CHAPTER 33 Primary Optic Nerve Sheath Meningiomas
INTRODUCTION
Optic nerve sheath meningiomas (ONSMs) represent 1% to 2% of all meningiomas, 1.7% of all orbital tumors, and about 35% of all intrinsic tumors of the optic nerve.1–3 Thus ONSMs represent a rare but nevertheless important entity due to their natural course of slowly progressive and unremitting loss of vision. Classical primary ONSM arise from the meningothelial cap cells of the arachnoids surrounding the intraorbital optic nerve and may extend along the optic canal intracranially. Secondary ONSM extends from the planum sphenoidale into the subdural or subarachnoid spaces surrounding the nerve within the optic canal and, ultimately, within the orbit.1,2,4–7 Most ONSMs are unilateral, with 5% manifested bilaterally.1 ONSM occurs predominantly in middle-aged women or in children.
The treatment of ONSM remains controversial, but includes surgery, radiotherapy, and plain observation. Complete surgical excision is thought to result in blindness in almost all cases.8 When intracranial extension that threatens the optic chiasm or the contralateral optic nerve is present, complete excision of the intracranial part via craniotomy has been described.3,7,9 Complete neurectomy and tumor resection are also performed in cases of severe unilateral loss of vision accompanied by disfiguring proptosis. As surgical reports revealed a high morbidity in terms of visual loss and recurrences due to incomplete removal, other treatment options were debated.10–17 In adults with good vision, observation was often chosen as first method because of the slow-growing nature of these tumors.18 During the past several years, treatment plans for ONSM changed with intervention of precise fractionated and stereotactic radiotherapy. The role of radiation became more and more important as several authors have reported stabilization or even improvement of vision.8,14–17,19,20 Radiation therapy is increasingly being offered to adults as primary therapy once mild to moderate vision loss develops. Despite the positive results reported in the literature, concerns regarding secondary complications have limited the acceptance of radiotherapy.21
In 2004, we introduced a new classification system in order to the possible manifestations and recommended optimal treatment modalities where surgical intervention and radiotherapy play a supplementary role.22 We overview a single center series of 90 optic nerve sheath meningiomas treated over a 17-year period (n = 70 surgery only, n = 5 radiation only, n = 18 surgery and postoperative radiation, n = 2 observation).
CLASSIFICATION
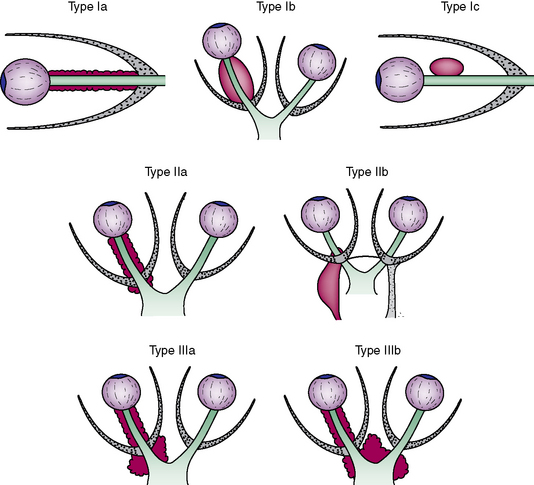
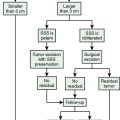
Our classification system based on the tumor location and extent is provided to clarify the possible manifestations of ONSM (Fig. 33-1).22 Treatment modalities are derived from the different types and subtypes.
Type II is located intraorbitally with extension through the optic canal or superior orbital fissure (n = 41; see Fig. 33-1). Type IIa is manifested as an intraorbital tumor with tumor growth through the optic canal (n = 37; Figs. 33-2A and 33-3B). Type IIb involves the orbital apex (n = 4) and the superior orbital fissure, and sometimes even infiltrates the cavernous sinus.
CLINICAL FEATURES
Slowly progressive, painless loss of vision and proptosis are the cardinal features of ONSMs.2,3,9,23 Commonly reported other clinical findings include afferent pupillary defect, color vision disturbance, visual field defect, optic disc edema, optic atrophy, and motility disturbance.23 Ophthalmoscopic examination may reveal optic nerve head swelling, contiguous macular edema, nerve pallor, or choroidal folds.8 Opticociliary shunt vessels may develop from compression of the central retinal vein in about one third of patients.3,8 The classic triad of optic atrophy, visual loss, and opticociliary shunt vessels is present only in a minority of patients. Mild to moderate proptosis (2–5 mm) is commonly present and may be the presenting sign.5 Chemosis, lid edema, and limitation of motility may also be found. Visual deterioration in ONSM usually is a question of time. In our series, visual acuity became worse with longer duration of preoperative symptoms and longer follow-up period. Optic atrophy is another negative predictor for recovery.
RADIOLOGIC FINDINGS
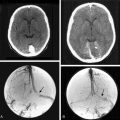
Magnetic resonance imaging (MRI) has become the gold standard for diagnosis of ONSMs, thus obviating the need for a tissue biopsy in most cases.3,9,10 High-field T1-weighted images with fat suppression and gadolinium contrast remain the procedure of choice for diagnosis of ONSM. The tumor is typically isointense to slightly hypointense to brain and optic nerve on T1-weighted images and hyper- or hypointense on T2-weighted images.16 The classic “tram-tracking” sign consists of the thickened optic nerve sheath containing the lesion surrounding the nonenhancing optic nerve. Neuroimaging characteristics may also show tubular, globular, or even fusiform enlargement of the optic nerve.24
C-scan ultrasound imaging as a noninvasive, quantitative, and inexpensive method provided optic nerve sheath diameters similar to those obtained by CT of the orbits.25 This ultrasound could image the optic nerve up to 15 mm behind the globe.
PATHOLOGY AND PATHOGENESIS
Primary ONSMs arise from the arachnoid cap cells surrounding the intracanalicular or intraorbital portion of the nerve and are almost always intimately associated with the nerve tending to surround the nerve.5,9,24,26 This results in a concentric thickening of the optic nerve diameter. ONSMs extend posteriorly into the annulus of Zinn. Therefore, ONSMs cannot be resected completely without compromising the integrity of the optic nerve.27
There are different mechanisms for preoperative optic nerve injury: ischemia, compression, demyelization and tumor invasion.10,27 Compressive mechanical injury leads to small vessel compromise and demyelization, especially in patients with a long duration of visual loss before surgery. Assuming that there is no additional intraoperative trauma to the optic nerve, incomplete or no recovery of visual function after surgery may imply chronic severe preoperative ischemic or compressive damage and demyelization. In our study, preoperative disc pallor and location in the optic canal were negative prognostic factors for visual improvement. The bony optic canal is not enlarged in ONSMs and the tumor in the canal compresses the optic nerve. This compressive injury can at least be reversed by surgical bony decompression of the optic canal. This is the main argument in favor of surgery.
Aggressive ONSMs are known with infiltration of the globe or optic nerve. Thus, deterioration of visual acuity may also result from direct tumor invasion into the intracranial optic nerve (n = 4, own series). Infiltration of the globe occurred in two patients and infiltration of the cavernous sinus in four patients (type IIb). Irregular margins in the orbit implied local invasion.26
LOCATION
Tumor location seems to have an important impact on the evolution of visual function. Onset of visual loss in ONSM at or near the orbital apex is thought to be followed by rapid and progressive further loss and a higher risk of intracranial extension.2,28 We were also able to identify intracanalicular location as a negative factor for visual acuity. Saeed and co-workers26 demonstrated that tumors with posterior components in the orbit had more frequent intracranial involvement. Intracranial extension was more frequent and had a greater growth rate in younger patients.26 In cases in which ONSM involves the intracanalicular portion of the nerve, up to 38% incidence of contralateral nerve involvement was reported.3 Bilateral presentations are most commonly found in patients with neurofibromatosis type 2.3,24
SURGICAL TECHNIQUES
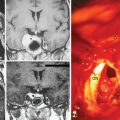
Almost all operations were performed via a unilateral frontotemporal approach. This pterional approach is well described in the literature.29–31 Craniotomy is extended to the middle of the orbital rim and 1 cm above the margin. For intradural procedures, the sylvian fissure was routinely opened. Drainage of cerebrospinal fluid (CSF) was performed by opening the basal cisterns and by lumbar drainage. The ipsilateral optic nerve and carotid artery were identified and the intracranial tumor was first coagulated and resected along the dura around the optic canal. Preservation of the small feeding vessels between the carotid artery and the optic nerve is important and can be reached by sucking the tumor within the arachnoidal plane. At this step mainly irrigation instead of coagulation should be used. The ipsilateral optic canal should always be opened. We favor early lateral opening of the dural part of the optic canal to avoid narrowing of the optic nerve through this band. The dura was resected around the optic canal and the bony optic canal was decompressed. The drilling should begin laterally until the floor of the optic canal is reached to prevent any contact to the nerve. Finally, the optic canal was unroofed. In case of opening the medially located sphenoid sinus or ethmoidal cells, subcutaneous tissue with fibrine glue were inserted. The tumor around the optic nerve and the dura was carefully removed. In tumors infiltrating the nerve, resection was limited to the exophytic part. In blind patients with disfiguring painful proptosis, a prechiasmatic transsection of the optic nerve was performed intradurally and the intraorbital part was removed as well. The intraorbital optic nerve was transected just behind the globe and deep in the apex.
OUTCOME
The natural history of ONSMs is a progressive loss of vision,1,3,9,11,14,18,19,22 but left untreated, ONSM may extend intracranially to the contralateral side, resulting in bilateral blindness. However, these tumors are not associated with mortality or significant neurologic morbidity except of visual deterioration.5,13
Outcome and Observation
Egan and Lessell18 reported the natural history of 16 untreated patients, 11 of whom developed visual loss over a mean of 10.2 years. Two of our patients were only observed and remained stable without further treatment. While observation may be warranted in certain cases, especially those with a visual acuity of 20/40 or better, close follow-ups should be performed. However, allowing progression of ONSM growth may decrease the opportunity for visual improvement after treatment.13 Pediatric and young patients are at increased risk for progressive visual decline due to the more aggressive behavior of the tumor. Thus, no general recommendation for observation without treatment should be given.
Outcome and Surgery
Only rare cases of visual improvement after microsurgical resection of ONSMs have been reported, but these cases represent a subset that are located far anteriorly, just posterior the globe.9–11 Other surgical series reported a high rate of visual complications (30%–40%), such as central retinal artery occlusion, motility disturbances, visual field defects, and a high rate of recurrence (65%).2,3,9 Therefore, surgical excision has been used to treat blind, uncomfortable, or unsightly eyes; to reduce the risk of intracranial extension or contralateral extension; and to treat young patients, in whom a higher biologic activity is presumed.2,3,7,9,23
Optic sheath opening along the length of the nerve within the optic canal to the annulus of Zinn was thought to relieve any focal circumferential pressure on the optic nerve.27 Controversially, Saeed and co-workers26 could not detect preservation of vision by optic sheath decompression. Despite reports of some successful cases, in the majority of patients visual function even declined due to orbital invasion.
In patients with tumors confined to the optic canal, decompression of the canal may be associated with vision stabilization for years.22
If there is evidence of tumor spread across the planum sphenoidale and useful vision is still present, only the intracranial part of the tumor with preservation of the optic nerve can be removed to prevent spread to the contralateral optic nerve.7,22,32 Resection may also be warranted in cases with disfiguring orbital proptosis with significantly compromised visual function.
In larger surgical series,33,34 only 2 of 21 patients with anteriorly located tumors showed improved vision, although 30% respectively 62% remained with functional visual acuity. In Kennerdell’s9 series, 7 patients with functional visual acuity remained stable for 2 to 10 years after subtotal resection. However, his group experienced loss of vision in the majority of cases. Delfini and co-workers28 support surgery in patients with progressive symptoms, despite resulting in more than 80% decline in vision. Verheggen and co-workers35 demonstrated an improvement of visual acuity in intracanalicular and intraorbital meningiomas in 89% postoperatively. Rosenberg and co-workers36 evaluated 20 surgically treated patients with 69% stable or improved vision postoperatively in correlation to the duration of preoperative symptoms. In the series of Roser and colleagues32 patients with sudden visual loss (<5 months before surgery) showed improvement through surgery, due to decompression of the optic nerve with subtotal resection of the tumor. These patients gained visual function for a mean time of 60 months with acceptable risk of morbidity and still having the feasibility of radiotherapy in the future.
Outcome and Surgery (Personal Data)
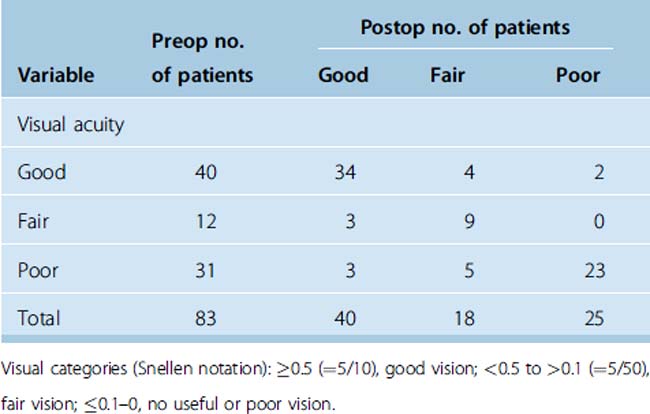
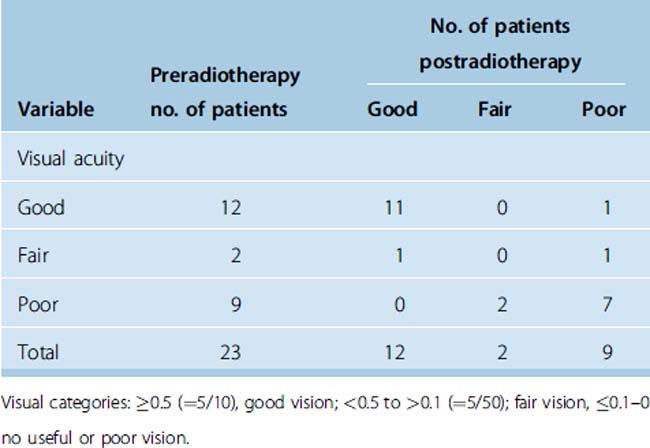
The postoperative visual acuity in 83 surgical patients did not significantly differ from the preoperative visual acuity (Wilcoxon test, n.s.). Patients remained stable within the 3 different visual categories (Table 33-1), χ2 test, P = 5.8 × 10−5. Patients with good preoperative vision preserved this quality (n = 34), only 6 patients’ vision worsened (see Table 33-1). Poor vision did improve in 8 patients postoperatively and remained unchanged in 23 patients (see Table 33-1). Only in the group with fair vision did the vision of three patients improve (see Table 33-1). Sixty-four of 83 patients remained unchanged. Thus, we were able to demonstrate that it is possible to perform surgery without it resulting in significant visual loss.
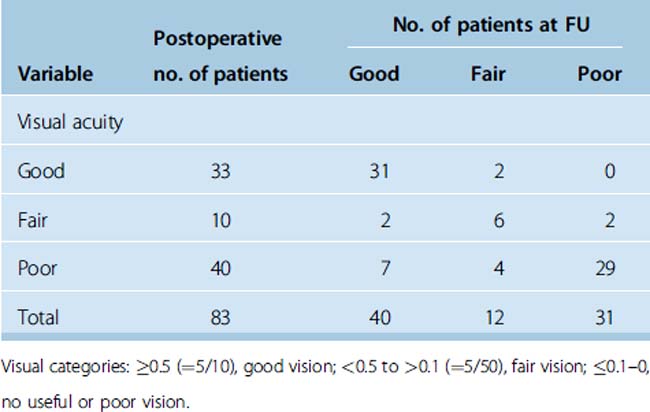
Vision worsened during an extended follow-up period (Spearman’s correlation coefficient r = –0.3, P = 0.001). In contrast to the postoperative findings, vision at follow-up significantly differed from the postoperative vision (Wilcoxon test, P = 0.03). However, most patients still remained stable within the three different groups (χ2 test, P = 1.15 × 10−13). Patients with good postoperative vision preserved this vision (n = 31), but the vision of two patients worsened (Table 33-2). Astonishingly, poor vision could improve at follow-up (n = 11). In the group with fair vision, the vision of two patients improved and two patients’ vision worsened (see Table 33-2). Sixty-six of 83 surgical patients remained unchanged.
Outcome and Radiotherapy
Several different techniques, namely stereotactic fractionated radiosurgery, intensity-modulated radiotherapy, stereotactic fractionated radiation therapy (SFRT), and three-dimensional conformal radiotherapy, provide increasing evidence for the benefit of radiotherapy in ONSM.5,8,11–17,19–21,37 These studies have shown promising results with stable or improved vision at follow-up times of up to 2 years.
Turbin and colleagues8 provided substantial data indicating that conventional radiation therapy was associated with the best visual outcome during follow-up period. They recommended fractionated external beam radiation (5000–5500 cGy). Visual acuity fell significantly in the observed only group (13 patients), only surgery (12 patients), and surgery with radiation group (16 patients). Eighteen patients had received only radiation and did not show a significant decrease in visual acuity. The role of surgery was restricted to patients with severe disc edema and rapid visual loss.17 Nevertheless, 33% of these patients developed complications from conventional radiotherapy, including retinopathy, retinal vascular occlusion, iritis, and temporal lobe atrophy. These complications were reduced by other groups using a more focused, conformal beam.
Liu and co-workers11 presented a series of five patients undergoing stereotactic radiotherapy by use of 1.8-Gy fractions to a cumulative dose of 45 to 54 Gy with dramatic improvement in visual acuity, visual field, and color vision within 3 months after SRT in four patients.
Andrews and co-workers19 reported a series of 30 patients with different locations of meningiomas (29% intraorbital, 36% optic canal, 3% chiasm, 18% chiasm and optic canal, 9% middle and posterior fossa) who underwent CF-SRT with a 6-MeV LINAC. Of 24 optic nerves with useful vision before CF-SRT, 22 demonstrated either stability (12 nerves) or improvement (10 nerves).
Pitz and colleagues15 report the results of 15 patients with ONSMs undergoing stereotactic fractionated conformal radiation with 54 Gy. Visual acuity improved in one patient and the visual field in six patients. Visual outcome in the other patients remained unchanged. This study is remarkable because of the visual improvement after radiation without side effects. Stereotactic three-dimensional conformal fractionated radiation seems to be superior to conventional fractionated radiation.
Narayan and co-workers14 demonstrated the effectiveness of 3D-conformal radiation therapy (n = 14) in controlling tumor growth while improving (n = 5) or preserving (n = 7) vision in most patients.
Berman and Miller38 summarized the data from 7 large series in which a total of 75 patients underwent SFRT. The reported disease control was 94.6% and visual improvement 54.7% within the first 3 months. Complications included headache, nausea, erythema, alopecia, and radiation retinopathy.
Some patients with ONSM have a stable course for many years, and a few may even show slight improvement. The routine application of radiation therapy may unnecessarily expose some patients to complications and should be reserved for those patients whose visual function declines under observation.18 Radiotherapy is still associated with relevant treatment related morbidity up to 33% like retinopathy, persistent retinitis, dry eye, neuronal damage to the optic nerve resulting in visual loss, and late pituitary dysfunction.8,14,16,20,39 Radiation optic neuropathy may occur from months to years after exposure of the nerve. Factors that contribute to optic neuropathy are in excess of 60 Gy and fraction doses greater than 1.9 Gy.40 The mechanism of injury is unknown but has been postulated to be caused by damage to endothelial cells of blood vessels.1 Landert and co-workers41 used slightly lower doses of 50.4 to 54 Gy and demonstrated visual acuity improvement in 86% and visual field improvement in 57% of seven eyes.
As these effects can occur even very long after treatment, the clinical data to definitively determine the long-term incidence of complications is still maturing.42
Radiotherapy does not lead to an acute decrease in tumor volume in all cases and therefore cannot be recommended in cases of rapid visual decline, where surgery is indicated to immediately alleviate pressure to visual structures.17,32 Postoperative tumor remnants provide smaller and safer targets for adjuvant radiotherapy.32
Outcome and Radiotherapy (Personal Data)
Vision improved in 3 patients between categories, in 2 patients within the category with good vision, remained unchanged in 16 patients, and deteriorated in 2 patients (Table 33-3). Poor vision could be improved in two patients to fair vision. Normalization of vision was seen in two patients with good and one with fair vision. Deterioration into poor vision occurred in one patient with fair and one with good vision.
LESSONS LEARNED
In the past, 10 intracranial extensions were detected intraoperatively and not suspected on preoperative MRI scans. On the other hand intracranial extension could not be confirmed in three patients. Today, fat-suppression techniques and high magnetic field MRI scans focused on the optic nerve usually answer the question of intracranial involvement. On very rare occasions, small tumors located within the optic canal remain impossible to detect using neuroimaging. Such lesions are discovered only during exploratory craniotomy.5,13
TREATMENT RECOMMENDATIONS
Considering radiotherapeutic results of the last 6 years8,14–17,19,20,42–47 we would like to recommend radiotherapy without biopsy as the treatment of choice in flat purely intraorbital ONSM (type Ia; see Fig. 33-1) once mild vision loss develops or tumor enlargement is determined by serial imaging. Otherwise these tumors should only be observed. Purely intraorbital tumors manifesting as large mass (type Ib) around the optic nerve should only be operated on to treat painfully uncomfortable eyes without useful vision. Otherwise these tumors can be observed and radiated once visual decline begins to occur. Type Ic tumors with large exophytic parts should be operated on.
[1] Cantore W.A. Neural orbital tumors. Curr Opin Ophthalmol. 2000;11:367-371.
[2] Castel A., Boschi A., Renard L., et al. Optic nerve sheath meningiomas: clinical features, functional prognosis and controversial treatment. Bull Soc Belge Ophtalmol. 2000;275:73-78.
[3] Dutton J.J. Optic nerve sheath meningiomas. Surv Ophthalmol. 1992;37:167-183.
[4] Mafee M.F., Goodwin J., Dorodi S. Optic nerve sheath meningiomas: role of MR imaging. Radiol Clin NA. 1999;37:37-58.
[5] Miller N.R. Primary tumours of the optic nerve and its sheath. Eye. 2004;18:1026-1037.
[6] Shimano H., Nagasawa S., Kawabata S., et al. Surgical strategy for meningioma extension into the optic canal. Neurol Med Chir (Tokyo). 2000;40:447-452.
[7] Volpe N.J., Gausas R.E. Optic nerve and orbital tumors. Neurosurg Clin NA. 1999;10:699-715.
[8] Turbin R.E., Thompson C.R., Kenderell J.S., et al. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002;109:890-900.
[9] Kennerdell J.S., Maroon J.C., Malton M., et al. The management of optic nerve sheath meningiomas. Am J Ophthalmol. 1988;106:450-457.
[10] Fineman M.S., Augsburger J.J. A new approach to an old problem. Surv Ophthalmol. 1999;43:519-524.
[11] Liu J.K., Forman S., Hershewe G.L., et al. Optic nerve sheath meningiomas: visual improvement after stereotactic radiotherapy. Neurosurgery. 2002;50:950-957.
[12] Miller N.R. Radiation for optic nerve meningiomas: is this the answer? Ophthalmology. 2002;109:833-834.
[13] Miller N.R. New concepts in the diagnosis and management of optic nerve sheath meningioma. J Neuroophthalmol. 2006;26:200-208.
[14] Narayan S., Cornblath W.T., Sandler H.M., et al. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2003;56:537-543.
[15] Pitz S., Becker G., Schiefer U., et al. Stereotactic fractionated irradiation of optic nerve sheath meningioma: a new treatment alternative. Br J Ophthalmol. 2002;86:1265-1268.
[16] Turbin R.E., Pokorny K. Diagnosis and treatment of orbital optic nerve sheath meningioma. Cancer Control. 2004;11:334-341.
[17] Turbin R.E., Wladis E.J., Frohman L.P., et al. Ophthalmol Plast Reconstr Surg. 2006;22:278-282.
[18] Egan R.A., Lessell S. A contribution to the natural history of optic nerve sheath meningiomas. Arch Ophthalmol. 2002;120:1505-1508.
[19] Andrews D.W., Faroozan R., Yang B.P., et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without surgery. Neurosurgery. 2002;51:890-904.
[20] Jeremic B., Pitz S. Primary optic nerve sheath meningioma: stereotactic fractionated radiation therapy as an emerging treatment of choice. Cancer. 2007;25:714-722.
[21] Eddleman C.S., Liu J.K. Optic nerve sheath meningioma: current diagnosis and treatment. Neurosurg Focus. 2007;23:E4.
[22] Schick U., Dott U., Hassler W. Surgical management of meningiomas involving the optic nerve sheath. J Neurosurg. 2004;101:951-959.
[23] Wright J.E., McNab A.A., McDonald W.I. Primary optic nerve sheath meningioma. Br J Ophthalmol. 1989;73:960-966.
[24] Carrasco J.R., Penne R.B. Optic nerve sheath meningiomas and advanced treatment options. Curr Opin Ophthalmol. 2004;15:406-410.
[25] Garcia J.P., Finger P.T., Kurli M., et al. 3D ultrasound coronal C-scan imaging for optic nerve sheath meningioma. Br J Ophthalmol. 2005;89:244-245.
[26] Saeed P., Rootman J., Nugent R.A., et al. Optic nerve sheath meningiomas. Ophthalmol. 2003;110:2019-2030.
[27] Lee J.H., Jeun S.S., Evans J., et al. Surgical management of clinoidal meningiomas. Neurosurgery. 2001;48:1012-1017.
[28] Delfini R., Missori P., Tarantino R. Primary benign tumors of the orbital cavity: Comparative data in a series of patients with optic nerve glioma, sheath meningioma or neurinoma. Surg Neurol. 1996;45:147-154.
[29] Hassler W.E., Eggert H. Extradural and intradural microsurgical approaches to lesions of the optic canal and the superior orbital fissure. Acta Neurochir (Vienna). 1985;74:87-93.
[30] Mauriello J.A., Flanagan J.C. Surgical approaches to the orbit. In: Mauriello J.A., Flanagan J.C., editors. Management of Orbital and Ocular Adnexal Tumors and Inflammations. Heidelberg: Springer-Verlag; 1990:149-169.
[31] Rohde V., Schaller K., Hassler W. The combined pterional and orbitocygomatic approach to extensive tumors of the lateral and latero-basal orbit and orbital apex. Acta Neurochir (Vienna). 1995;132:127-130.
[32] Roser F., Nakamura M., Martini-Thomas R., et al. The role of surgery in meningiomas involving the optic nerve sheath. Clin Neurol Neurosurg. 2006;108:470-476.
[33] Cristante L. Surgical treatment of meningiomas of the orbit and optic canal: a retrospective study with particular attention to the visual outcome. Acta Neurochir (Wien). 1994;126:27-32.
[34] Ito M., Ishizawa A., Miyaoka M., et al. Intraorbital meningiomas. Surgical management and role of radiation therapy. Surg Neurol. 1988;29:448-453.
[35] Verheggen R., Markakis E., Muhlendyck H., et al. Symptomatology, surgical therapy and postoperative results of sphenoorbital, intraorbital-intracanalicular and optic sheath meningiomas. Acta Neurochir Suppl (Wien). 1996;65:95-98.
[36] Rosenberg L.F., Miller N.R. Visual results after microsurgical removal of meningiomas involving the anterior visual system. Arch Ophthalmol. 1984;102:1019-1023.
[37] Moyer P.D., Golnik K.C., Breneman J. Treatment of optic nerve sheath meningioma with three-dimensional conformal radiation. Am J Ophthalmol. 2000;5:694-696.
[38] Berman D., Miller N.R. New concepts in the management of optic nerve sheath meningiomas. Ann Acad Med Singapore. 2006;35:168-174.
[39] Subramanian P.S., Bressler N.M., Miller N.R. Radiation retinopathy after fractionated radiotherapy for optic nerve sheath meningioma. Ophthalmol. 2004;111:565-567.
[40] Parson J.T., Bova F.J., Fitzgerald C.R. Radiation optic neuropathy after megavoltage external-beam irradiation: analysis of time-dose. Int J Radiat Oncol Biol Phys. 1994;30:753-763.
[41] Landert M., Baumert B.G., Bosch M.M. The visual impact of fractionated stereotactic conformal radiotherapy on seven eyes with optic nerve sheath meningiomas. J Neuroophthalmol. 2005;24:86-91.
[42] Melian E., Jay M. Primary radiotherapy for optic nerve sheath meningioma. Semin Ophthalmol. 2004;19:130-140.
[43] Baumert B.G., Villa S., Studer G., et al. Early improvement in vision after fractionated stereotactic radiotherapy for primary optic nerve sheath meningioma. Radiother Oncol. 2004;72:169-174.
[44] Kwon Y., Bae J.S., Lee do H., et al. Visual changes after gamma knife surgery for optic nerve tumours. Report of three cases. J Neurosurg. 2005;102(Suppl.):143-146.
[45] Moster M.L. Detection and treatment of optic nerve sheath meningioma. Curr Neurol Neurosci Rep. 2005;5:367-375.
[46] Radhakrishnan S., Lee M.S. Optic nerve sheath meningiomas. Curr Treat Options Neurol. 2005;7:51-55.
[47] Richards J.C., Roden D., Harper C.S. Management of sight-threatening optic nerve sheath meningioma with fractionated stereotactic radiotherapy. Clin Exp Ophthalmol. 2005;33:137-141.