Primary aldosteronism
1. Define primary aldosteronism.
Primary aldosteronism (PA) is a generic term for a group of disorders in which excessive production of aldosterone by the zona glomerulosa of the adrenal cortex occurs independently of normal renin-angiotensin stimulation. These primary disorders of the adrenal system are distinct from forms of secondary hyperaldosteronism due to excessive renin (renal artery stenosis, renin-producing tumors). The four most important clinical entities constituting PA are bilateral hyperplasia of the zona glomerulosa (commonly termed idiopathic hyperaldosteronism [IHA]), solitary aldosterone-producing adenoma (APA), adrenal carcinoma, and glucocorticoid-remediable aldosteronism. IHA and APA are the most important causes of PA.
2. How common are these disorders?
The most common manifestation of excess aldosterone secretion is hypertension. Cross-sectional and prospective studies indicate that up to 12% of the hypertensive population may have PA.
3. Aside from hypertension, what are the common clinical manifestations of primary aldosteronism?
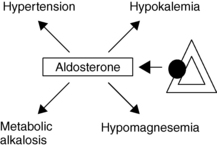
Aldosterone normally acts at the renal distal convoluted tubule to stimulate reabsorption of sodium ions (Na), as well as secretion of potassium (K) and hydrogen ions (H) and at the cortical and medullary collecting ducts to cause direct secretion of H. Excess secretion of aldosterone in PA results in hypertension, potassium loss, and metabolic alkalosis; hypomagnesemia may also occur (Fig. 27-1). Spontaneous hypokalemia (K < 3.5 mmol/L), however, is an uncommon presenting manifestation of PA, occurring in only 9% to 37% of cases of PA. Therefore, normokalemic hypertension is the most common presentation. Vague symptoms are manifestations of hypokalemia: weakness, muscle cramping, paresthesias, headaches, hyperglycemia (insulinopenia), palpitations, polyuria, and polydipsia.
4. Who should be screened for primary aldosteronism?
Hypertension affects 29% of the adult U.S. population; screening for PA must be judicious. Case detection should be targeted to four groups of patients:
 Patients with moderate to severe hypertension: Joint National Commission (JNC) stage 2 (BP 160-179 systolic/100-109 diastolic mm Hg) or stage 3 (> 180/> 110 mm Hg); PA prevalence 8% to 13%.
Patients with moderate to severe hypertension: Joint National Commission (JNC) stage 2 (BP 160-179 systolic/100-109 diastolic mm Hg) or stage 3 (> 180/> 110 mm Hg); PA prevalence 8% to 13%.
 Patients with resistant hypertension: BP higher than 140/90 mm Hg despite treatment with three antihypertensive medications; PA prevalence 17% to 23%.
Patients with resistant hypertension: BP higher than 140/90 mm Hg despite treatment with three antihypertensive medications; PA prevalence 17% to 23%.
 Hypertensive patients with spontaneous or diuretic-induced hypokalemia; PA prevalence 50%.
Hypertensive patients with spontaneous or diuretic-induced hypokalemia; PA prevalence 50%.
 Patients with adrenal incidentalomas who have hypertension; PA prevalence 1% to 10%.
Patients with adrenal incidentalomas who have hypertension; PA prevalence 1% to 10%.
Hypertension due to aldosterone excess causes enhanced perivascular inflammation and myocardial fibrosis; end-organ damage is therefore more severe than in essential hypertension. Screening and confirmation of the diagnosis are described in questions 14 and 16.
5. What is the most common form of primary aldosteronism?
Of the four causes mentioned in question 1, IHA is most common, accounting for up to 70% of cases in most series. IHA, also known as bilateral adrenal hyperplasia, is characterized by bilateral hyperplasia (diffuse and focal) of the zona glomerulosa layer of both adrenal glands. The most likely cause is supranormal sensitivity of the zona glomerulosa in affected adrenal glands to physiologic concentrations of angiotensin II.
6. What is the second most common cause of primary aldosteronism?
APAs account for up to 30% of cases of PA. APAs are small (< 2 cm), occur more commonly in the left adrenal gland, and are composed of zona glomerulosa cells, zona reticularis cells, and hybrid cells with characteristics of both layers. APAs are also known as Conn’s syndrome.
7. Why differentiate between IHA and APA?
APAs are a surgically curable form of PA; IHA is not. APAs produce greater amounts of aldosterone than other forms of PA; consequently, the degrees of hypertension and biochemical abnormalities tend to be more severe. APAs demonstrate partial autonomy of function, secreting aldosterone in response to stimulation by corticotropin (ACTH) but not by angiotensin II. Aldosterone synthesis by these tumors, therefore, parallels the normal circadian rhythm of ACTH secretion, with the highest serum aldosterone concentrations occurring in the mornings and the lowest in the evenings.
8. How do symptoms of IHA differ from symptoms of APA?
Aldosterone is produced in smaller amounts in IHA than in APA; therefore the degree of hypertension, hypokalemia, hypomagnesemia, and metabolic alkalosis is less dramatic. Serum aldosterone levels tend to rise during upright posture, perhaps owing to greater sensitivity to angiotensin II.
9. How commonly does adrenal cancer cause primary aldosteronism?
Adrenal carcinoma as a cause of PA is rare. The tumors are very large (> 6 cm) and commonly metastatic at the time of diagnosis. All cases of PA should be imaged with computed tomography (CT) to exclude this rare cause of PA.
10. What is glucocorticoid-remediable aldosteronism?
In this rare cause of PA, production of mineralocorticoid is stimulated solely by ACTH. The disorder is inherited in an autosomal-dominant fashion.
11. How is aldosterone synthesis normally regulated in the zona glomerulosa?
Humans possess two mitochondrial 11β-hydroxylase isoenzymes that are responsible for cortisol and aldosterone synthesis (designated CYP11B1 and CYP11B2). Both are encoded on chromosome 8. CYP11B1, which is responsible for conversion of 11-deoxycortisol to cortisol, is expressed only in the zona reticularis. CYP11B2, which is responsible for the conversion of corticosterone to aldosterone, is expressed only in the zona glomerulosa. CYP11B1 activity is stimulated by ACTH, whereas CYP11B2 is stimulated by angiotensin II or hyperkalemia.
12. Explain the genetic basis of glucocorticoid-remediable aldosteronism.
Glucocorticoid-remediable aldosteronism results from a heritable mutation that causes the fusion of the promoter region of the CYP11B1 gene with the structural region of the CYP11B2 gene. The resulting chimeric gene responds to ACTH with overproduction of aldosterone, as well as precursors 18-hydroxycortisol and 18-oxocortisol. These metabolites of the cortisol C-18 oxidation pathway are biochemical markers that facilitate identification of affected kindreds. Excessive aldosterone secretion may be inhibited by administration of glucocorticoids that suppress ACTH secretion by the pituitary.
13. How is primary aldosteronism diagnosed?
The diagnosis of PA is based on the demonstration of inappropriately elevated plasma aldosterone concentration (PAC) with concomitantly suppressed plasma renin activity (PRA). Although hypokalemia is suggestive of PA, normokalemic hypertension is the most common presentation.
14. How are patients screened for primary aldosteronism?
The most sensitive screening test is the aldosterone/renin ratio (ARR). Concomitant PAC and PRA values are obtained from a specimen collected in the office (PAC in ng/dL; PRA in ng/mL/h) from a patient who has been out of bed for at least 2 hours and seated for 5 to 15 minutes. Preparation prior to screening includes correction of hypokalemia to more than 3.5 mmol/L, a sodium-unrestricted diet, and withdrawal of medications proven to alter the ARR (spironolactone, eplerenone, amiloride, triamterene, potassium wasting diuretics) for 4 weeks. ARR greater than 30 with PAC exceeding 15 ng/dL raises the possibility of PA.
15. Will antihypertensive agents alter the ARR results?
Nearly every patient screened for PA has begun therapy with one or more antihypertensives. Medications mentioned in question 14 must be withdrawn. Potentially, beta-adrenergic blockers, central alpha-2-receptor agonists, and renin inhibitors all greatly reduce PRA and slightly reduce PAC. Potentially, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and dihydropyridine calcium channel blockers all greatly increase PRA and slightly reduce PAC. These commonly used medications rarely alter the results of ARR, but if a nondiagnostic result is encountered, alternative antihypertensive drugs may be substituted for the potential offending agents for 2 weeks, and the test repeated. The following medications have little effect on the ARR and may be substituted for temporary control during ARR testing: verapamil–slow release, hydralazine, prazosin, doxazosin, and terazosin.
16. How is the diagnosis of primary aldosteronism confirmed?
Several tests confirm PA. The most commonly used is the oral sodium loading test. Intravascular volume expansion should normally suppress aldosterone secretion. In the oral sodium loading test, the patient consumes more than 200 mmol (6 g) of dietary sodium for 3 days, and then from day 3 through day 4, a 24-hour urine collection for aldosterone and sodium is collected. Urinary excretion of aldosterone (high-performance liquid chromatography–tandem mass spectrometry) that exceeds 12 μg/day confirms a diagnosis of PA. Urinary sodium excretion of at least 200 mmol/day ensures adequacy of the test. An alternative test is acute volume expansion by intravenous administration of 2 L of normal saline over 4 hours with measurement of PAC at baseline and at the end of the saline infusion. Failure of the PAC at 4 hours to fall to less than 50% of the baseline PAC also confirms the diagnosis.
17. After confirmation of primary aldosteronism, why is it important to differentiate APA from IHA?
APA is amenable to surgical resection of the involved adrenal gland, whereas IHA is usually treated medically.
18. Does CT or magnetic resonance imaging (MRI) aid in differentiation?
To a limited extent, both localizing procedures may aid in identifying the cause of PA. A large APA may be discernible on high-resolution CT, which at some institutions can identify adenomas as small as 5 mm. MRI at present performs as well as CT in identifying APA but involves higher cost and longer scan time. The diagnostic accuracy of MRI or CT in preoperatively localizing an APA has been reported to be 70% to 85%, but accuracy declines in older populations, in whom incidental hormonally inactive adrenal masses are more common. Some experts believe that biochemically silent adrenal masses are so rare in patients younger than 40 years that no further evaluation is necessary. In patients older than 40 years, adrenal venous sampling (AVS) must be performed to verify unilateral aldosterone production (see question 19). Adrenal carcinoma, a rare cause of PA, is easily identified with either CT or MRI. See also Chapter 29 for more on this topic.
19. Which localizing test is required if CT or MRI suggests an APA in a patient older than 40 years?
A more invasive localizing procedure to differentiate a normal adrenal gland from one containing an adenoma is AVS. Many institutions believe AVS should be performed before surgical intervention for an APA is considered. In this procedure, catheters are introduced into the left and right adrenal veins and the inferior vena cava. Levels of PAC are determined from these sites, along with concomitant cortisol levels following infusion of cosyntropin (synthetic ACTH). Cortisol levels are determined to ensure that the adrenal veins are properly catheterized. PAC/cortisol is referred to as “cortisol-corrected” aldosterone. APAs produce large amounts of aldosterone; the normal adrenal vein PAC is 100 to 400 ng/dL, whereas APAs may generate concentrations of 1000 to 10,000 ng/dL. The ratio of PAC/cortisol produced on the affected side versus the unaffected side always exceeds 4:1. When compared with CT scan results, discordant AVS results are found in up 30% of cases.
20. Explain the difficulty with adrenal venous sampling.
Collection of an aldosterone and cortisol specimen from the left adrenal gland is relatively simple, because the venous effluent drains directly into the left renal vein. The venous flow from the right adrenal, however, goes directly into the inferior vena cava. Catheterization of the right adrenal vein is difficult because of the few angiographic landmarks. Contrast material used to localize the right adrenal gland can cause corticomedullary hemorrhage during the procedure.
21. How accurate is adrenal venous sampling?
22. How is the patient with APA managed?
The patient undergoes screening tests, as described in question 15. The diagnosis of PA is confirmed with 24-hour urine collection for aldosterone measurement during oral salt loading, as described in question 16. AVS reveals a 4:1 gradient between the adenoma and the “normal” adrenal, and surgical resection of the affected adrenal is considered.
23. What should be done after the APA is localized?
After the APA is localized, unilateral adrenalectomy is performed. Laparoscopic resection is now widely available and is preferable to the open posterior approach. One year postoperatively, 70% of patients so treated are normotensive. By the fifth postoperative year, only 53% remain normotensive. Normal potassium balance tends to be permanent.
24. Do all patients with APA require surgery?
No. Although surgical resection is preferred, patients who have other comorbid conditions that preclude surgery may be successfully treated medically as described in question 28.
25. How is a patient with IHA managed?
The patient undergoes screening and confirmatory tests, as described in questions 15 and 16. CT does not reveal unilateral enlargement of the adrenals, and AVS does not show a unilateral abnormality. After the diagnosis of IHA is made, the patient is scrupulously sequestered from surgical colleagues.
26. What is the agent of choice for pharmacologic treatment of IHA?
Pharmacologic therapy is effective. The agent of choice is spironolactone (25-200 mg bid), a competitive mineralocorticoid receptor antagonist. Hypokalemia corrects dramatically, whereas hypertension responds after 4 to 8 weeks. Unfortunately, spironolactone also interferes with the action of androgens, causing decreased libido, impotence, and gynecomastia in men and menstrual irregularities in women. Eplerenone (50 mg b.i.d.) is a selective mineralocorticoid receptor antagonist with 60% of the potency of spironolactone, but without many of the side effects of the latter. Eplerenone is more costly and there are fewer long-term data available for this agent.
27. What other pharmacologic options are available?
In patients intolerant of the agents discussed in question 26, amiloride (5-15 mg b.i.d.) corrects hypokalemia within several days. A concomitant antihypertensive agent is usually necessary to reduce blood pressure. Success also has been reported in IHA treated with calcium channel blockers (calcium is involved in the final common pathway for aldosterone production) and angiotensin-converting enzyme inhibitors (IHA appears to be sensitive to low concentrations of angiotensin II).
28. How is a patient with glucocorticoid-remediable aldosteronism managed?
Dluhy, RG, Lifton, RP. Glucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 1999;84:4341–4344.
Fardella, CE, Mosso, L, Gomez-Sanchez, C, et al, Primary hyperaldosteronism in essential hypertensives. prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab 2000;85:1863–1867.
Funder, JW, Carey, RM, Fardella, CE, et al, Case detection diagnosis, and treatment of patients with primary aldosteronism. an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008;93:3266–3281.
Jossart, GH, Burpee, SE, Gagner, M. Surgery of the adrenal glands. Endocrinol Metab N Am. 2000;29:57–68.
Liftin, RP, Dluhy, RG, Powers, M, et al. A chimaeric 11-hydroxylase/aldosterone synthetase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265.
Lim, PO, Young, WF, MacDonald, TM. A review of the medical treatment of primary aldosteronism. J Hypertens. 2001;19:353–361.
Magill, SB, Raff, H, Shaker, JL, et al. Comparison of adrenal vein sampling and computed tomography in the differentiation of primary aldosteronism. J Clin Endocrinol Metab. 2001;86:1066–1071.
Milliez, P, Girerd, X, Plouin, PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248.
Mulatero, P, Rabbia, F, Milan, A, et al. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension. 2002;40:897–902.
Mulatero, P, Stowasser, M, Loh, K, et al. Increased diagnoses of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050.
Rossi, G, Bernini, G, Desideri, G, et al, Renal damage in primary aldosteronism. results of the PAPY study. Hypertension 2006;48:232–238.
Rossi, P. Diagnosis and treatment of primary aldosteronism. Endocrinol Metab N Am. 2011;40:313–332.
Schwartz, GL, Screening for adrenal-endocrine hypertension. overview of accuracy and cost-effectiveness. Endocrinol Metab Clin N Am 2011;40:279–294.
Schwartz, GL, Turner, ST, Screening for primary aldosteronism in essential hypertension. diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem 2005;51:386–394.
Tan, YY, Ogilvie, JB, Triponez, E, et al. Selective use of adrenal venous sampling in the lateralization of aldosterone-producing adenomas. World J Surg. 2006;30:879–885.
Tanabe, A, Naruse, M, Takagi, S, et al. Variability in the renin/aldosterone profile under random and standardized sampling conditions in primary aldosteronism. J Clin Endocrinol Metab. 2003;88:2489–2492.
Tiu, SC, Choi, CH, Shek, CC, et al. The use of aldosterone-renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different conditions of blood sampling. J Clin Endocrinol Metab. 2005;90:72–78.