11 Primary and Secondary Headache
Primary Headache Disorders
Migraine
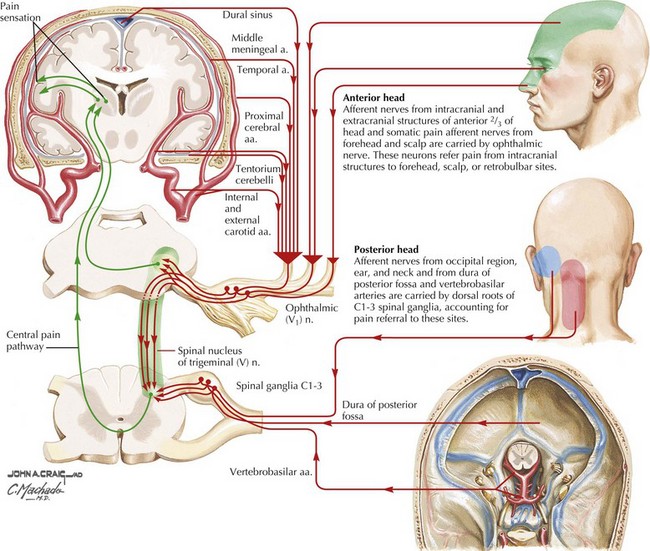
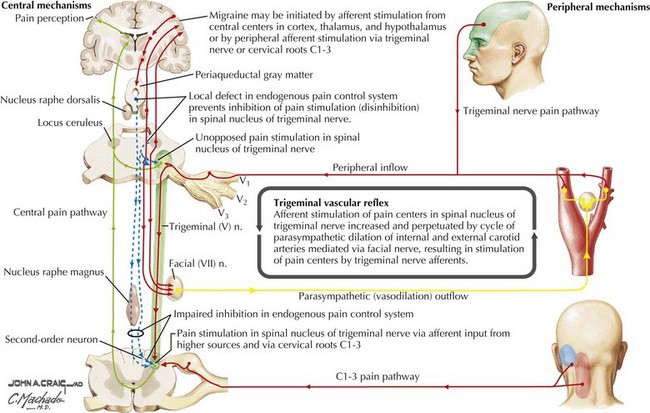
Migraine pathophysiology includes a combination of cortical hyperexcitability and discharge followed by cation and neurotransmitter release with secondary activation of trigeminal pathways, and subsequent release of vasoactive neuropeptides and proinflammatory substances. These promote meningeal blood vessel dilatation and neurogenic inflammation in the primary pain nerve endings of the head lying in the arteries, leptomeninges, and nasal sinuses (Figs. 11-1 and 11-2). In migraine with aura, the pathophysiology of the aura is thought to be related to slow neuronal discharge of the cortex in a sequential pattern or “spreading depression” followed by a concomitant decrease in cerebral blood flow.
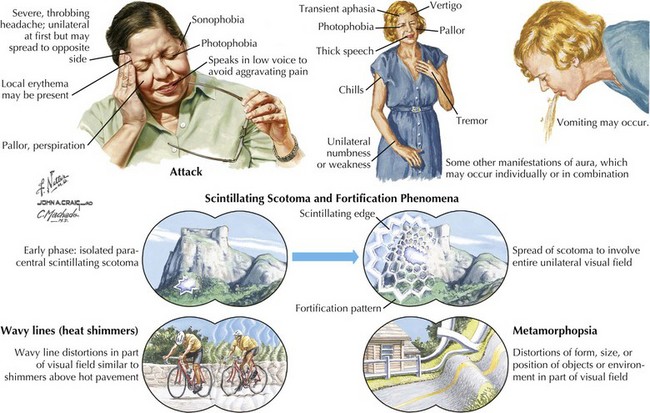
Patients with migraine often have prodromal symptoms that herald the oncoming headache. These include fatigue, thirst, anorexia, fluid retention, food cravings, gastrointestinal symptoms, and emotional or mood disturbances such as irritability, elation or depression. Approximately 15% of migraine patients have an aura preceding the pain phase. This presentation known presently as migraine with aura (formerly classic migraine) comprises focal neurologic symptoms, most commonly visual (>95% of all auras), that typically evolve and then regress over minutes before headache onset. These visual phenomena can occur in a homonymous or hemifield distribution. Typical migraine scotomata include scintillating flashes or stars (phosphenes) and geometric patterns known as fortification spectra (Fig. 11-3). Auras may also involve sensory, motor or rarely higher cortical pathways, including language. Most auras develop slowly over several to 20 minutes, last less than 1 hour, and may spread over different anatomic areas. For example, the patient may initially experience numbness in the fingers that gradually spreads up the arm to the face and sometimes even down the leg. In some individuals, the auras may not be necessarily followed by the headache phase. Less commonly, the aura phase consists of complex symptomatology with a more abrupt onset. For example, in basilar artery migraine, symptoms may include dysarthria, vertigo, ataxia, diplopia, hearing deficits, and even altered mentation or loss of consciousness. In confusional migraine, cognitive deficits are more prominent. Ocular nerve palsies are the hallmark of ophthalmoplegic migraine. Hemiplegic migraine is associated with variable but prominent unilateral weakness, and there is often a family history with an inherited voltage-dependent calcium channelopathy.
Management and Therapy
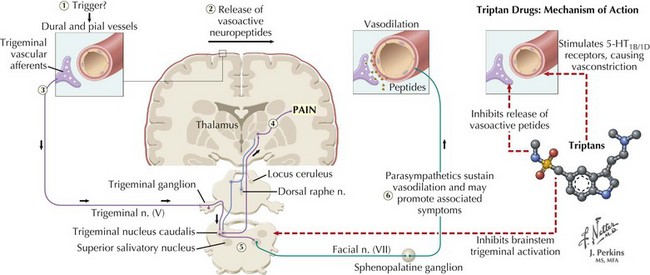
However, in patients with more severe disabling migraines, oral, injectable, intranasal, or quick-dissolve sublingual serotonin 1B/1D receptor agonist (“triptan”) preparations are the medications of choice. Their success is attributed to the multiple sites of action triptans have on the migraine cascade that include decreasing cortical hyperexcitability, decreasing tissue leakage of neuropeptides and blocking their dural neurovascular effects, tempering trigeminal afferent input, suppressing or down-regulating brainstem activation, gating thalamic pain response, and finally countering progressive vasodilation (Fig. 11-4). The rapidly acting triptans include almotriptan, eletriptan, rizatriptan, sumatriptan, and zolmitriptan. The longer acting triptans include naratriptan and frovatriptan. These preparations are also recommended for patients with milder migraines that are not initially disabling but are refractory to simple analgesics. Butalbital, ergotamine, and isometheptene/dichloralphenazone preparations are also commonly used for abortive therapy. When the above-mentioned therapeutic options are ineffective in patients with the most severe migraines or if those treatments are contraindicated, nonnarcotic treatments, such as ketorolac and antiemetics, are attempted. When these fail, opiate-category medications are often used, primarily in the emergency room. However, the possibility of sedation and, more importantly, subsequent overuse and dependence must be considered.
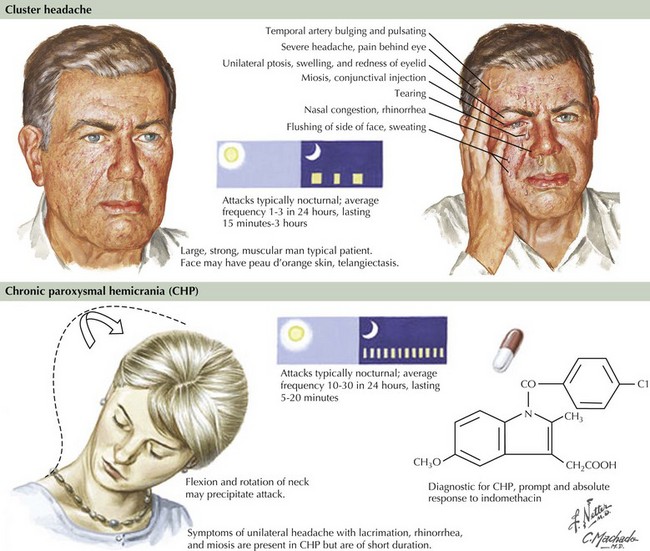
Cluster Headache
Clinical Vignette
The distinctive clinical features, as summarized in the clinical vignette, assist in diagnosing cluster headaches (Fig. 11-5). The underlying pathophysiology is related to activation of the trigeminal vascular and parasympathetic systems. The first two divisions of the trigeminal pathway are more commonly involved. Recent positron emission tomographic scan studies by Goadsby and colleagues revealed activation of the medial hypothalamic gray matter, an area involved in the control of circadian rhythms. It is felt that dysfunction of neurons in this area leads to activation of a trigeminal-autonomic loop in the brainstem. These pathophysiologic mechanisms would explain the cardinal symptoms of cluster headache that include the episodic/circadian nature of the attacks, the distribution and quality of pain, and associated autonomic symptoms.
Other Trigeminal Autonomic Cephalgias
Paroxysmal Hemicranias
These are unusual primary headaches—unilateral and short-lived (2–45 minutes), which occur in a chronic or episodic manner. Typically, the pain has a severe throbbing or boring quality and often recurs several times during the same day. These headaches are associated with ipsilateral cranial autonomic dysfunction but, unlike cluster headaches, occur more often in women than in men. Furthermore, these headaches have daily recurrences and tend not to conglomerate over a few days such as in cluster headaches. They usually respond well to 25–50 mg indomethacin 2–3 times daily for at least 48 hours. These headaches by definition are “indomethacin responsive,” and a trial is always warranted if there are no medical contraindications to its use (Fig. 11-5). There are reports of good response as well to acetazolamide. Calcium channel blockers, such as verapamil, are used for long-term prophylactic treatment.
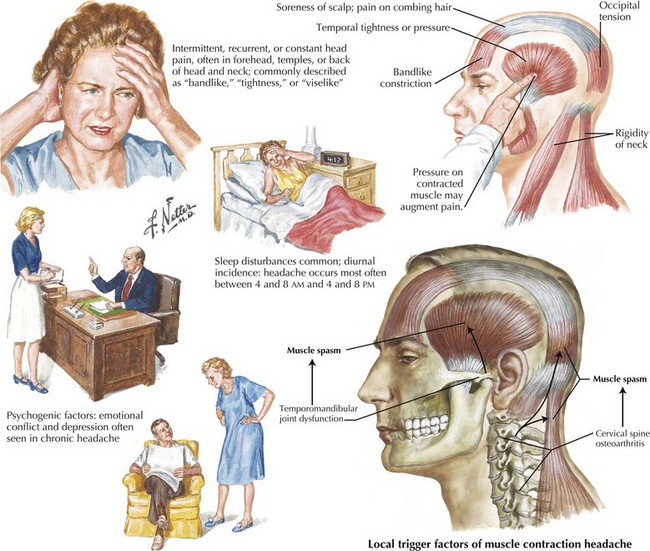
Tension-Type Headache
Careful evaluation is indicated in every patient suspected of having tension-type headache. Exclusion of structural, infectious, or metabolic disorders is essential. Although sometimes features of migraine are present, they are a minor part of the clinical picture. Specific triggers are less common than with migraine. The precise pathophysiology is unknown. It is likely a heterogeneous disorder with various etiologic factors that ultimately lead to pericranial and nuchal muscular tension or spasm (Fig. 11-6). Disrupted sleep, psychosocial stress, anxiety, depression, and analgesic drug overuse are contributing factors. The treatment of tension headache usually requires only over-the-counter analgesics and NSAIDs, nonpharmacologic intervention, that include relaxation and biofeedback techniques massage, and heat application. Prophylactic medication is indicated for frequent recurrence or when abortive therapies are ineffective or contraindicated. The best available evidence supports the use of tricyclic antidepressants, specifically amitriptyline. This medication is best tolerated when started at a nightly low dose (10–25 mg) and increased gradually if needed.
Secondary Headache Disorders
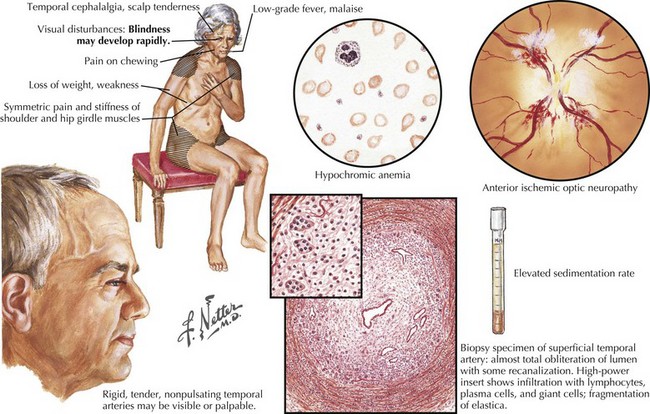
Giant Cell (Temporal) Arteritis
Clinical Vignette
Headache is the most common and prominent presenting symptom of giant cell or temporal arteritis, a serious disorder of the elderly with potentially devastating complications such as permanent blindness. As in the above vignette, early identification and prompt treatment prevents blindness from developing. The pain of temporal arteritis is usually bilateral and nonspecific, being throbbing or continuous, and with variable intensity, at times so mild that its potential significance is easily overlooked. Systemic complaints including anorexia, general malaise, myalgias, and arthralgias are common, and severe as important diagnostic clues. Polymyalgia rheumatica, a condition characterized by proximal musculoskeletal pain and morning stiffness, frequently accompanies the headache. Jaw or tongue claudication and rarely facial tissue ischemia have been described and reflect external carotid artery involvement. Patients may have subtle and intermittent visual blurring or frank episodes of monocular visual loss mimicking transient ischemic attacks. Although often present, temporal artery tenderness may be relatively minor (Fig. 11-7). Early diagnosis is paramount as arteritis may precipitously cause unilateral or sequential bilateral anterior ischemic optic neuropathy with permanent visual loss. Although posterior ciliary arteries are most commonly involved, visual loss may less commonly result from ophthalmic artery involvement and rarely retinal artery arteritis. Furthermore, the arteritis may be widespread with involvement beyond the temporal arteries to the aorta and its branches. Infrequently, ischemic stroke may occur as arteritis affects the extracranial carotid or vertebral arteries. The intracranial circulation is generally spared. Erythrocyte sedimentation rate (ESR) and C-reactive protein provide the laboratory means to support the diagnosis. Typically, the ESR is significantly increased to 60–110 mm/hour, although there are exceptions. Biopsy of a long temporal artery segment is indicated in every patient suspected of having temporal arteritis. Because the arteritis is patchy, a unilateral biopsy may fail to show the inflammatory changes of giant cell arteritis and bilateral biopsies may be required to make the diagnosis. A mixed infiltrate of neutrophils and T lymphocytes involves the media with concurrent intimal hyperplasia and gradual luminal narrowing. Granulomatous, inflammatory arteritis with giant cell formation and marked disruption of the internal elastic lamina are classic for temporal arteritis (see Fig. 11-7).
Brain Hemorrhage, Infections, And Tumors
Every individual with a precipitous onset of “the worst headache of my life” warrants immediate careful medical and neurologic evaluation by their physician or in the emergency room. Funduscopic examination should evaluate for papilledema or subhyaloid hemorrhages. Regardless of the findings, emergency imaging is indicated. Cranial CT offers a rapid and readily available means to evaluate for hemorrhage and mass lesions. MRI and MR angiography may be indicated but are generally not required for the urgent evaluation of thunderclap headache. If no mass lesion is found on imaging, CSF analysis is indicated to evaluate for subarachnoid hemorrhage and infection (Chapters 48, 49, and 57).
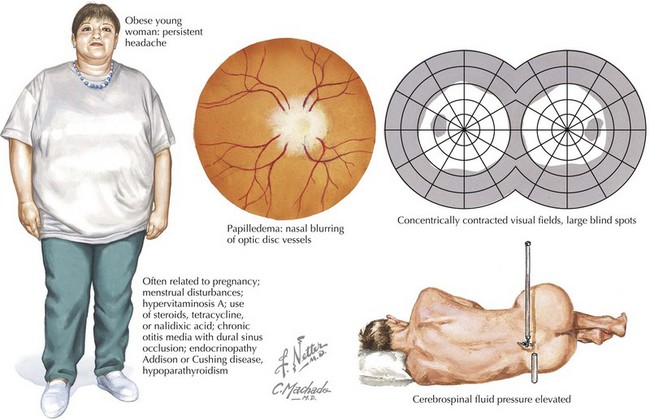
Idiopathic Intracranial Hypertension
Clinical Vignette
Idiopathic intracranial hypertension, also called pseudotumor cerebri, is a unique syndrome of relatively severe poorly defined and often progressive headaches with associated horizontal diplopia. In addition, transient visual obscurations and pulsatile tinnitus may be part of the clinical picture. It primarily presents in healthy, usually overweight, young women. Pseudotumor cerebri is associated with increased intracranial CSF pressure about 250 mm H2O (Fig. 11-8).
Low Csf Pressure Headache
Diagnosis
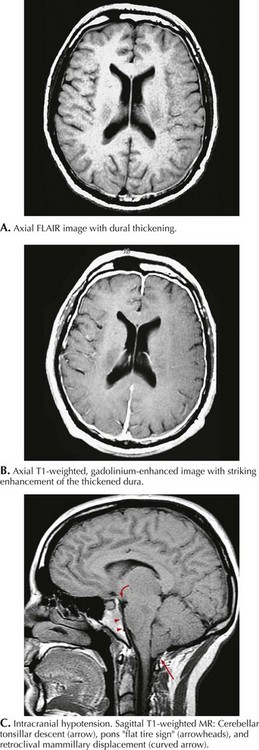
MRI with gadolinium demonstrates diffuse pachymeningeal enhancement in most low–CSF pressure headaches. This is often accompanied by dural thickening that can be striking and may be confused with leptomeningeal inflammatory or neoplastic processes (Fig. 11-9). In more severe cases, subdural fluid collections and descent of the brain with downward displacement of the cerebellar tonsils can be seen. Lumbar puncture demonstrates decreased intracranial pressure, usually less than 50 mm H2O. CSF analysis may be normal, but slight elevation of protein and a mild lymphocytic pleocytosis may be seen. Radioisotope cisternography or contrast myelography can be used to detect sites of CSF leakage.
Cranial Neuralgias
Trigeminal Neuralgia
Clinical Vignette
A careful history is the key to the diagnosis of this uncommon but eminently treatable facial neuralgia. Trigeminal neuralgia, also called tic douloureux, is a disabling, lancinating, or electrical facial pain that occurs in the trigeminal nerve distribution (see also Chapter 6). It is one of the worst pains humans experience. This condition is not defined by any test but requires the clinician to recognize it by its primary historical attributes. There are no associated neurologic deficits.
Occipital Neuralgia
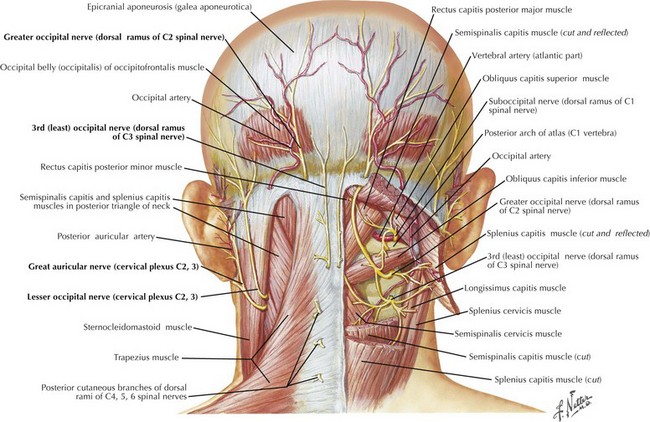
Although similar in pain characteristics to trigeminal neuralgia, occipital neuralgia is differentiated by location to the posterior scalp innervated by the greater and lesser occipital nerves from the C2 dermatome (Fig. 11-10). Occipital neuralgia is probably underdiagnosed. Pain is generally localized to the base of the skull but may extend to the vertex, behind the ear or into the neck and upper back. Pain may be initially incited by hyperextension of the neck or whiplash injury. Patients are commonly seen after motor vehicle accident, sports trauma, or work-related injury. The neuralgia is usually unilateral, at times bilateral, and occurs in brief paroxysms of jabbing pain superimposed on a more chronic dull occipital ache.
Obstructive Sleep Apnea
Headache may be the presenting symptom in sleep apnea. Patients frequently complain of daily headaches that are worse upon awakening. Invariably, these patients are fatigued and experience excessive daytime sleepiness. A careful history, including discussion of snoring, may prove vital in making this diagnosis. The neurologic examination is generally normal, although certain clues on physical exam may include obesity and abnormalities or tissue redundancy of the palate, uvula, or tongue. When correctly identified, this headache syndrome may improve dramatically with nocturnal continuous positive airway pressure treatment. Sleep apnea is discussed in more detail in Chapter 15.
Infectious Mechanisms
Meningitis must be considered in anyone experiencing an acute-onset headache. Typically, these individuals have a concomitant fever and stiff neck (meningismus) as detailed in Chapter 48.
Cranial herpes zoster (shingles) is a “reactivation” of the varicella-zoster virus within the gasserian ganglion or upper cervical dorsal root ganglion. Typically, these patients report severe, sometimes excruciating, boring head pain or neuralgia. The rash usually precedes the onset of the headache, but, in some instances, intense head pain can antedate skin lesions by a few days. The classic vesicles can vary from a few easily overlooked lesions to an extensive vesicular rash. After the dermatologic changes appear, treatment with antiviral medications, such as acyclovir, valacyclovir, and Famvir, is indicated. When herpes zoster ophthalmicus occurs, an ophthalmology consultation should be obtained and along with antivirals, coadministration of corticosteroids may be considered, when no contraindication exists. There is an increased incidence of shingles in the elderly and the immunosuppressed. Postherpetic neuralgia may occur in the distribution of the trigeminal nerve or upper cervical roots and may require prolonged treatment. Treatment is discussed in Chapter 49.
Attal N, Cruccu G, Haanpaa M, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153-1169.
Bennetto L, Patel N, Fuller G. Trigeminal neuralgia and its management. Br Med J. 2007;334:201-205.
Bigal ME, Lipton RB, Tepper SJ, et al. Primary chronic daily headache and its subtypes in adolescents and adults. Neurology. 2004;63:843-847.
Cady R, Dodick DW. Diagnosis and treatment of migraine. Mayo Clin Proc. 77, 2002. 266–261
Chole R, Patil R, Degwekar S, et al. Drug treatment of trigeminal neuralgia: a systemic review of the literature. J Oral Maxillofac Surg. 2007;65:40-45.
Dodick D. Chronic daily headache. N Engl J Med. 2006;354:158-165.
Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgia, and facial pain. Cephalalgia. 1988;8(Suppl. 7):1-96. Classic reference providing a framework from which to approach headache and treatment
Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. 2nd ed. Cephalalgia. 2004;24(Suppl. 1):9-160. This second edition of headache classification contains some minor changes reflecting recent advances in understanding the pathophysiology and therapeutics of headaches
Hoffman G, Cid M, Rendt-Zagar K, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621-630.
Lance JW, Goadsby PJ. Mechanism and Management of Headache, 7th ed. Philadelphia, PA: Elsevier; 2005.
Lipton RB, Bigel ME, Diamond M, et alfor the AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Loder E, Rizzoli P. Tension-type headache. Br Med J. 2008;336:88-92.
Olesen J, Tfelt-Hansen P, Welch KMA. The Headaches, 2nd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2000.
Pipitone N, Salvarani C. Improving therapeutic options for patients with giant cell arteritis. Curr Opin Rheumatol. 2008;20(1):17-22.
Ramadan NM, Silberstein SD, Freitag FG, et alfor the US Headache Consortium. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for prevention of migraine. Available from www.aan.com/professionals/practice/pdfs/gl0090.pdf Provides evidence-based guidelines for the treatment of migraine headache
Salcarani C, Macchioni P, Manzini C, et al. Infliximab plus Prednisone or Placebo plus prednisone for the initial treatment of polymyalgia rheumatica: a randomized trial. Ann Intern Med. 2007;146(9):631-639.
Salvarani C, Cantini F, Boiardi L, et al. Polymyalgia rheumatica and giant cell arteritis. N Engl J Med. 2002;347(4):261-271.
Silberstein SD, Lipton RB, Dalessio DJ. Wolff’s headache and other head pain, 7th ed. Oxford, UK: Oxford University Press; 2001.
Straube A, Empl M, Ceballos-Baumann A, et al. Pericranial injection of botulinum toxin type A (Dysport) for tension-type headache—a multicentre, double-blinded, randomized, placebo-controlled study. Eur J Neurol. 2008;15(3):205-213.
US Headache Consortium. Multispecialty Consensus on Diagnosis and Treatment of Headache. www.aan.com. Provides practice guidelines for the diagnosis and treatment of different headache types
Wareham D, Breuer J. Herpes zoster. Br Med J. 2007;334:1211-1215.