CHAPTER 42 Primary surgical treatment for developmental glaucomas
Introduction
After Otto Barkan described the use of goniotomy as a primary treatment for congenital glaucoma in 1942, the prognosis for infantile glaucoma was markedly improved1,2. Barkan’s method of using a goniolens for incisional angle surgery under direct visualization continues to be useful with little modification. Trabeculotomy is an alternative approach that was independently described by Burian and Smith in 19603–4. This procedure involves canulation of Schlemm’s canal through a scleral incision with a trabeculotome, which is then rotated to rupture the trabecular meshwork. More recently, some glaucoma surgeons have utilized combined trabeculotomy and trabeculectomy as a primary procedure in patients judged to have a poor prognosis for success with initial goniotomy or trabeculotomy. This approach has been used most commonly in infants in the Middle East and India as well as in patients over 2 to 3 years of age.
Epidemiologic considerations and terminology
Primary congenital glaucoma is the most common form of glaucoma in children, occurring in approximately 1 in 10 000 births in western developed countries5. This condition results from an isolated maldevelopment of the trabecular meshwork (trabeculodysgenesis) and is not associated with other developmental ocular anomalies or ocular disease. Typically, it manifests at birth or early childhood, before age 3 years. The majority of patients are diagnosed by age 6 months, and 80% are diagnosed within the first year of life.
Clinical features, diagnosis, and differential diagnosis
Primary congenital glaucoma presents classically with a triad of symptoms: epiphora, photophobia, and blepharospasm6. These symptoms arise due to corneal irritation accompanying corneal epithelial edema, which is secondary to increased intraocular pressure. Patients may also present with a ‘red eye’ that mimics conjunctivitis7.
Several clinical signs may be evident in primary congenital glaucoma. Ocular enlargement (buphthalmos) may occur under the influence of increased intraocular pressure, with the major enlargement occurring at the corneo-scleral junction6–8. The cornea may appear hazy or opacified, and breaks in Descemet’s membrane (Haab’s striae) may be present. Finally, optic nerve cupping can occur rapidly and early in infants with primary congenital glaucoma. Cupping of the optic nerve head may be reversible with normalization of the intraocular pressure in infants9. This finding is uncommon in an adult with glaucomatous optic nerve head damage.
Many of the clinical features of primary congenital glaucoma can be found in other conditions and must be considered in the differential diagnosis2. Other conditions may cause red eye, corneal enlargement, corneal edema, and optic nerve abnormalities. However, these clinical entities cannot be completely characterized by photophobia, tearing, blepharospasm, increased intraocular pressure, and generalized ocular enlargement with optic nerve cupping. Recognition of the type of glaucoma present in a pediatric patient often requires experience and knowledge.
Anatomic considerations
The anterior chamber angle exhibits characteristic ultrastructural changes in eyes with primary congenital glaucoma. Anterior iris insertion is accompanied by compact trabecular beams containing excessive extracellular matrix material. Proliferation of fibrous tissue at the inner wall of Schlemm’s canal has also been described10,11. These findings are consistent with non-differentiation of the trabecular meshwork and persistence of embryonic characteristics. Secondary forms of congenital glaucoma may demonstrate additional anomalies of the iris, cornea, and lens. Pigmentation of the trabecular meshwork is acquired with age; therefore, recognition of the lightly or non-pigmented trabecular meshwork in an infant may be difficult. Severe ocular enlargement, sclerocornea, and other abnormalities may render external landmarks for internal structures unreliable. In these instances, imaging techniques such as ultrasound and optical coherence tomography (OCT) or endoscopic techniques may be helpful.
Fundamental principles
Surgical intervention provides the most effective and definitive treatment for most developmental glaucomas. Medical therapy may serve a supportive role in temporarily reducing intraocular pressure and clearing the cornea until surgery can be performed. A minority of patients with congenital glaucoma may respond to medical therapy alone12. Some secondary glaucomas may respond to medical therapy, such as glaucoma associated with aphakia or pseudophakia. Commonly used glaucoma medications in pediatric patients include topical beta-blockers, carbonic anhydrase inhibitors, and prostaglandin analogs. Oral acetazolamide elixir (5–15 mg/kg/day) may also be administered. Alpha-2-agonists are avoided in young children due to problems with sedation and other untoward side effects.
Indications for surgery
The primary developmental glaucomas are highly responsive to both goniotomy and trabeculotomy. Goniotomy is the classic procedure for primary congenital glaucoma and can be useful in cases of uveitic glaucoma as well as to prevent glaucoma development in patients with aniridia13–15. It requires a clear or nearly clear cornea and gonioscopy surgical skills. Trabeculotomy is an alternative to goniotomy which is particularly useful in eyes with cloudy corneas that preclude a gonioscopic view of the angle. In recent years, combined trabeculotomy and trabeculectomy has been advocated as an alternative approach in refractory cases. When extensive iris structural defects accompany the trabeculodysgenesis, careful examination of the angle is important. In aniridia, for example, peripheral anterior synechiae formation and anterior stromal insertion add complexity to surgical decisions.
Preoperative assessment
Anesthesia
All anesthetics have the potential to alter intraocular pressure. Halogenated ethers such as halothane, sevoflurane, and desflurane cause rapid lowering of IOP16,17. Ketamine is known to cause a modest elevation in IOP18,19. Induction with ketamine may allow more accurate and consistent IOP measurements, particularly during the first 10 minutes following onset of anesthesia20. Standardization of anesthesia for intraocular pressure measurements for diagnosis and follow-up of primary developmental glaucoma is highly desirable. Inconsistent readings should always be interpreted with consideration of the patient’s general state of anesthesia and the specific anesthetic used.
In about two-thirds of patients, congenital glaucoma is bilateral, which sometimes raises the question of simultaneous bilateral surgery2. Although potentially devastating, the risks are low for bilateral sight-threatening complications such as endophthalmitis. The risk of general anesthesia in infants, however, is also a concern, and can be reduced by performing simultaneous bilateral surgery. The surgeon should be aware of the risk of anesthesia for infants at their institution, which varies in different regions. The decision for simultaneous bilateral surgery should only be made with full participation of the patient’s family in the decision-making process.
Operation techniques
Goniotomy
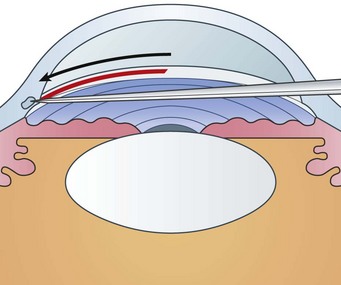
The goniotomy knife enters the anterior chamber through peripheral clear cornea, approximately 1 mm inside the corneo-limbal junction opposite the planned incision site. The blade is guided across the anterior chamber toward the opposing trabecular meshwork. Care is taken to remain parallel to the iris and away from the pupil and lens. The tip of the knife is engaged slightly anterior to the middle of the trabecular meshwork (Fig. 42.1). Under the operating microscope, the tip of the knife can be seen to indent the trabecular meshwork before it is incised by circumferential movement of the knife.
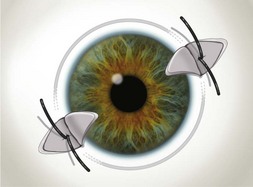
The tip of the goniotomy knife should remain in a superficial position, cutting at a uniform depth along the incision. There should be little or no perceptible tactile sensation of cutting trabecular tissue. If a coarse, grating sensation is felt, the knife is likely positioned too deep. As the incision proceeds, a white line develops behind the blade, the peripheral iris falls posteriorly, and the angle deepens (Fig. 42.2). Great care should be taken to avoid touching the iris on the knife edge or damaging the lens. The incision may encompass 90–120o circumferentially. However, with rotation of the globe by the assistant surgeon, up to 4 to 6 clock hours of meshwork can be incised in total (Fig. 42.3).
Once the incision has been completed, the blade is carefully withdrawn avoiding the corneal endothelium, iris, and lens and maintaining the anterior chamber. A single 10-0 nylon or Biosorb suture can be used to close the incision site if the wound leaks. A moderate amount of bleeding is typical and indicates that communication between the anterior chamber and Schlemm’s canal has been achieved. This bleeding is innocuous and usually rapidly clears following surgery21,22. The entire procedure normally requires approximately 10 minutes to complete. A modification to the above approach is the use of coaxial endoscopy, which allows for goniotomy in patients with cloudy corneas23–25.
Trabeculotomy
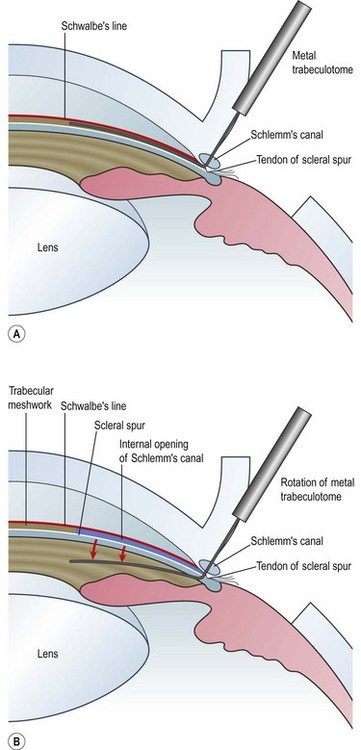
An initial fornix- or limbus-based conjunctival flap is followed by a paracentesis to facilitate maintaining the anterior chamber. Next, a limbus-based partial thickness scleral flap is made to a depth of approximately one-half scleral thickness, bearing in mind that the sclera in buphthalmic eyes is usually much thinner than in the adult eye (Fig. 42.4A).
The junction of the posterior border of the trabecular band and the sclera is the landmark for the scleral spur and for finding Schlemm’s canal. In most eyes this is situated between 2 and 2.5 mm behind the surgical limbus. A radial incision is then made across the scleral spur with the goal of cutting the external wall of Schlemm’s canal without entering the anterior chamber (Fig. 42.4B). Because Schlemm’s canal is separated from the anterior chamber only by the trabecular meshwork, this is the most delicate step in the operation and demands the most microsurgical skill.
Under high magnification, the radial incision is gradually deepened with a sharp blade until it is carried through the external wall of Schlemm’s canal, at which point there is ooze of aqueous, occasionally mixed with blood. The inner wall is identified by its mild pigmentation and composition of crisscrossing fibers. Confirmation of Schlemm’s canal may be made by passing a blunt canula or 6-0 nylon suture into the canal without resistance26.
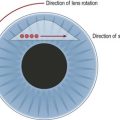
The internal arm of the trabeculotome is introduced into the canal using the external arm as a guide (Fig. 42.4C). Once 90% of the trabeculotome resides in the canal, it is rotated into the anterior chamber until 75% of the probe arm length has entered the chamber (Fig. 42.5). Care must be taken to avoid trauma to the iris and peripheral cornea. The rotation is reversed and the instrument is withdrawn. Approximately 2–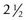 clock hours of the internal wall of Schlemm’s canal and trabecular meshwork are disrupted by this maneuver. The trabeculotome is then passed into Schlemm’s canal on the other side of the radial incision and the procedure is repeated. In total, 100–120° of trabecular meshwork are ruptured.
clock hours of the internal wall of Schlemm’s canal and trabecular meshwork are disrupted by this maneuver. The trabeculotome is then passed into Schlemm’s canal on the other side of the radial incision and the procedure is repeated. In total, 100–120° of trabecular meshwork are ruptured.
If the probe does not slip easily down the canal, it should be withdrawn and dissection of the outer wall continued until the surgeon is satisfied that all fibers of the outer wall are removed. Forceful introduction of the probe in the region of Schlemm’s canal can create a false passage. During rotation of the trabeculotome into the anterior chamber, some light resistance is expected, and intracameral bleeding from the inner wall may occur27–29. As with goniotomy, this bleeding is usually transient, resolving within a few days after surgery.
Numerous modifications to trabeculotomy have been described. Redmond Smith developed a modification that he called ‘nylon filament trabeculotomy’, which involved cannulating Schlemm’s canal and threading the suture circumferentially, withdrawing it at another site, and pulling it tight like a bowstring30. Beck and Lynch described a 360° trabeculotomy using a blunted 6-0 polypropylene suture31. The suture was threaded through Schlemm’s canal and retrieved either through the original radial incision or through a second radial incision 180° away (Fig. 42.6). This technique can be performed via a single nasal surgical site with the two ends of the passed suture tightened until the suture is pulled into the anterior chamber. More recently, ophthalmic microcatheters have been used in suture trabeculotomy to provide visual cues during cannulation and allow viscodilation of Schlemm’s canal32.
Combined trabeculotomy–trabeculectomy
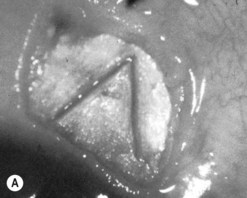
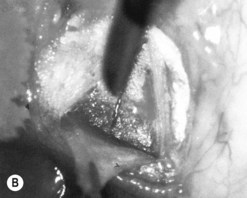
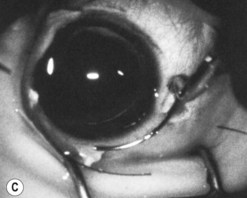
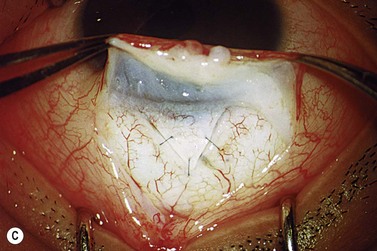
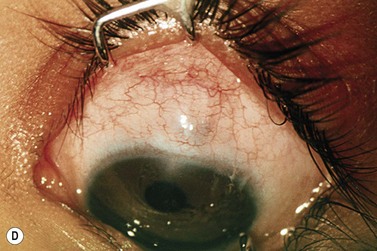
The operative procedure and postoperative appearance are shown in Fig. 42.7. Following an initial trabeculotomy as described above, a trabeculectomy may be performed by making an incision at the surgical limbus with a sharp blade under the original scleral flap. A Descemet’s punch is used to create a sclerostomy. Alternatively, a block of sclera may be removed using a sharp blade and Vannas scissors. After performing a surgical iridectomy, the partial thickness flap is closed with interrupted 10-0 nylon sutures. Alternatively, one may use absorbable suture material such as 9-0 Vicryl (polyglactin) to encourage aqueous flow during the postoperative period. Conjunctiva and Tenon’s capsule are closed in a running fashion with absorbable 9-0 polyglactin sutures on a BV needle.
If mitomycin C is used during the procedure, it should be applied after the initial partial-thickness scleral flap for trabeculotomy. Successful outcomes have been reported after trabeculotomy-trabeculectomy with and without mitomycin C2. When used intraoperatively, the usual concentration of mitomycin C ranges from 0.2 to 0.5 mg/cc applied for 1–3 minutes.
Intraoperative complications
The efficacy and safety profile of goniotomy surgery are dependent on surgical experience and technique. Serious complications are rare. A transient hyphema occurs in the majority of cases but usually resolves within 1 week. Potential complications that have been reported include iridodialysis, cyclodialysis, shallowing of the anterior chamber, retinal detachment, iris incarceration, and epithelial ingrowth33,34. Damage to the lens and progression of cataract formation can also occur.
The risks of trabeculotomy are often related to performing angle surgery without direct visualization. Identifying key surgical landmarks can be challenging in eyes with marked buphthalmos or significant corneal edema. Potential complications include cyclodialysis, iris trauma, Descemet’s detachment, prolonged hyphema, intraocular pressure spike, shallowing of the anterior chamber, postoperative hypotony, retinal detachment and progression of cataract formation. False passages and inadvertent bleb formation have also been reported29,35. Combined trabeculotomy–trabeculectomy has a risk profile similar to trabeculotomy with perhaps an increased risk of vitreous prolapse during sclerostomy formation and surgical iridectomy36.
Assessment of surgery
Goniotomy for developmental glaucoma has a high success rate of approximately 80%, ranging from 70% to 93% in most large series21,33,37.
It appears that goniotomy is most successful in patients whose glaucoma is recognized early and treated between 1 month and 1 year of age, though good success rates are achieved in patients up to 2 years of age33. Russell-Eggitt et al.37 reported a relapse rate of approximately 2–3% per year over 30 years in eyes that had initially been controlled for IOP after one or more goniotomies. Thus, regular surveillance of these patients is needed.
Trabeculotomy in patients with primary developmental glaucoma shows similar results compared with goniotomy. McPherson and Berry reported that 22 (96%) of 23 eyes following trabeculotomy had an intraocular pressure under 21 mmHg at a mean follow-up of 5.6 years27. Ikeda et al.38 studied the long-term outcome of 149 patients with primary or secondary developmental glaucoma who underwent one or more trabeculotomy surgeries. Patients with primary developmental glaucoma had a 47% mean reduction in IOP following a single trabeculotomy with an average follow-up of 9.5 years. Likewise, patients with secondary developmental glaucoma had a 38% mean reduction in IOP following a single trabeculectomy with an average follow-up of 8.1 years. A trend of effective intraocular pressure control was maintained over 18 years in both groups. Success probabilities at 20 years were 80.8% for eyes with primary developmental glaucoma and 64.2% for eyes with secondary developmental glaucoma.
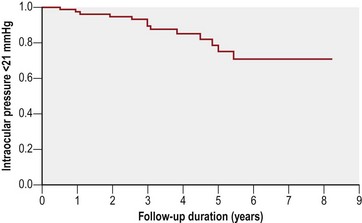
Long-term results of combined trabeculotomy and trabeculectomy have been described by Mandal et al.36 who reported long-term outcomes of 299 eyes of 157 consecutive Indian patients with primary congenital glaucoma. Success probabilities were 94.4% at 1 year and 63% at 8 years (Fig. 42.8). Mullaney et al.39 reviewed 100 consecutive eyes in 60 patients with infantile glaucoma treated with combined surgery (most received mitomycin C, 0.2 or 0.4 mg/cc). Successful control of IOP was achieved in 78% of eyes with primary developmental glaucoma, but in just 45% of eyes with anterior segment anomalies, with an average follow up of approximately 10 months. Combined trabeculotomy–trabeculectomy is a safe and effective alternative procedure for patients with developmental glaucoma who present with hazy media and respond poorly to primary trabeculotomy alone, as in India and parts of the Middle East. Moreover, the combined surgery establishes two major outflow pathways for IOP control, a theoretical advantage in poor prognosis patients.
1 Barkan O. Operation for congenital glaucoma. Am J Ophthalmol. 1942;25:552-568.
2 Mandal AK, Netland PA. The Pediatric Glaucomas. New York, NY: Elsevier; 2006.
3 Burian HM. A case of Marfan’s syndrome with bilateral glaucoma with a description of a new type of operation for developmental glaucoma. Am J Ophthalmol. 1960;50:1187-1192.
4 Smith R. A new technique for opening the canal of Schlemm. Br J Ophthalmol. 1960;44:370-373.
5 Francois J. Congenital glaucoma and its inheritance. Ophthalmologica. 1972;181:61-73.
6 Hoskins HDJr, Kass MA. Becker–Shaffer’s Diagnosis and Therapy of the Glaucomas, 6th edn. St. Louis: CV Mosby; 1989.
7 Shaffer RN, Weiss DI. The Congenital and Pediatric Glaucomas. St. Louis: CV Mosby; 1973.
8 Kwitko ML. The pediatric glaucomas. Int Ophthalmol Clin. 1981;21:199-222.
9 Quigley HA. The pathogenesis of reversible cupping in congenital glaucoma. Am J Ophthalmol. 1977;84:358-370.
10 Shaffer RN. Pathogenesis of congenital glaucoma. Gonioscopic and microscopic anatomy. Trans Am Acad Ophthalmol Otolaryngol. 1955;59:297-308.
11 Worst JGF. Congenital glaucoma. Remarks on the aspect of chamber angle, ontogenic and pathogenic background, and mode of action of goniotomy. Invest Ophthalmol. 1968;7:127-134.
12 Maris PJGJr, Mandal AK, Netland PA. Medical therapy of pediatric glaucoma and glaucoma in pregnancy. Ophthalmol Clin N Am. 2005;18:461-468.
13 Ho CL, Wong EY, Walton DS. Goniosurgery for glaucoma complicating chronic childhood uveitis. Arch Ophthalmol. 2004;6:838-844. 122
14 Ho CL, Walton DS. Goniosurgery for glaucoma secondary to chronic anterior uveitis: prognostic factors and surgical technique. J Glaucoma. 2004;6:445-449. 13
15 Chen TC, Walton DS. Goniosurgery for prevention of aniridic glaucoma. Arch Ophthalmol. 1999;9:1144-1148. 177
16 Ausinsch B, Munson ES, Levy NS. Intraocular pressure in children with glaucoma during halothane anesthesia. Ann Ophthalmol. 1977;9:1391-1394.
17 Hetherington J, Shaffer RN. Tonometry and tonography in congenital glaucoma. Invest Ophthalmol. 1968;7:134-137.
18 Peuler M, Glass DD, Arens JF. Ketamine and intraocular pressure. Anesthesiology. 1975;43:575-578.
19 Ausinsch B, Rayburn RL, Munson ES, et al. Ketamine and intraocular pressure in children. Anesth Analg. 1976;55:773-775.
20 Blumberg D, Congdon N, Jampel H, et al. The effects of sevoflurane and ketamine on intraocular pressure in children during examination under anesthesia. Am J Ophthalmol. 2007;143:494-499.
21 Barkan O. Surgery of congenital glaucoma. Review of 196 eyes operated by goniotomy. Am J Ophthalmol. 1953;36:1523-1534.
22 Hass J. Principles and problems of therapy in congenital glaucoma. Invest Ophthalmol. 1968;7:140-146.
23 Medow NB, Sauer HL. Endoscopic goniotomy for congenital glaucoma. J Pediatr Ophthalmol Strabismus. 1997;34:258-259.
24 Bayraktar S, Koseoglu T. Endoscopic goniotomy with anterior chamber maintainer: Surgical technique and one year results. Ophthalmic Surg Lasers. 2001;32:496-502.
25 Joos KM, Shen JH. An ocular endoscope enables a goiniotomy despite a cloudy cornea. Arch Ophthalmol. 2001;119:134-135.
26 Smith R. A new technique for opening the canal of Schlemm. Br J Ophthalmol. 1960;44:370-373.
27 McPherson SDJr, Berry DP. Goniotomy vs external trabeculotomy for developmental glaucoma. Am J Ophthalmol. 1983;95:427-431.
28 McPherson SDJr. Results of external trabeculotomy. Am J Ophthalmol. 1973;76:918-920.
29 McPherson SD, McFarland D. External trabeculotomy for developmental glaucoma. Ophthalmology. 1980;87:302-305.
30 Smith R. A new technique for opening the canal of Schlemm. Br J Ophthalmol. 1960;44:373-378.
31 Beck AD, Lynch MG. 360° trabeculotomy for primary congenital glaucoma. Arch Ophthalmol. 1995;113:1200-1202.
32 Reynolds AC, Debry P, Marcus C, et al. Use of the iTrack catheter for 360 degree trabeculotomy ab externo for infantile congenital glaucoma. Poster presented at: The 18th Annual Meeting of the American Glaucoma Society; March 6, 2008; Washington, DC.
33 Shaffer RN. Prognosis of goniotomy in primary infantile glaucoma (trabeculodysgenesis). Trans Am Ophthalmol Soc. 1982;80:321-325.
34 Rice NSC. The surgical management of congenital glaucoma. Aust J Ophthalmol. 1977;5:174-179.
35 Akimoto M, Tanihara H, Negi A, et al. Surgical results of trabeculotomy ab externo for developmental glaucoma. Arch Ophthalmol. 1994;112:1540-1544.
36 Mandal AK, Bhatia PG, Bhaskar A, et al. Long-term surgical and visual outcomes in Indian children with developmental glaucoma operated on within 6 months of birth. Ophthalmology. 2004;111:283-290.
37 Russell-Eggitt IM, Rice NS, Jay B, et al. Relapse following goniotomy for congenital glaucoma due to trabecular dysgenesis. Eye. 1992;6:197-200.
38 Ikeda H, Ishigooka H, Muto T, et al. Long-term outcome of trabeculotomy for the treatment of developmental glaucoma. Arch Ophthalmol. 2004;122:1122-1128.
39 Mullaney PB, Selleck C, Al-Awad A, et al. Combined trabeculotomy and trabeculectomy as an initial procedure in uncomplicated congenital glaucoma. Arch Ophthalmol. 1999;117:457-460.