
Cautions: Less bioavailable than amoxicillin, causing greater diarrhea. Drug interaction: Probenecid. |
| Children: 100-200 mg/kg/24 hr divided q 6 hr IV or IM (meningitis: 200-400 mg/kg/24 hr divided q 4-6 hr IV or IM). | ||
| Adults: 250-500 mg q 4-8 hr IV or IM. | ||
| Ampicillin-sulbactam Unasyn. Injection. |
β-Lactam (ampicillin) and β-lactamase inhibitor (sulbactam) enhances ampicillin activity against penicillinase-producing bacteria: S. aureus, H. influenzae, M. catarrhalis, E. coli, Klebsiella, B. fragilis. | Cautions: Drug dosed on ampicillin component. May cause diarrhea, rash. Drug eliminated renally. Note: Higher dose may be active against penicillin-tolerant/resistant S. pneumoniae. |
| Children: 100-200 mg/kg/24 hr divided q 4-8 hr IV or IM. | Drug interaction: Probenecid. | |
| Adults: 1-2 g q 6-8 hr IV or IM (max daily dose: 8 g). | ||
| Azithromycin Zithromax. Tablet: 250 mg. Suspension: 100 mg/5 mL, 200 mg/5 mL. |
Azalide antibiotic with activity against S. aureus, Streptococcus, H. influenzae, Mycoplasma, Legionella, Chlamydia trachomatis. Children: 10 mg/kg PO on day 1 (max dose: 500 mg) followed by 5 mg/kg PO q 24 hr for 4 days. Group A streptococcus pharyngitis: 12 mg/kg/24 hr PO (max dose: 500 mg) for 5 days. Adults: 500 mg PO day 1 followed by 250 mg for 4 days. Uncomplicated C. trachomatis infection: single 1 g dose PO. |
Note: Very long half-life permitting once-daily dosing. No metabolic-based drug interactions (unlike erythromycin and clarithromycin), limited gastrointestinal distress. Shorter-course regimens (e.g., 1-3 days) under investigation. 3-day, therapy (10 mg/kg/24 hr × 3 days) and single-dose therapy (30 mg/kg): use with increasing frequency (not for streptococcus pharyngitis). |
| Aztreonam Azactam. |
β-Lactam (monobactam) antibiotic with activity against gram-negative aerobic bacteria, Enterobacteriaceae, and Pseudomonas aeruginosa. | Cautions: Rash, thrombophlebitis, eosinophilia. Renally eliminated. Drug interaction: Probenecid. |
| Injection. | Neonates: Postnatal age ≤7 days ≤2,000 g: 60 mg/kg/24 hr divided q 12 hr IV or IM; >2,000 g: 90 mg/kg/24 hr divided q 8 hr IV or IM; postnatal age >7 days <1,200 g: 60 mg/kg/24 hr divided q 12 hr IV or IM; 1,200-2,000 g: 90 mg/kg/24 hr divided q 8 hr IV or IM; >2,000 g: 120 mg/kg/24 hr divided q 6-8 hr IV or IM. | |
| Children: 90-120 mg/kg/24 hr divided q 6-8 hr IV or IM. For cystic fibrosis up to 200 mg/kg/24 hr IV. | ||
| Adults: 1-2 g IV or IM q 8-12 hr (max dose: 8 g/24 hr). | ||
| Carbenicillin Geopen Injection. Geocillin oral tablet. |
Extended-spectrum penicillin (remains susceptible to penicillinase destruction) active against Enterobacter, indole-positive Proteus, and Pseudomonas. | Cautions: Painful given intramuscularly; rash; each gram contains 5.3 mEq sodium. Interferes with platelet aggregation at high doses, increases in liver transaminase levels. Renally eliminated. Oral tablet for treatment of urinary tract infection only. Drug interaction: Probenecid. |
| Neonates: Postnatal age ≤7 days ≤2,000 g: 225 mg/kg/24 hr divided q 8 hr IV or IM; >2,000 g: 300 mg/kg/24 hr divided q 6 hr IV or IM; >7 days: 300-400 mg/kg/24 hr divided q 6 hr IV or IM. | ||
| Children: 400-600 mg/kg/24 hr divided q 4-6 hr IV or IM. | ||
| Cefaclor Ceclor. Capsule: 250, 500 mg. |
2nd generation cephalosporin active against S. aureus, Streptococcus including S. pneumoniae, H. influenzae, E. coli, Klebsiella, and Proteus. | Cautions: β-Lactam safety profile (rash, eosinophilia) with high incidence of serum sickness reaction. Renally eliminated. Drug interaction: Probenecid. |
| Suspension: 125 mg/5 mL, 187 mg/5 mL, 250 mg/5 mL, 375 mg/5 mL. | Children: 20-40 mg/kg/24 hr divided q 8-12 hr PO (max dose: 2 g). Adults: 250-500 mg q 6-8 hr PO. |
|
| Cefadroxil Duricef, Ultracef. |
1st-generation cephalosporin active against S. aureus, Streptococcus, E. coli, Klebsiella, and Proteus. | Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Long half-life permits q 12-24 hr dosing. |
| Capsule: 500 mg. | Children: 30 mg/kg/24 hr divided q 12 hr PO (max dose: 2 g). | Drug interaction: Probenecid. |
| Tablet: 1,000 mg. | ||
| Suspension: 125 mg/5 mL, 250 mg/5 mL, 500 mg/5 mL. | Adults: 250-500 mg q 8-12 hr PO. | |
| Cefazolin Ancef, Kefzol. |
1st generation cephalosporin active against S. aureus, Streptococcus, E. coli, Klebsiella, and Proteus. | Caution: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Does not adequately penetrate CNS. |
| Injection. | Neonates: Postnatal age ≤7 days 40 mg/kg/24 hr divided q 12 hr IV or IM; >7 days 40-60 mg/kg/24 hr divided q 8 hr IV or IM. | Drug interaction: Probenecid. |
| Children: 50-100 mg/kg/24 hr divided q 8 hr IV or IM. | ||
| Adults: 0.5-2g q 8 hr IV or IM (max dose: 12 g/24 hr). | ||
| Cefdinir Omnicef. Capsule: 300 mg. Oral suspension: 125 mg/5 mL. |
Extended-spectrum, semi-synthetic cephalosporin. Children 6 mo-12 yr: 14 mg/kg/24 hr in 1 or 2 doses PO (max dose: 600 mg/24 hr). Adults: 600 mg q 24 hr PO. |
Cautions: Reduce dosage in renal insufficiency (creatinine clearance <60 mL/min). Avoid taking concurrently with iron-containing products and antacids because absorption is markedly decreased; take at least 2 hr apart. |
| Drug interaction: Probenecid. | ||
| Cefepime Maxipime. Injection. |
Expanded-spectrum, 4th generation cephalosporin active against many gram-positive and gram-negative pathogens, including Pseudomonas aeruginosa many multidrug-resistant pathogens. | Adverse events: Diarrhea, nausea, vaginal candidiasis Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. |
| Children: 100-150 mg/kg/24 hr q 8-12 hr IV or IM. | Drug interaction: Probenecid. | |
| Adults: 2-4 g/24 hr q 12 hr IV or IM. | ||
| Cefixime Suprax. Tablet: 200, 400 mg. |
3rd generation cephalosporin active against Streptococci, H. influenzae, M. catarrhalis, Neisseria gonorrhoeae, Serratia marcescens, and P. vulgaris. No antistaphylococcal or antipseudomonal activity. | Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Does not adequately penetrate CNS. Drug interaction: Probenecid. |
| Suspension: 100 mg/5 mL. | Children: 8 mg/kg/24 hr divided q 12-24 hr PO. | |
| Adults: 400 mg/24 hr divided q 12-24 hr PO. | ||
| Cefoperazone sodium Cefobid. Injection. |
3rd generation cephalosporin active against many gram-positive and gram-negative pathogens. Neonates: 100 mg/kg/24 hr divided q 12 hr IV or IM. Children: 100-150 mg/kg/24 hr divided q 8-12 hr IV or IM. Adults: 2-4 g/24 hr divided q 8-12 hr IV or IM (max dose: 12 g/24 hr). |
Cautions: Highly protein bound cephalosporin with limited potency reflected by weak antipseudomonal activity. Variable gram-positive activity. Primarily hepatically eliminated in bile. Drug interaction: Disulfiram-like reaction with alcohol. |
| Cefotaxime sodium Claforan. Injection. |
3rd generation cephalosporin active against gram-positive and gram-negative pathogens. No antipseudomonal activity. Neonates: ≤7 days: 100 mg/kg/24 hr divided q 12 hr IV or IM; >7 days: <1,200 g 100 mg/kg/24 hr divided q 12 hr IV or IM; >12,000 g: 150 mg/kg/24 hr divided q 8 hr IV or IM. |
Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Each gram of drug contains 2.2 mEq sodium. Active metabolite. Drug interaction: Probenecid. |
| Children: 150 mg/kg/24 hr divided q 6-8 hr IV or IM (meningitis: 200 mg/kg/24 hr divided q 6-8 hr IV). | ||
| Adults: 1-2 g q 8-12 hr IV or IM (max dose: 12 g/24 hr). | ||
| Cefotetan disodium Cefotan. Injection. |
2nd generation cephalosporin active against S. aureus, Streptococcus, H. influenzae, E. coli, Klebsiella, Proteus, and Bacteroides. Inactive against Enterobacter. Children: 40-80 mg/kg/24 hr divided IV or IM q 12 hr. |
Cautions: Highly protein-bound cephalosporin, poor CNS penetration; β-Lactam safety profile (rash, eosinophilia), disulfiram-like reaction with alcohol. Renally eliminated (∼20% in bile). |
| Adults: 2-4 g/24 hr divided q 12 hr IV or IM (max dose: 6 g/24 hr). | ||
| Cefoxitin sodium Mefoxin. Injection. |
2nd generation cephalosporin active against S. aureus, Streptococcus, H. influenzae, E. coli, Klebsiella, Proteus, and Bacteroides. Inactive against Enterobacter. | Cautions: Poor CNS penetration; β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Painful given intramuscularly. |
| Neonates: 70-100 mg/kg/24 hr divided q 8-12 hr IV or IM. | Drug interaction: Probenecid. | |
| Children: 80-160 mg/kg/24 hr divided q 6-8 hr IV or IM. | ||
| Adults: 1-2 g q 6-8 hr IV or IM (max dose: 12 g/24 hr). | ||
| Cefpodoxime proxetil Vantin. Tablet: 100 mg, 200 mg. Suspension: 50 mg/5 mL, 100 mg/5 mL. |
3rd generation cephalosporin active against S. aureus, Streptococcus, H. influenzae, M. catarrhalis, N. gonorrhoeae, E. coli, Klebsiella, and Proteus. No antipseudomonal activity. Children: 10 mg/kg/24 hr divided q 12 hr PO. Adults: 200-800 mg/24 hr divided q 12 hr PO (max dose: 800 mg/24 hr). Uncomplicated gonorrhea: 200 mg PO as single-dose therapy. |
Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Does not adequately penetrate CNS. Increased bioavailability when taken with food. Drug interaction: Probenecid; antacids and H-2 receptor antagonists may decrease absorption. |
| Cefprozil Cefzil. Tablet: 250, 500 mg. Suspension: 125 mg/5 mL, 250 mg/5 mL. |
2nd generation cephalosporin active against S. aureus, Streptococcus, H. influenzae, E. coli, M. catarrhalis, Klebsiella, and Proteus. Children: 30 mg/kg/24 hr divided q 8-12 hr PO. Adults: 500-1,000 mg/24 hr divided q 12 hr PO (max dose: 1.5 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Good bioavailability; food does not affect bioavailability. Drug interaction: Probenecid. |
| Ceftazidime Fortaz, Ceptaz, Tazicer, Tazidime. Injection. |
3rd generation cephalosporin active against gram-positive and gram-negative pathogens, including Pseudomonas aeruginosa. Neonates: Postnatal age ≤7 days: 100 mg/kg/24 hr divided q 12 hr IV or IM; >7 days ≤1,200 g: 100 mg/kg/24 hr divided q 12 hr IV or IM; >1,200 g: 150 mg/kg/24 hr divided q 8 hr IV or IM. Children: 150 mg/kg/24 hr divided q 8 hr IV or IM (meningitis: 150 mg/kg/24 hr IV divided q 8 hr). Adults: 1-2 g q 8-12 hr IV or IM (max dose: 8-12 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Increasing pathogen resistance developing with long-term, widespread use. Drug interaction: Probenecid. |
| Ceftiaoxime Cefizox. Injection. |
3rd generation cephalosporin active against gram-positive and gram-negative pathogens. No antipseudomonal activity. Children: 150 mg/kg/24 hr divided q 6-8 hr IV or IM. Adults: 1-2 g q 6-8 hr IV or IM (max dose: 12 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Drug interaction: Probenecid. |
| Ceftriaxone sodium Rocephin. Injection. |
3rd generation cephalosporin active against gram-positive and gram-negative pathogens. No antipseudomonal activity. Neonates: 50-75 mg/kg q 24 hr IV or IM. Children: 50-75 mg/kg q 24 hr IV or IM (meningitis: 75 mg/kg dose 1 then 80-100 mg/kg/24 hr divided q 12-24 hr IV or IM). Adults: 1-2 g q 24 hr IV or IM (max dose: 4 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia). Eliminated via kidney (33-65%) and bile; can cause sludging. Long half-life and dose-dependent protein binding favors q 24 hr rather than q 12 hr dosing. Can add 1% lidocaine for IM injection. |
| Cefuroxime (cefuroxime axetil for oral administration) Ceftin, Kefurox, Zinacef. Injection. Suspension: 125 mg/5 mL. Tablet: 125, 250, 500 mg. |
2nd generation cephalosporin active against S. aureus, Streptococcus, H. influenzae, E. coli, M. catarrhalis, Klebsiella, and Proteus. Neonates: 40-100 mg/kg/24 hr divided q 12 hr IV or IM. Children: 200-240 mg/kg/24 hr divided q 8 hr IV or IM; PO administration: 20-30 mg/kg/24 hr divided q 8 hr PO. Adults: 750-1,500 mg q 8 hr IV or IM (max dose: 6 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Food increases PO bioavailability. Drug interaction: Probenecid. |
| Cephalexin Keflex, Keftab. Capsule: 250, 500 mg Tablet: 500 mg, 1 g. Suspension: 125 mg/5 mL, 250 mg/5 mL, 100 mg/mL drops. |
1st generation cephalosporin active against S. aureus, Streptococcus, E. coli, Klebsiella, and Proteus. Children: 25-100 mg/kg/24 hr divided q 6-8 hr PO. Adults: 250-500 mg q 6 hr PO (max dose: 4 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Drug interaction: Probenecid. |
| Cephradine Velosef Capsule: 250, 500 mg. Suspension: 125 mg/5 mL, 250 mg/5 mL. |
1st generation cephalosporin active against S. aureus, Streptococcus, E. coli, Klebsiella, and Proteus. Children: 50-100 mg/kg/24 hr divided q 6-12 hr PO. Adults: 250-500 mg q 6-12 hr PO (max dose: 4 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Drug interaction: Probenecid. |
| Chloramphenicol Chloromycetin. Injection. Capsule: 250 mg. Ophthalmic, otic solutions. Ointment. |
Broad-spectrum protein synthesis inhibitor active against many gram-positive and gram-negative bacteria, Salmonella, vancomycin-resistant Enterococcus faecium, Bacteroides, other anaerobes, Mycoplasma, Chlamydia, and Rickettsia; usually inactive against Pseudomonas. Neonates: Initial loading dose 20 mg/kg followed 12 hr later by: postnatal age ≤7 days: 25 mg/kg/24 hr q 24 hr IV; >7 days: ≤2,000 g: 25 mg/kg/24 hr q 24 hr IV; >2,000 g: 50 mg/kg/24 hr divided q 12 hr IV. Children: 50-75 mg/kg/24 hr divided q 6-8 hr IV or PO (meningitis: 75-100 mg/kg/24 hr IV divided q 6 hr). Adults: 50 mg/kg/24 hr divided q 6 hr IV or PO (max dose: 4 g/24 hr). |
Cautions: Gray-baby syndrome (from too-high dose in neonate), bone marrow suppression aplastic anemia (monitor hematocrit, free serum iron). Drug interactions: Phenytoin, phenobarbital, rifampin may decrease levels. Target serum concentrations: Peak 20-30 mg/L; trough 5-10 mg/L. |
| Ciprofloxacin Cipro. Tablet: 100, 250, 500, 750 mg. Injection. Ophthalmic solution and ointment. Otic suspension. Oral suspension: 250 and 500 mg/5 mL. |
Quinolone antibiotic active against P. aeruginosa, Serratia, Enterobacter, Shigella, Salmonella, Campylobacter, N. gonorrhoeae, H. influenzae, M. catarrhalis, some S. aureus, and some Streptococcus. Neonates: 10 mg/kg q 12 hr PO or IV. Children: 15-30 mg/kg/24 hr divided q 12 hr PO or IV; cystic fibrosis: 20-40 mg/kg/24 hr divided q 8-12 hr PO or IV. Adults: 250-750 mg q 12 hr; 200-400 mg IV q 12 hr PO (max dose: 1.5 g/24 hr). |
Cautions: Concerns of joint destruction in juvenile animals not seen in humans; tendonitis, superinfection, dizziness, confusion, crystalluria, some photosensitivity. Drug interactions: Theophylline; magnesium-, aluminum-, or calcium-containing antacids; sucralfate; probenecid; warfarin; cyclosporine. |
| Clarithromycin Biaxin. Tablet: 250, 500 mg. Suspension: 125 mg/5 mL, 250 mg/5 mL. |
Macrolide antibiotic with activity against S. aureus, Streptococcus, H. influenzae, Legionella, Mycoplasma, and C. trachomatis. Children: 15 mg/kg/24 hr divided q 12 hr PO. Adults: 250-500 mg q 12 hr PO (max dose: 1 g/24 hr). |
Cautions: Adverse events less than erythromycin; gastrointestinal upset, dyspepsia, nausea, cramping. Drug interactions: Same as erythromycin: astemizole carbamazepine, terfenadine cyclosporine, theophylline, digoxin, tacrolimus. |
| Clindamycin Cleocin. Capsule: 75, 150, 300 mg. Suspension: 75 mg/5 mL. Injection. Topical solution, lotion, and gel. Vaginal cream. |
Protein synthesis inhibitor active against most gram-positive aerobic and anaerobic cocci except Enterococcus. Neonates: Postnatal age ≤7 days <200 g; 10 mg/kg/24 hr divided q 12 hr IV or IM; >2,000 g: 15 mg/kg/24 hr divided q 8 hr IV or IM; >7 days <1,200 g: 10 mg/kg/24 hr IV or IM divided q 12 hr; 1,200-2,000 g: 15 mg/kg/24 hr divided q 8 hr IV or IM; >2,000 g: 20 mg/kg/24 hr divided q 8 hr IV or IM. Children: 10-40 mg/kg/24 hr divided q 6-8 hr IV, IM, or PO. Adults: 150-600 mg q 6-8 hr IV, IM, or PO (max dose: 5 g/24 hr IV or IM or 2 g/24 hr PO). |
Cautions: Diarrhea, nausea, Clostridium difficile–associated colitis, rash. Administer slow IV over 30-60 min. Topically active as an acne treatment. |
| Cloxacillin sodium Tegopen. Capsule: 250, 500 mg. Suspension: 125 mg/5 mL. |
Penicillinase-resistant penicillin active against S. aureus and other gram-positive cocci except Enterococcus and coagulase-negative staphylococci. Children: 50-100 mg/kg/24 hr divided q 6 hr PO. Adults: 250-500 mg q 6 hr PO (max dose: 4 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia). Primarily hepatically eliminated; requires dose reduction in renal disease. Food decreases bioavailability. Drug interaction: Probenecid. |
| Co-trimoxazole (trimethoprim-sulfamethoxazole; TMP-SMZ) Bactrim, Cotrim, Septra, Sulfatrim. Tablet: SMZ 400 mg and TMP 80 mg. Tablet DS: SMZ 800 mg and TMP 160 mg. Suspension: SMZ 200 mg and TMP 40 mg/5 mL. Injection. |
Antibiotic combination with sequential antagonism of bacterial folate synthesis with broad antibacterial activity: Shigella, Legionella, Nocardia, Chlamydia, Pneumocystis jiroveci. Dosage based on TMP component. Children: 6-20 mg TMP/kg/24 hr or IV divided q 12 hr PO. Pneumocystis carinii pneumonia: 15-20 mg TMP/kg/24 hr divided q 12 hr PO or IV. P. carinii prophylaxis: 5 mg TMP/kg/24 hr or 3 times/wk PO. Adults: 160 mg TMP q 12 hr PO. |
Cautions: Drug dosed on TMP (trimethoprim) component. Sulfonamide skin reactions: rash, erythema multiforme, Stevens-Johnson syndrome, nausea, leukopenia. Renal and hepatic elimination; reduce dose in renal failure. Drug interactions: Protein displacement with warfarin, possibly phenytoin, cyclosporine. |
| Demeclocycline Declomycin. Tablet: 150, 300 mg. Capsule: 150 mg. |
Tetracycline active against most gram-positive cocci except Enterococcus, many gram-negative bacilli, anaerobes, Borrelia burgdorferi (Lyme disease), Mycoplasma, and Chlamydia. Children: 8-12 mg/kg/24 hr divided q 6-12 hr PO. Adults: 150 mg PO q 6-8 hr. Syndrome of inappropriate antidiuretic hormone secretion: 900-1,200 mg/24 hr or 13-15 mg/kg/24 hr divided q 6-8 hr PO with dose reduction based on response to 600-900 mg/24 hr. |
Cautions: Teeth staining, possibly permanent (if administered <8 yr of age) with prolonged use; photosensitivity, diabetes insipidus, nausea, vomiting, diarrhea, superinfections. Drug interactions: Aluminum-, calcium-, magnesium-, zinc- and iron-containing food, milk, dairy products may decrease absorption. |
| Dicloxacillin Dynapen, Pathocil. Capsule: 125, 250, 500 mg. Suspension: 62.5 mg/5 mL. |
Penicillinase-resistant penicillin active against S. aureus and other gram-positive cocci except Enterococcus and coagulase-negative staphylococci. Children: 12.5-100 mg/kg/24 hr divided q 6 hr PO. Adults: 125-500 mg q 6 hr PO. |
Cautions: β-Lactam safety profile (rash, eosinophilia). Primarily renally (65%) and bile (30%) elimination. Food may decrease bioavailability. Drug interaction: Probenecid. |
| Doripenem Doribax. Injection. |
Carbapenem antibiotic with broad-spectrum activity against gram-positive cocci and gram-negative bacilli, including P. aeruginosa and anaerobes. Children: dose unknown. Adults: 500 mg q 8 hr IV. |
Cautions: β-Lactam safety profile; does not undergo hepatic metabolism. Renal elimination (70-75%); dose adjustment for renal failure. Drug interactions: Valproic acid, probenecid. |
| Doxycycline Vibramycin, Doxy. Injection. Capsule: 50, 100 mg. Tablet: 50, 100 mg. Suspension: 25 mg/5 mL. Syrup: 50 mg/5 mL. |
Tetracycline antibiotic active against most gram-positive cocci except Enterococcus, many gram-negative bacilli, anaerobes, B. burgdorferi (Lyme disease), Mycoplasma, and Chlamydia. Children: 2-5 mg/kg/24 hr divided q 12-24 hr PO or IV (max dose: 200 mg/24 hr). Adults: 100-200 mg/24 hr divided q 12-24 hr PO or IV. |
Cautions: Teeth staining, possibly permanent (<8 yr of age) with prolonged use; photosensitivity, nausea, vomiting, diarrhea, superinfections. Drug interactions: Aluminum-, calcium-, magnesium-, zinc-, iron-, kaolin-, and pectin-containing products, food, milk, dairy products may decrease absorption. Carbamazepine, rifampin, barbiturates may decrease half-life. |
| Erythromycin E-Mycin, Ery-Tab, Ery-C, Ilosone. Estolate 125, 500 mg. Tablet EES: 200 mg. Tablet base: 250, 333, 500 mg. Suspension: estolate 125 mg/5 mL, 250 mg/5 mL, EES 200 mg/5 mL, 400 mg/5 mL. Estolate drops: 100 mg/mL. EES drops: 100 mg/2.5 mL. Available in combination with sulfisoxazole (Pediazole), dosed on erythromycin content. |
Bacteriostatic macrolide antibiotic most active against gram-positive organisms, Corynebacterium diphtheriae, and Mycoplasma pneumoniae. Neonates: Postnatal age ≤7 days: 20 mg/kg/24 hr divided q 12 hr PO; >7 days <1,200 g: 20 mg/kg/24 hr divided q 12 hr PO; <1,200 g: 30 mg/kg/24 hr divided q 8 hr PO (give as 5 mg/kg/dose q 6 hr to improve feeding intolerance). Children: Usual max dose 2 g/24 hr. Base: 30-50 mg/kg/24 hr divided q 6-8 hr PO. Estolate: 30-50 mg/kg/24 hr divided q 8-12 hr PO. Stearate: 20-40 mg/kg/24 hr divided q 6 hr PO. Lactobionate: 20-40 mg/kg/24 hr divided q 6-8 hr IV. Gluceptate: 20-50 mg/kg/24 hr divided q 6 hr IV; usual max dose 4 g/24 hr IV. Adults: Base: 333 mg PO q 8 hr; estolate/stearate/base: 250-500 mg q 6 hr PO. |
Cautions: Motilin agonist leading to marked abdominal cramping, nausea, vomiting, diarrhea. Associated with hypertrophic pyloric stenosis in young infants. Many different salts with questionable tempering of gastrointestinal adverse events. Rare cardiac toxicity with IV use. Dose of salts differ. Topical formulation for treatment of acne. Drug interactions: Antagonizes hepatic CYP 3A4 activity: astemizole, carbamazepine, terfenadine, cyclosporine, theophylline, digoxin, tacrolimus, carbamazepine. |
| Gentamicin Garamycin. Injection. Ophthalmic solution, ointment, topical cream. |
Aminoglycoside antibiotic active against gram-negative bacilli, especially E. coli, Klebsiella, Proteus, Enterobacter, Serratia, and Pseudomonas. Neonates: Postnatal age ≤7 days 1,200-2,000 g: 2.5 mg/kg q 12-18 hr IV or IM; <2,000 g: 2.5 mg/kg q 12 hr IV or IM; postnatal age >7 days 1,200-2,000 g: 2.5 mg/kg q 8-12 hr IV or IM; >2,000 g: 2.5 mg/kg q 8 hr IV or IM. Children: 2.5 mg/kg/24 hr divided q 8-12 hr IV or IM. Alternatively may administer 5-7.5 mg/kg/24 hr IV once daily. Intrathecal: Preservative-free preparation for intraventricular or intrathecal use: neonate: 1 mg/24 hr; children: 1-2 mg/24 hr IT; adults: 4-8 mg/24 hr. Adults: 3-6 mg/kg/24 hr divided q 8 hr IV or IM. |
Cautions: Anaerobes, S. pneumoniae, and other Streptococcus are resistant. May cause ototoxicity and nephrotoxicity. Monitor renal function. Drug eliminated renally. Administered IV over 30-60 min. Drug interactions: May potentiate other ototoxic and nephrotoxic drugs. Target serum concentrations: Peak 6-12 mg/L; trough >2 mg/L with intermittent daily dose regimens only. |
| Imipenem-cilastatin Primaxin. Injection. |
Carbapenem antibiotic with broad-spectrum activity against gram-positive cocci and gram-negative bacilli, including P. aeruginosa and anaerobes. No activity against Stenotrophomonas maltophilia. Neonates: Postnatal age ≤7 days <1,200 g: 20 mg/kg q 18-24 hr IV or IM; >1,200 g: 40 mg/kg divided q 12 hr IV or IM; postnatal age >7 days 1,200-2,000 g: 40 mg/kg q 12 hr IV or IM; >2,000 g: 60 mg/kg q 8 hr IV or IM. Children: 60-100 mg/kg/24 hr divided q 6-8 hr IV or IM. Adults: 2-4 g/24 hr divided q 6-8 hr IV or IM (max dose: 4 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia), nausea, seizures. Cilastatin possesses no antibacterial activity; reduces renal imipenem metabolism. Primarily renally eliminated. Drug interaction: Possibly ganciclovir. |
| Linezolid Zyvox. Tablet: 400, 600 mg. Oral suspension: 100 mg/5 mL. Injection: 100 mg/5 mL. |
Oxazolidinone antibiotic active against gram-positive cocci (especially drug-resistant organisms), including Staphylococcus, Streptococcus, E. faecium, and Enterococcus faecalis. Interferes with protein synthesis by binding to 50S ribosome subunit. Children: 10 mg/kg q 12 hr IV or PO. Adults: Pneumonia: 600 mg q 12 hr IV or PO; skin infections: 400 mg q 12 hr IV or PO. |
Adverse events: Myelosuppression, pseudomembranous colitis, nausea, diarrhea, headache. Drug interaction: Probenecid. |
| Loracarbef Lorabid. Capsule: 200 mg. Suspension: 100 mg/5 mL, 200 mg/5 mL. |
Carbacephem very closely related to cefaclor (2nd generation cephalosporin) active against S. aureus, Streptococcus, H. influenzae, M. catarrhalis, E. coli, Klebsiella, and Proteus. Children: 30 mg/kg/24 hr divided q 12 hr PO (max dose: 2 g). Adults: 200-400 mg q 12 hr PO (max dose: 800 mg/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia). Renally eliminated. Drug interaction: Probenecid. |
| Meropenem Merrem. Injection. |
Carbapenem antibiotic with broad-spectrum activity against gram-positive cocci and gram-negative bacilli, including P. aeruginosa and anaerobes. No activity against Stenotrophomonas maltophilia. Children: 60 mg/kg/24 hr divided q 8 hr IV meningitis: 120 mg/kg/24 hr (max dose: 6 g/24 hr) q 8 hr IV. Adults: 1.5-3 g q 8 hr IV. |
Cautions: β-Lactam safety profile; appears to possess less CNS excitation than imipenem. 80% renal elimination. Drug interaction: Probenecid. |
| Metronidazole Flagyl, Metro-IV, generic. Topical gel, vaginal gel. Injection. Tablet: 250, 500 mg. |
Highly effective in the treatment of infections due to anaerobes. Neonates: <1,200 g: 7.5 mg/kg 48 hr PO or IV; postnatal age ≤7 days 1,200-2,000 g: 7.5 mg/kg/24 hr q 24 hr PO or IV; 2,000 g: 15 mg/kg/24 hr divided q 12 hr PO or IV; postnatal age <7 days 1,200-2,000 g: 15 mg/kg/24 hr divided q 12 hr PO or IV; >2,000 g: 30 mg/kg/24 hr divided q 12 hr PO or IV. Children: 30 mg/kg/24 hr divided q 6-8 hr PO or IV. Adults: 30 mg/kg/24 hr divided q 6 hr PO or IV (max dose: 4 g/24 hr). |
Cautions: Dizziness, seizures, metallic taste, nausea, disulfiram-like reaction with alcohol. Administer IV slow over 30-60 min. Adjust dose with hepatic impairment. Drug interactions: Carbamazepine, rifampin, phenobarbital may enhance metabolism; may increase levels of warfarin, phenytoin, lithium. |
| Mezlocillin sodium Mezlin. Infection. |
Extended-spectrum penicillin active against E. coli, Enterobacter, Serratia, and Bacteroides; limited antipseudomonal activity. Neonates: Postnatal age ≤7 days: 150 mg/kg/24 hr divided q 12 hr IV; >7 days: 225 mg/kg divided q 8 hr IV. Children: 200-300 mg/kg/24 hr divided q 4-6 hr IV; cystic fibrosis 300-450 mg/kg/24 hr IV. Adults: 2-4 g/dose q 4-6 hr IV (max dose: 12 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia); painful given intramuscularly; each gram contains 1.8 mEq sodium. Interferes with platelet aggregation with high doses; increases noted in liver function test results. Renally eliminated. Inactivated by β-lactamase enzyme. Drug interaction: Probenecid. |
| Mupirocin Bactroban. Ointment. |
Topical antibiotic active against Staphylococcus and Streptococcus. Topical application: Nasal (eliminate nasal carriage) and to the skin 2-4 times per day. |
Caution: Minimal systemic absorption as drug metabolized within the skin. |
| Nafcillin sodium Nafcil, Unipen. Injection. Capsule: 250 mg. Tablet: 500 mg. |
Penicillinase-resistant penicillin active against S. aureus and other gram-positive cocci, except Enterococcus and coagulase-negative staphylococci. Neonates: Postnatal age ≤7 days 1,200-2,000 g: 50 mg/kg/24 hr divided q 12 hr IV or IM; >2,000 g: 75 mg/kg/24 hr divided q 8 hr IV or IM; postnatal age >7 days 1,200-2,000 g: 75 mg/kg/q 8 hr; >2,000 g: 100 mg/kg divided q 6-8 hr IV (meningitis: 200 mg/kg/24 hr divided q 6 hr IV). Children: 100-200 mg/kg/24 hr divided q 4-6 hr IV. Adults: 4-12 g/24 hr divided q 4-6 hr IV (max dose: 12 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia), phlebitis; painful given intramuscularly; oral absorption highly variable and erratic (not recommended). Adverse effect: Neutropenia. |
| Nalidixic acid NegGram. Tablet: 250, 500, 1,000 mg. Suspension: 250 mg/5 mL. |
1st generation quinolone effective for short-term treatment of lower urinary tract infections caused by E. coli, Enterobacter, Klebsiella, and Proteus. Children: 50-55 mg/kg/24 hr divided q 6 hr PO; suppressive therapy 25-33 mg/kg/24 hr divided q 6-8 hr PO. Adults: 1 g q 6 hr PO; suppressive therapy: 500 mg q 6 hr PO. |
Cautions: Vertigo, dizziness, rash. Not for use in systemic infections. Drug interactions: Liquid antacids. |
| Neomycin sulfate Mycifradin, generic. Tablet: 500 mg. Topical cream, ointment. Solution: 125 mg/5 mL. |
Aminoglycoside antibiotic used for topical application or orally before surgery to decrease gastrointestinal flora (nonabsorbable) and hyperammonemia. Infants: 50 mg/kg/24 hr divided q 6 hr PO. Children: 50-100 mg/kg/24 hr divided q 6-8 hr PO. Adults: 500-2,000 mg/dose q 6-8 hr PO. |
Cautions: In patients with renal dysfunction because small amount absorbed may accumulate. Adverse events: Primarily related to topical application, abdominal cramps, diarrhea, rash. Aminoglycoside ototoxicity and nephrotoxicity if absorbed. |
| Nitrofurantoin Furadantin, Furan, Macrodantin. Capsule: 50, 100 mg. Extended-release capsule: 100 mg. Macrocrystal: 50, 100 mg. Suspension: 25 mg/5 mL. |
Effective in the treatment of lower urinary tract infections caused by gram-positive and gram-negative pathogens. Children: 5-7 mg/kg/24 hr divided q 6 hr PO (max dose: 400 mg/24 hr); suppressive therapy 1-2.5 mg/kg/24 hr divided q 12-24 hr PO (max dose: 100 mg/24 hr). Adults: 50-100 mg/24 hr divided q 6 hr PO. |
Cautions: Vertigo, dizziness, rash, jaundice, interstitial pneumonitis. Do not use with moderate to severe renal dysfunction. Drug interactions: Liquid antacids. |
| Ofloxacin Ocuflox 0.3% ophthalmic solution: 1, 5, 10 mL. Floxin 0.3% otic solution: 5, 10 mL. |
Quinolone antibiotic for treatment of conjunctivitis or corneal ulcers (ophthalmic solution) and otitis externa or chronic suppurative otitis media (otic solution) caused by susceptible gram-positive, gram-negative, anaerobic bacteria, or Chlamydia trachomatis. Child >1-12 yr: Conjunctivitis: 1-2 drops in affected eye(s) q 2-4 hr for 2 days, then 1-2 drops qid for 5 days. Corneal ulcers: 1-2 drops q 30 min while awake and at 4 hours at night for 2 days, then 1-2 drops hourly for 5 days while awake, then 1-2 drops q 6 hr for 2 days. Otitis externa (otic solution): 5 drops into affected ear bid for 10 days. Chronic suppurative otitis media: treat for 14 days. Child >12 yr and adults: Ophthalmic solution doses same as for younger children. Otitis externa (otic solution): Use 10 drops bid for 10 or 14 days as for younger children. |
Adverse events: Burning, stinging, eye redness (ophthalmic solution), dizziness with otic solution if not warmed. |
| Oxacillin sodium Prostaphlin. Injection. Capsule: 250, 500 mg. Suspension: 250 mg/5 mL. |
Penicillinase-resistant penicillin active against S. aureus and other gram-positive cocci, except Enterococcus and coagulase-negative staphylococci. Neonates: Postnatal age ≤7 days 1,200-2,000 g: 50 mg/kg/24 hr divided q 12 hr IV; >2,000 g: 75 mg/kg/24 hr IV divided q 8 hr IV; postnatal age >7 days <1,200 g: 50 mg/kg/24 hr IV divided q 12 hr IV; 1,200-2,000 g: 75 mg/kg/24 hr divided q 8 hr IV; >2,000 g: 100 mg/kg/24 hr IV divided q 6 hr IV. Infants: 100-200 mg/kg/24 hr divided q 4-6 hr IV. Children: PO 50-100 mg/kg/24 hr divided q 4-6 hr IV. Adults: 2-12 g/24 hr divided q 4-6 hr IV (max dose: 12 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia). Moderate oral bioavailability (35-65%). Primarily renally eliminated. Drug interaction: Probenecid. Adverse effect: Neutropenia. |
| Penicillin G Injection. Tablets. |
Penicillin active against most gram-positive cocci; S. pneumoniae (resistance is increasing), group A streptococcus, and some gram-negative bacteria (e.g., N. gonorrhoeae, N. meningitidis). Neonates: Postnatal age ≤7 days 1,200-2,000 g: 50,000 units/kg/24 hr divided q 12 hr IV or IM (meningitis: 100,000 units/kg/24 hr divided q 12 hr IV or IM); >2,000 g: 75,000 units/kg/24 hr divided q 8 hr IV or IM (meningitis: 150,000 units/kg/24 hr divided q 8 hr IV or IM); postnatal age >7 days ≤1,200 g: 50,000 units/kg/24 hr divided q 12 hr IV (meningitis: 100,000 units/kg/24 hr divided q 12 hr IV); 1,200-2,000 g: 75,000 units/kg/24 hr q 8 hr IV (meningitis: 225,000 units/kg/24 hr divided q 8 hr IV); >2,000 g: 100,000 units/kg/24 hr divided q 6 hr IV (meningitis: 200,000 units/kg/24 hr divided q 6 hr IV). Children: 100,000-250,000 units/kg/24 hr divided q 4-6 hr IV or IM (max dose: 400,000 units/kg/24 hr). Adults: 2-24 million units/24 hr divided q 4-6 hr IV or IM. |
Cautions: β-Lactam safety profile (rash, eosinophilia), allergy, seizures with excessive doses particularly in patients with marked renal disease. Substantial pathogen resistance. Primarily renally eliminated. Drug interaction: Probenecid. |
| Penicillin G, benzathine Bicillin. Injection. |
Long-acting repository form of penicillin effective in the treatment of infections responsive to persistent, low penicillin concentrations (1-4 wk), e.g., group A streptococcus pharyngitis, rheumatic fever prophylaxis. Neonates >1,200 g: 50,000 units/kg IM once. Children: 300,000-1.2 million units/kg q 3-4 wk IM (max dose: 1.2-2.4 million units/dose). Adults: 1.2 million units IM q 3-4 wk. |
Cautions: β-Lactam safety profile (rash, eosinophilia), allergy. Administer by IM injection only. Substantial pathogen resistance. Primarily renally eliminated. Drug interaction: Probenecid. |
| Penicillin G, procaine Crysticillin. Injection. |
Repository form of penicillin providing low penicillin concentrations for 12 hr. Neonates >1,200 g: 50,000 units/kg/24 hr IM. Children: 25,000-50,000 units/kg/24 hr IM for 10 days (max dose: 4.8 million units/dose). Gonorrhea: 100,000 units/kg (max dose: 4.8 million units/24 hr) IM once with probenecid 25 mg/kg (max dose: 1 g) Adults: 0.6-4.8 million units q 12-24 hr IM. |
Cautions: β-Lactam safety profile (rash, eosinophilia) allergy. Administer by IM injection only. Substantial pathogen resistance. Primarily renally eliminated. Drug interaction: Probenecid. |
| Penicillin V Pen VK, V-Cillin K. Tablet: 125, 250, 500 mg. Suspension: 125 mg/5 mL, 250 mg/5 mL. |
Preferred oral dosing form of penicillin, active against most gram-positive cocci; S. pneumoniae (resistance is increasing), other Streptococcus, and some gram-negative bacteria (e.g., N. gonorrhoeae, N. meningitidis). Children: 25-50 mg/kg/24 hr divided q 4-8 hr PO. Adults: 125-500 mg q 6-8 hr PO (max dose: 3 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia), allergy, seizures with excessive doses particularly in patients with renal disease. Substantial pathogen resistance. Primarily renally eliminated. Inactivated by penicillinase. Drug interaction: Probenecid. |
| Piperacillin Pipracil. Injection. |
Extended-spectrum penicillin active against E. coli, Enterobacter, Serratia, P. aeruginosa, and Bacteroides. Neonates: Postnatal age ≤7 days 150 mg/kg/24 hr divided q 8-12 hr IV; >7 days; 200 mg/kg divided q 6-8 hr IV. Children: 200-300 mg/kg/24 hr divided q 4-6 hr IV; cystic fibrosis: 350-500 mg/kg/24 hr IV. Adults: 2-4 g/dose q 4-6 hr (max dose: 24 g/24 hr) IV. |
Cautions: β-Lactam safety profile (rash, eosinophilia); painful given intramuscularly; each gram contains 1.9 mEq sodium. Interferes with platelet aggregation/serum sickness-like reaction with high doses; increases in liver function tests. Renally eliminated. Inactivated by penicillinase. Drug interaction: Probenecid. |
| Piperacillin-tazobactam Zosyn. Injection. |
Extended-spectrum penicillin (piperacillin) combined with a β-lactamase inhibitor (tazobactam) active against S. aureus, H. influenzae, E. coli, Enterobacter, Serratia, Acinetobacter, P. aeruginosa, and Bacteroides. Children: 300-400 mg/kg/24 hr divided q 6-8 hr IV or IM. Adults: 3.375 g q 6-8 hr IV or IM. |
Cautions: β-Lactam safety profile (rash, eosinophilia); painful given intramuscularly; each gram contains 1.9 mEq sodium. Interferes with platelet aggregation, serum sickness–like reaction with high doses, increases in liver function test results. Renally eliminated. Drug interaction: Probenecid. |
| Quinupristin/dalfopristin Synercid. IV injection: powder for reconstitution, 10 mL contains 150 mg quinupristin, 350 mg dalfopristin. |
Streptogramin antibiotic (quinupristin) active against vancomycin-resistant E. faecium (VRE) and methicillin-resistant S. aureus (MRSA). Not active against E. faecalis. Children and adults: VRE: 7.5 mg/kg q 8 hr IV for VRE; skin infections: 7.5 mg/kg q 12 hr IV. |
Adverse events: Pain, edema, or phlebitis at injection site, nausea, diarrhea. Drug interactions: Synercid is a potent inhibitor of CYP 3A4. |
| Sulfadiazine Tablet: 500 mg. |
Sulfonamide antibiotic primarily indicated for the treatment of lower urinary tract infections due to E. coli, P. mirabilis, and Klebsiella. Toxoplasmosis: Neonates: 100 mg/kg/24 hr divided q 12 hr PO with pyrimethamine 1 mg/kg/24 hr PO (with folinic acid). Children: 120-200 mg/kg/24 hr divided q 6 hr PO with pyrimethamine 2 mg/kg/24 hr divided q 12 hr PO ≥3 days then 1 mg/kg/24 hr (max dose: 25 mg/24 hr) with folinic acid. Rheumatic fever prophylaxis: ≤30 kg: 500 mg/24 hr q 24 hr PO; >30 kg: 1 g/24 hr q 24 hr PO. |
Cautions: Rash, Stevens-Johnson syndrome, nausea, leukopenia, crystalluria. Renal and hepatic elimination; avoid use with renal disease. Half-life ∼10 hr. Drug interactions: Protein displacement with warfarin, phenytoin, methotrexate. |
| Sulfamethoxazole Gantanol. Tablet: 500 mg. Suspension: 500 mg/5 mL. |
Sulfonamide antibiotic used for the treatment of otitis media, chronic bronchitis, and lower urinary tract infections due to susceptible bacteria. Children: 50-60 mg/kg/24 hr divided q 12 hr PO. Adults: 1 g/dose q 12 hr PO (max dose: 3 g/24 hr). |
Cautions: Rash, Stevens-Johnson syndrome, nausea, leukopenia, crystalluria. Renal and hepatic elimination; avoid use with renal disease. Half-life 12 hr. Initial dose often a loading dose (doubled). Drug interactions: Protein displacement with warfarin, phenytoin, methotrexate. |
| Sulfisoxazole Gantrisin. Tablet: 500 mg. Suspension: 500 mg/5 mL. Ophthalmic solution, ointment. |
Sulfonamide antibiotic used for the treatment of otitis media, chronic bronchitis, and lower urinary tract infections due to susceptible bacteria. Children: 120-150 mg/kg/24 hr divided q 4-6 hr PO (max dose: 6 g/24 hr). Adults: 4-8 g/24 hr divided q 4-6 hr PO. |
Cautions: Rash, Stevens-Johnson syndrome, nausea, leukopenia, crystalluria. Renal and hepatic elimination; avoid use with renal disease. Half-life ∼7-12 hr. Initial dose often a loading dose (doubled). Drug interactions: Protein displacement with warfarin, phenytoin, methotrexate. |
| Ticarcillin Ticar. Injection. |
Extended-spectrum penicillin active against E. coli, Enterobacter, Serratia, P. aeruginosa, and Bacteroides. Neonates: Postnatal age ≤7 days <2,000 g: 150 mg/kg/24 hr divided q 8-12 hr IV; >7 days <2,000 g: 225 mg/kg/24 hr divided q 8 hr IV; >7 days <1,200 g: 150 mg/kg/24 hr divided q 12 hr IV; 1,200-2,000 g: 225 mg/kg/24 hr divided q 8 hr IV; >2,000 g: 300 mg/kg/24 hr divided q 6-8 hr IV. Children: 200-400 mg/kg/24 hr divided q 4-6 hr IV; cystic fibrosis: 400-600 mg/kg/24 hr IV. Adults: 2-4 g/dose q 4-6 hr IV (max dose: 24 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia); painful given intramuscularly; each gram contains 5-6 mEq sodium. Interferes with platelet aggregation; increases in liver function tests. Renally eliminated. Inactivated by penicillinase. Drug interaction: Probenecid. |
| Ticarcillin-clavulanate Timentin. Injection. |
Extended-spectrum penicillin (ticarcillin) combined with a β-lactamase inhibitor (clavulanate) active against S. aureus, H. influenzae, Enterobacter, E. coli, Serratia, P. aeruginosa, Acinetobacter, and Bacteroides. Children: 280-400 mg/kg/24 hr q 4-8 hr IV or IM. Adults: 3.1 g q 4-8 hr IV or IM (max dose: 18-24 g/24 hr). |
Cautions: β-Lactam safety profile (rash, eosinophilia); painful given intramuscularly; each gram contains 5-6 mEq sodium. Interferes with platelet aggregation; increases in liver function tests. Renally eliminated. Drug interaction: Probenecid. |
| Tigecycline Tygacil. Injection. |
Tetracycline-class antibiotic (glycylcycline) active against Enterobacteriaceae, including ESBL producers; streptococci (including VRE); staphylococci (including MRSA); and anaerobes. Children: unknown. Adults: 100 mg loading dose followed by 50 mg q 12 hr IV. |
Cautions: Pregnancy; children under 8 yr of age; photosensitivity; hypersensitivity to tetracyclines; hepatic impairment (~60% hepatic clearance). Drug interaction: Warfarin; mycophenolate mofetil. |
| Tobramycin Nebcin, Tobrex. Injection. Ophthalmic solution, ointment. |
Aminoglycoside antibiotic active against gram-negative bacilli, especially E. coli, Klebsiella, Enterobacter, Serratia, Proteus, and Pseudomonas. Neonates: Postnatal age ≤7 days, 1,200-2,000 g: 2.5 mg/kg q 12-18 hr IV or IM; >2,000 g: 2.5 mg/kg q 12 hr IV or IM; postnatal age >7 days, 1,200-2,000 g: 2.5 mg/kg q 8-12 hr IV or IM; >2,000 g: 2.5 mg/kg q 8 hr IV or IM. Children: 2.5 mg/kg/24 hr divided q 8-12 hr IV or IM. Alternatively may administer 5-7.5 mg/kg/24 hr IV. Preservative-free preparation for intraventricular or intrathecal use: neonate: 1 mg/24 hr; children: 1-2 mg/24 hr; adults: 4-8 mg/24 hr. Adults: 3-6 mg/kg/24 hr divided q 8 hr IV or IM. |
Cautions: S. pneumoniae, other Streptococcus, and anaerobes are resistant. May cause ototoxicity and nephrotoxicity. Monitor renal function. Drug eliminated renally. Administered IV over 30-60 min. Drug interactions: May potentiate other ototoxic and nephrotoxic drugs. Target serum concentrations: Peak 6-12 mg/L; trough <2 mg/L. |
| Trimethoprim Proloprim, Trimpex. Tablet: 100, 200 mg |
Folic acid antagonist effective in the prophylaxis and treatment of E. coli, Klebsiella, P. mirabilis, and Enterobacter urinary tract infections; P. carinii pneumonia. Children: For urinary tract infection: 4-6 mg/kg/24 hr divided q 12 hr PO. Children >12 yr and adults: 100-200 mg q 12 hr PO. P. carinii pneumonia (with dapsone): 15-20 mg/kg/24 hr divided q 6 hr for 21 days PO. |
Cautions: Megaloblastic anemia, bone marrow suppression, nausea, epigastric distress, rash. Drug interactions: Possible interactions with phenytoin, cyclosporine, rifampin, warfarin. |
| Vancomycin Vancocin, Luphocin. Injection. Capsule: 125 mg, 250 mg. Suspension. |
Glycopeptide antibiotic active against most gram-positive pathogens including Staphylococcus (including MRSA and coagulase-negative staphylococci), S. pneumoniae including penicillin-resistant strains, Enterococcus (resistance is increasing), and C. difficile–associated colitis. Neonates: Postnatal age ≤7 days, <1,200 g: 15 mg/kg/24 hr divided q 24 hr IV; 1,200-2,000 g: 15 mg/kg/24 hr divided q 12-18 hr IV; >2,000 g: 30 mg/kg/24 hr divided q 12 hr IV; postnatal age >7 days, <1,200 g: 15 mg/kg/24 hr divided q 24 hr IV; 1,200-2,000 g: 15 mg/kg/24 hr divided q 8-12 hr IV; >2,000 g: 45 mg/kg/24 hr divided q 8 hr IV. Children: 45-60 mg/kg/24 hr divided q 8-12 hr IV; C. difficile–associated colitis; 40-50 mg/kg/24 hr divided q 6-8 hr PO. 40-50 mg/kg/24 hr divided q 6-8 hr PO. |
Cautions: Ototoxicity and nephrotoxicity particularly when co-administered with other ototoxic and nephrotoxic drugs. Infuse IV over 45-60 min. Flushing (red man syndrome) associated with rapid IV infusions, fever, chills, phlebitis (central line is preferred). Renally eliminated. Target serum concentrations: Peak (1 hr after 1 hr infusion) 30-40 mg/L; trough 5-10 mg/L. |
Penicillins
Resistance to penicillin is mediated by a variety of mechanisms (see Table 173-1). The production of β-lactamase is a common mechanism exhibited by many organisms that may be overcome, with variable success, by including a β-lactamase inhibitor with the penicillin. Such combination products (ampicillin-sulbactam, amoxicillin-clavulanate, piperacillin-tazobactam) are potentially very useful for management of resistant isolates, but only if the resistance is β-lactamase mediated. Notably, S. aureus and S. pneumoniae mediate β-lactam resistance through mechanisms other than β-lactamase production, rendering these combination agents of little value for the management of β-lactam–resistant S. aureus and S. pneumoniae infections.
| TYPE OF REACTION | FREQUENCY (%) | OCCURS MOST FREQUENTLY WITH* |
|---|---|---|
| ALLERGIC | ||
| IgE antibody | 0.004-0.4 | Penicillin G |
| Anaphylaxis | ||
| Early urticaria (<72 hr) | ||
| Cytotoxic antibody | Rare | Penicillin G |
| Hemolytic anemia | ||
| Antigen-antibody complex disease | Rare | Penicillin G |
| Serum sickness | ||
| Delayed hypersensitivity | 4-8 | Ampicillin |
| Contact dermatitis | ||
| IDIOPATHIC | 4-8 | Ampicillin |
| Skin rash | ||
| Fever | ||
| Late-onset urticaria | ||
| GASTROINTESTINAL | 2-5 | |
| Diarrhea | 2-5 | Ampicillin |
| Enterocolitis | <1 | Ampicillin |
| HEMATOLOGIC | ||
| Hemolytic anemia | Rare | Penicillin G |
| Neutropenia | 1-4 | Penicillin G, nafcillin, oxacillin, piperacillin |
| Platelet dysfunction | 3 | Carbenicillin, ticarcillin |
| HEPATIC | ||
| Elevated serum aspartate transaminase level | 1-4 | Oxacillin, nafcillin, carbenicillin |
| ELECTROLYTE DISTURBANCE | ||
| Sodium overload | Variable | Ticarcillin |
| Hypokalemia | Variable | Ticarcillin |
| Hyperkalemia—acute | Rare | Penicillin G |
| NEUROLOGIC | ||
| Seizures | Rare | Penicillin G |
| Bizarre sensations | Procaine penicillin | |
| RENAL | ||
| Interstitial nephritis | 1-2 | Methicillin |
| Hemorrhagic cystitis | Rare | Methicillin |
* All the reactions can occur with any of the penicillins.
From Mandell GL, Bennett JE, Dolin R, editors: Principles and practice of infectious diseases, vol 1, ed 6, Philadelphia, 2005, Elsevier, p 286.
Cephalosporins
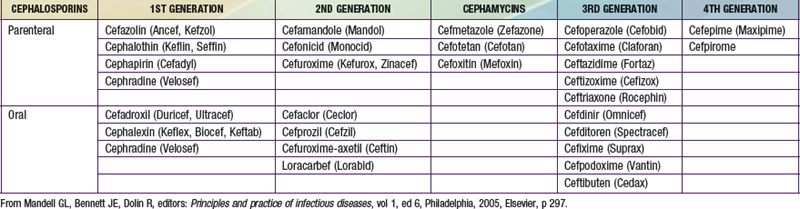
Cephalosporins differ structurally from penicillins insofar as the β-lactam ring exists as a 6 member ring, compared to the 5 member ring structure of the penicillins. These agents are widely used in pediatric practice, both in oral and parenteral formulations (Table 173-5). The 1st generation cephalosporins (e.g., cefazolin, a parenteral formulation, and cephalexin, an oral equivalent) are commonly used for management of skin and soft tissue infections caused by susceptible strains of S. aureus and group A streptococcus. The 2nd generation cephalosporins (e.g., cefuroxime, cefoxitin) have better activity against gram-negative infections than do 1st generation cephalosporins and are used to treat respiratory tract infections, urinary tract infections, and skin and soft-tissue infections. A variety of orally administered 2nd generation agents (cefaclor, cefprozil, loracarbef, cefpodoxime) are commonly used in the outpatient management of sinopulmonary infections and otitis media. The 3rd generation cephalosporins (cefotaxime, ceftriaxone, and ceftazidime) are typically used for serious pediatric infections, including meningitis and sepsis. Ceftazidime is highly active against most strains of P. aeruginosa, making this a useful agent for febrile, neutropenic oncology patients. Another cephalosporin class, so-called 4th generation cephalosporins (e.g., cefepime) is indicated for treatment of pediatric meningitis, has activity against P. aeruginosa, and retains good activity against methicillin-susceptible staphylococcal infections.
Adverse reactions to cephalosporins are noted in Table 173-6.
| TYPE | SPECIFIC | FREQUENCY |
|---|---|---|
| Hypersensitivity | Rash | 1-3% |
| Urticaria | <1% | |
| Serum sickness | <1% | |
| Anaphylaxis | 0.01% | |
| Gastrointestinal | ||
| Diarrhea | 1-19% | |
| Nausea, vomiting | 1-6% | |
| Transient transaminase elevation | 1-7% | |
| Biliary sludge | 20-46%* | |
| Hematologic | ||
| Eosinophilia | 1-10% | |
| Neutropenia | <1% | |
| Thrombocytopenia | <1-3% | |
| Hypoprothrombinemia | <1% | |
| Impaired platelet aggregation | <1% | |
| Hemolytic anemia | <1% | |
| Renal | ||
| Interstitial nephritis | <1% | |
| Central nervous system | ||
| Seizures | <1% | |
| False-positive laboratory | ||
| Coombs positive | 3% | |
| Glucosuria | Rare | |
| Serum creatinine | Rare | |
| Other | ||
| Drug fever | Rare | |
| Disulfiram-like reaction† | Rare | |
| Superinfection | Rare | |
| Phlebitis | Rare | |
† Cephalosporins with thiomethyl tetrazole ring (MTT) side chain.
From Mandell GL, Bennett JE, Dolin R, editors: Principles and practice of infectious diseases, vol 1, ed 6, Philadelphia, 2005, Elsevier, p 303.
Glycopeptides
Glycopeptide antibiotics include vancomycin and teicoplanin, the less commonly available analog. These agents are bactericidal and act via inhibition of cell wall biosynthesis. The antimicrobial activity of the glycopeptides is limited to gram-positive organisms, including S. aureus, coagulase-negative staphylococci, pneumococcus, enterococci, Bacillus, and Corynebacterium. Vancomycin is frequently employed in pediatric practice and is of particular value for serious infections, including meningitis, caused by MRSA and penicillin- and cephalosporin-resistant S. pneumoniae. Vancomycin is also commonly used for infections in the setting of fever and neutropenia in oncology patients, in combination with other antibiotics (Chapter 171), and for infections associated with indwelling medical devices (Chapter 172). Oral formulations of vancomycin are occasionally used to treat pseudomembranous colitis due to Clostridium difficile infections; intrathecal therapy may also be used for selected CNS infections. Vancomycin must be administered with care because of its propensity to produce red man syndrome, which is a reversible adverse effect that is rare in young children and can typically be readily managed by slowing the rate of infusion of the drug. Newer glycopeptides antibiotics that appear to be approaching licensure include oritavancin, dalbavancin, and the glycolipodepsipeptide agent, ramoplanin.
Bowlware KL, Stull T. Antibacterial agents in pediatrics. Infect Dis Clin North Am. 2004;18:513-531.
Bradley JS. Newer antistaphylococcal agents. Curr Opin Pediatr. 2005;17:71-77.
Buckley J, Coffin SE, Lautenbach E, et al. Outcome of Escherichia coli and/or Klebsiella bloodstream infection in children with central venous catheters. Infect Control Hosp Epidemiol. 2007;28(11):1308-1310.
Buescher ES. Community-acquired methicillin-resistant Staphylococcus aureus in pediatrics. Curr Opin Pediatr. 2005;17:67-70.
Chemaly RF, Hanmod SS, Jiang Y, et al. Tigecycline use in cancer patients wit serious infections. A report on 110 cases from a single institution. Medicine. 2009;88:211-220.
Cohen R. Approaches to reduce antibiotic resistance in the community. Pediatr Infect Dis J. 2006;25:977-980.
Committee on Infectious Diseases. The use of systemic fluoroquinolones. Pediatrics. 2006;118:1287-1292.
Dagan R, Barkai G, Leibovitz E, et al. Will reduction of antibiotic use reduce antibiotic resistance? Pediatr Infect Dis. 2006;25:981-986.
Dancer SJ. How antibiotics can make us sick: the less obvious adverse effects of antimicrobial chemotherapy. Lancet. 2004;4:611-619.
Elliott DJ, Zaoutis TE, Troxel AB, et al. Empiric antimicrobial therapy for pediatric skin and soft-tissue infections in the era of methicillin-resistant Staphylococcus aureus. Pediatrics. 2009;123(6):e959-966.
Gendrel D, Chalumeau M, Moulin F, et al. Fluoroquinolones in paediatrics: A risk for the patient or for the community? Lancet Infect Dis. 2003;3:537-546.
Hancock REW. Mechanisms of action of newer antibiotics for gram-positive pathogens. Lancet Infect Dis. 2005;5:209-218.
Jacoby GA, Munoz-Price LS. The new β-lactamases. N Engl J Med. 2005;352:380-391.
Kline RM, Baorto EP. Treatment of pediatric febrile neutropenia in the era of vancomycin-resistant microbes. Pediatr Blood Cancer. 2005;44:207-214.
Low DE, Pichichero ME, Schaad UB. Optimizing antibacterial therapy for community-acquired respiratory tract infections in children in an era of bacterial resistance. Clin Pediatr North Am. 2004;43:135-151.
Malhotra-Kumar S, Lammens C, Coenen S, et al. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet. 2007;369:482-490.
The Medical Letter. Extended release amoxicillin for strep throat. Med Lett. 2009;51:17-18.
Okeke IN, Laxminarayan R, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part 1: recent trends and current status. Lancet Infect Dis. 2005;5:481-492.
Pankey GA, Steele RW. Tigecycline: a single antibiotic for polymicrobial infections. Pediatr Infect Dis J. 2007;26:77-78.
Pong AL, Bradley JS. Guidelines for the selection of antibacterial therapy in children. Pediatr Clin North Am. 2005;52:869-894.
Rubino CM, Bradley JS. Optimizing therapy with antibacterial agents: use of pharmacokinetic-pharmacodynamic principles in pediatrics. Paediatr Drugs. 2007;9(6):361-369.
Sabharwal V, Marchant CD. Fluoroquinolone use in children. Pediatr Infect Dis J. 2006;25:257-258.
Samaha-Kfoury JN, Araj GF. Recent developments in β-lactamases and extended spectrum β-lactamases. Br Med J. 2003;327:1209-1213.
Wagner T, Burns JL. Anti-inflammatory properties of macrolides. Pediatr Infect Dis J. 2007;26:75-76.