Chapter 41 Prevention and Treatment of Knee Arthrofibrosis
INTRODUCTION
Loss of a normal range of knee motion after an injury or ligament reconstruction is a potentially devastating complication. Although a multitude of investigations and publications have appeared since the early 1990s regarding this problem, loss of knee motion continues to be one of the most frequently reported complications after surgical procedures.27,31,72,83,84 Although the definition and use of the term arthrofibrosis vary among authors, it implies a loss of knee flexion, extension, or both compared with the contralateral normal knee.
Primary arthrofibrosis is caused by an exaggerated inflammatory response to an injury or surgical procedure, followed by the production of fibroblastic cells and an increase in the deposition of extracellular matrix proteins.147 Proliferative scar tissue or fibrous adhesions form within the joint, which can result in either localized or diffuse involvement of all of the compartments of the knee and the extra-articular soft tissues (Fig. 41-1). In the most severe cases, dense scar tissue obliterates the normal peripatellar recesses, suprapatellar pouch, intercondylar notch, and articular surfaces. Formation of dense scar tissue in the infrapatellar region may result in a patella infera and permanent limitation of normal knee flexion and extension. The consequent pain and restricted knee motion resulting from arthrofibrosis may lead to disabling events including severe quadriceps atrophy, loss of patellar mobility, patellar tendon adaptive shortening, patella infera, and articular cartilage deterioration.105,106
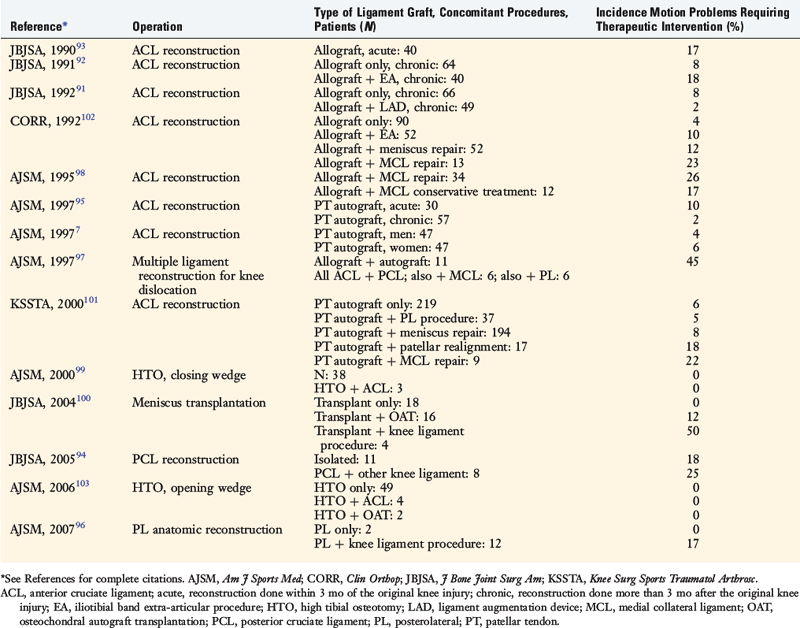
The incidence of arthrofibrosis after ligament reconstructive surgery varies according to several factors, which are discussed. Two of the most commonly noted factors are knee dislocations and major concurrent operative procedures performed with the ligament surgery such as reconstruction of other knee ligaments, complex meniscus repair, or high tibial osteotomy (Table 41-1).7,91–103 Publications show wide disparity among authors regarding the treatment of this problem, especially the time postoperatively that intervention is warranted and what type of treatment should be implemented for losses of knee extension and flexion and loss of patella mobility.
It is important to note that limitations in knee flexion and extension may occur owing to reasons other than arthrofibrosis. Any acute injury causing pain and swelling may cause a short-term loss of knee motion, which usually resolves as symptoms subside. Other factors influencing the restoration of knee motion include a mechanical block from a displaced bucket-handle meniscus tear, an impinged or improperly placed anterior cruciate ligament (ACL) graft, a cyclops lesion,65 improper graft tensioning that constrains normal knee joint motion,20 and graft fixation at 30° or more of extension.5 In addition, contracture of the posterior capsular structures may limit knee extension. If not resolved, these problems may result in the development of what is termed secondary arthrofibrosis, complicating the course of treatment.
Normal knee motion varies, but most individuals have some amount of hyperextension, which averages 5° in men and 6° in women.30,31,74 This degree of hyperextension is required so that the normal “screw-home” mechanism can occur that allows the knee to be stabilized in extension during the stance phase, with the quadriceps muscle relaxed. Normal knee flexion averages 140° in men and 143° in women. It is generally assumed that at least 125° of knee flexion is required for activities of daily living.31,74 Rowe and coworkers120 measured knee kinematics during functional activities in a group of 20 subjects aged 49 to 80 years and reported that 90° of flexion is required for gait and slopes, 90° to 120° for stairs and sitting, and 135° for bathing. Patients not involved in athletics tolerate small flexion deficits. Significant flexion deficits of less than 90° cause problems with squatting, stair-climbing, and sitting. Athletes have a poorer tolerance for even minor losses of flexion, which affect running and jumping. Cosgarea and associates24 reported that deficits of 10° or more of flexion were associated with decreased running speed.
All patients, regardless of their activity level, have greater problems with loss of knee extension. A loss of just 5° of extension may produce a flexed-knee gait, fatigue the quadriceps muscle, and cause patellofemoral pain.63,66,86,89,121 Limitation of more than 20° may cause a functional limb-length discrepancy.24 Many years ago, Perry and colleagues114 demonstrated the effect of a loss of extension on joint contact pressures, quadriceps muscle activity, and fatigue. These investigators measured the extensor forces required to stabilize the flexed knee during simulated weight-bearing in cadaver specimens. The quadriceps force required to stabilize the knee was 75% of the load on the head of the femur at 15° of flexion, which increased to 210% at 30° of flexion, and to 410% at 60° of flexion. The quadriceps force was equal to 20% of the average maximum quadriceps strength at 15° of flexion, but increased to 50% at 30° of flexion. Surface pressure in the tibiofemoral and patellofemoral joints also increased with greater amounts of knee flexion.
TERMINOLOGY AND CLASSIFICATION SYSTEMS
A variety of terms have been used to describe, define, or classify a limitation of knee flexion and extension including arthrofibrosis, flexion contracture, ankylosis, infrapatellar contracture syndrome, and motion loss. Some of the terms indicate a specific process whereas others simply imply a limitation of motion compared with the contralateral normal knee. Ankylosis is one such generic term that indicates stiffness of joints that occurs for any reason and may represent loss of knee flexion, extension, or both. The term motion loss is another general phrase used to describe deviations from the amount of flexion and extension compared with the contralateral normal knee. Conversely, arthrofibrosis describes a specific cause for a limitation of knee motion, which is the formation of diffuse scar tissue or fibrous adhesions within a joint.63,111,115 Flexion contracture indicates a loss of extension due to any cause and is accompanied by a relative shortening of the posterior soft tissues (in either the capsule or the muscles). Petsche and Hutchinson115 stated that arthrofibrosis and flexion contracture are general terms, and it is necessary to indicate a specific cause for loss of motion.
Critical Points TERMINOLOGY AND CLASSIFICATION SYSTEMS
Classification systems have been proposed by various authors to describe limitations in knee motion, based on either the anatomic location of scar tissue and adhesions or the amount of knee motion in the affected joint (Table 41-2).12,33,111,132,141 Sprague and coworkers141 first introduced a system based on the arthroscopically observed location of scar tissues and adhesions in 24 patients who had a severe limitation of knee motion. Paulos and associates111 described three stages of knee motion restrictions due to infrapatellar contracture syndrome. Del Pizzo and colleagues33 and Shelbourne and coworkers132 developed classification systems based on the deviation of knee motion compared to the opposite knee. Blauth and Jaeger12 described a system based on the total arc of motion in the affected knee.
| Reference | Classification System |
|---|---|
| Sprague et al., 1982141 |
The authors developed a classification system based on the anatomic sites at which scar tissue, adhesions, and adaptive shortening of soft tissues occur (Fig. 41-2 and Table 41-3). This system is advantageous because it highlights the areas that must be addressed surgically, as is discussed.
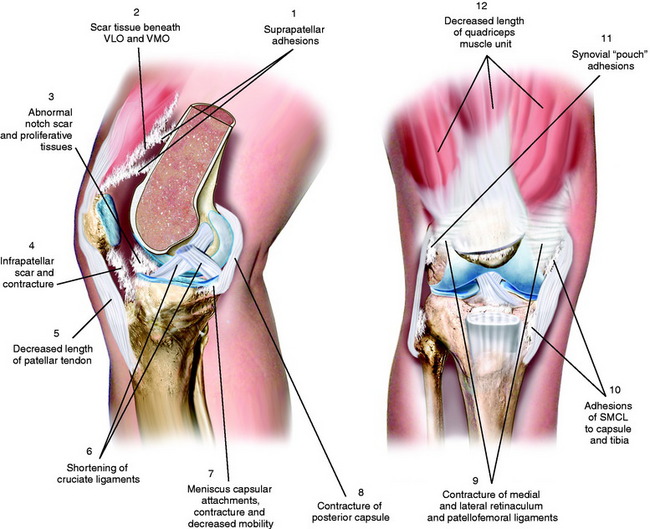
FIGURE 41-2 Multiple areas of soft tissue contracture, adhesions, and scar tissue formation with knee arthrofibrosis.
TABLE 41-3 Authors’ Anatomic Classification System of Arthrofibrosis
| Loss of Flexion |
| Quadriceps muscle |
| Suprapatellar pouch |
| Medial and lateral capsular pouches |
| Patellar retinaculum and associated ligaments |
| Patellar tendon |
| Medial and lateral extra-articular ligament structures |
| Loss of Extension |
| Posterior capsule |
| Femoral notch |
| Hamstrings muscle |
| Cruciate ligaments |
ACL, anterior cruciate ligament; PCL, posterior cruciate ligament; VLO, vastus lateralis obliquus; VMO, vastus medialis obliquus.
RISK FACTORS AFTER KNEE LIGAMENT RECONSTRUCTION
A variety of factors appear to influence the requirement for treatment intervention for knee motion limitations (Table 41-4) and the final amount of knee flexion and extension gained after ligament reconstruction. These factors are related to the severity of the injury, the timing of surgery, preoperative treatment, technical aspects of the ligament reconstruction, and the postoperative course and rehabilitation. Although it remains uncertain why some knees are more likely to form an abnormal scar response to trauma and surgery than others, knowledge of these factors may help reduce this problem.
TABLE 41-4 Risk Factors for the Development of Knee Arthrofibrosis
ACL, anterior cruciate ligament; MCL, medial collateral ligament.
Severity of the Injury
Patients who sustain knee dislocations are at increased risk for developing motion complications (Fig. 41-3).23,35,83,97,127,136 These injuries frequently occur from high-energy accidents that produce extreme soft tissue swelling and edema, hematomas, muscle damage, and other multiple trauma that must be resolved before consideration of knee soft tissue reconstructions. Early operative intervention when possible is advisable for multiple ligament injuries that involve the lateral and posterolateral structures where acute repair and augmentation procedures are performed (see Chapter 22, Posterolateral Ligament Injuries: Diagnosis, Operative Techniques, and Clinical Outcomes). For all other dislocations, surgery is delayed with the limb immobilized in a posterior plaster splint or bivalved cast with a posterior pad to prevent posterior tibial subluxation. Even in these severe knee injuries, it is possible to start immediate range of motion and prevent scar tissue formation that may compromise the outcome of a subsequent ligament reconstruction. Too often, these serious knee joint injuries are not treated with an early motion and function program. When surgical treatment is elected, the reconstructive and repair procedures of torn ligaments, capsular structures, and menisci are performed in a manner that allows immediate knee motion to be instituted postoperatively.97
Preoperative Issues
Performing ACL and other knee ligament reconstruction within a few weeks of the injury or before the resolution of swelling, pain, quadriceps muscle atrophy, abnormal gait mechanics, and motion limitations has been noted by many authors to correlate with postoperative knee motion problems.25,54,86,129,130,134,144,152 Shelbourne and associates134 noted an increased rate of arthrofibrosis in patients who underwent acute ACL reconstruction (within 1 week of the injury) compared with those in whom the reconstruction was delayed for at least 21 days. Similar findings were reported by Wasilewski and colleagues152 who noted that arthrofibrosis was found in 22% of acutely reconstructed knees versus 12.5% of knees reconstructed with chronic ACL deficiency. Mohtadi and coworkers86 found that ACL reconstructions performed less than 6 weeks postinjury had a higher rate of knee stiffness than those done more than 6 weeks postinjury (11% and 4%, respectively). Harner and associates54 reported a 37% rate of motion complications after acute ACL reconstructions (done within 1 month of the injury) compared with a 5% rate in chronic ACL-deficient knees.
Critical Points RISK FACTORS AFTER KNEE LIGAMENT RECONSTRUCTION
ACL, anterior cruciate ligament; MCL, medial collateral ligament.
Although Bach and colleagues6 did not observe a significant difference in knee motion complications between acute and chronic ACL-reconstructed knees, the authors stressed the need to delay surgery until patients achieved 120° of knee motion and swelling had resolved. The authors found “no advantage to performing urgent acute ACL reconstruction.”6 Sterett and coworkers143 found no association between surgical timing and postoperative motion problems. However, in their series, all patients had achieved at least 0° to 120°, had good quadriceps control, and could perform a straight leg raise without an extensor lag before undergoing surgery. Eight percent of the patients in this investigation’s acute subgroup required arthroscopic débridement of scar tissue. Meighan and associates81 also found no advantage in performing early ACL reconstruction and noted an increased rate of complications in patients who underwent surgery within 2 weeks of the injury compared with those who had surgery between 8 and 12 weeks postinjury. The delayed group had a faster return of knee motion and quadriceps strength.
A few authors have not found a higher rate of postoperative motion problems after acute ACL reconstruction.18,62,75 However, it appears that delaying surgery until knee motion is regained, swelling is resolved, and a good quadriceps contraction is demonstrated is advantageous in decreasing the risk of postoperative arthrofibrosis. The inflammatory response to the initial injury varies among patients. Whereas some have little effusion and swelling, others have an exaggerated inflammatory response characterized by pain, soft tissue edema, and redness and increased warmth to tissues surrounding the knee. These knees are placed into an initial conservative treatment program to resolve these problems first and are also carefully monitored after ACL reconstruction for a similar exaggerated inflammatory reaction postoperatively. Knees with isolated ACL ruptures that have little swelling and demonstrate normal motion early after injury may be considered for earlier reconstruction. In addition, knees with multiple ligament ruptures that include the posterolateral structures are also candidates for early surgical repair, as discussed in Chapter 22, Posterolateral Ligament Structures: Diagnosis, Operative Techniques, and Clinical Outcomes.
Technical Factors at Surgery
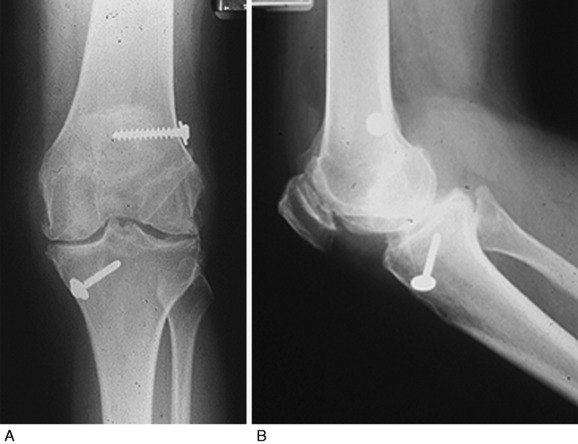
Improper ACL graft placement has been frequently noted as a cause of loss of knee motion.* On the tibia, grafts placed anterior to the native ACL insertion result in impingement on the roof of the intercondylar notch when the knee is extended.59,60,78 Yaru and colleagues154 recommended that with passive extension, there should be a 3-mm clearance between the anterior portion of the intercondylar notch and the graft to prevent impingement. Grafts placed lateral to the insertion site impinge on the lateral wall of the notch. In addition, Romano and coworkers119 found that ACL grafts placed too far anterior in the tibial tunnel can cause knee extension deficits, and grafts placed too far medially in the tibial tunnel can cause knee flexion deficits. On the femur, an excessive anterior graft placement causes deleterious forces on the graft, leading to limitations in flexion and potential graft failure (Fig. 41-4).
Overtensioning ACL grafts may lead to abnormal knee kinematics.84,115 In addition, the degree of knee extension during graft tensioning and fixation may affect postoperative motion.5,20,47,82,89 Austin and associates5 noted in a cadaver study that the amount of graft tension (44 N or 89 N) did not effect knee extension; however, tensioning the graft in knee flexion was associated with extension deficits. The authors reported that grafts tensioned and fixed at 30° of flexion had more than a 12° increase in knee flexion after ACL reconstruction compared with those tensioned and fixed at 0° of flexion. From a two-part biomechanical and clinical study, Nabors and colleagues89 suggested that grafts tensioned in full extension result in a low incidence of knee motion loss. In their series of 57 patients who underwent patellar tendon autograft ACL reconstruction, only 1 patient had a mild (5°) loss of extension.
Concurrent medial collateral ligament (MCL) repair with ACL reconstruction has been associated with an increased risk of knee arthrofibrosis.54,98,128 Harner and associates54 postulated that concurrent MCL primary repair may cause a limitation of knee motion due to the disruption of the medial capsule, because the procedure does not restore the normal tissue planes, resulting in scar formation and a heightened pain response. The studies of Shelbourne and coworkers128,133 and the authors of this chapter98 led to the recommendation many years ago to treat the majority of combined ACL-MCL ruptures conservatively. This allows healing of the medial-side injury, followed by ACL reconstruction in appropriately indicated patients (see Chapter 24, Medial and Posteromedial Ligament Injuries: Diagnosis, Operative Techniques, and Clinical Outcomes). There are exceptions to this rule, and any surgical procedure on the medial side of the knee joint should be carefully observed postoperatively for a limitation of motion or, in acute knee injuries, the development of heterotopic soft tissue ossificiation requiring treatment.126,150
Postoperative Course and Rehabilitation
There is consensus in the literature that immobilization is detrimental to all of the knee joint structures and may result in a permanent limitation of knee motion, prolonged muscle atrophy, patella infera, and articular cartilage deterioration.25,51,67,104–106,112,122 Early knee joint motion decreases pain and postoperative joint effusions, aids in the prevention of scar tissue formation and capsular contractions that can limit normal knee flexion and extension, decreases muscle disuse effects, maintains articular cartilage nutrition, and benefits the healing ACL graft.† Modern rehabilitation programs (see Chapters 12, Scientific Basis of Rehabilitation after Anterior Cruciate Ligament Autogenous Reconstruction; 13, Rehabilitation of Primary and Revision Anterior Cruciate Ligament Reconstructions; and 14, Neuromuscular Retraining after Anterior Cruciate Ligament Reconstruction) incorporate immediate knee motion and muscle-strengthening exercises the day after surgery, both of which have been shown to be safe and not deleterious to healing grafts. Importantly, the immediate motion program must include patellar mobilization (inferior, superior, medial, and lateral directions) to avoid an infrapatellar contracture.
Patient compliance with postoperative rehabilitation is essential in recovering full knee motion. In the authors’ experience, the small percentage of patients who have permanent restrictions in extension or flexion have often been unwilling to perform the required motion, strengthening, and patellar mobilization exercises postoperatively. In addition, in instances in which an early postoperative limitation of motion has been recognized, these patients are also unwilling to undergo treatment recommendations such as overpressure exercises, extension casts, and other modalities that are usually effective in resolving these problems.101,102 Thus, in a majority of cases, the inflammatory and fibrotic response that follows surgery and initially limits knee motion is treatable if no delay occurs in instituting a gentle motion and overpressure program along with appropriate anti-inflammatory medications. There is a distinct group of patients, probably in the range of 1% to 2%, who demonstrate a pathologic exaggerated fibrous tissue proliferative response from a genetic basis, in which the treatment is prolonged and may not be successful. This unique group of patients are discussed in a later section of this chapter.
Significant lower extremity muscle atrophy represents an unresolved problem after ACL reconstruction, because the magnitude of quadriceps atrophy and strength loss may exceed 20% to 30% in the first few months postoperatively.39,40,50,81,117 Prolonged quadriceps atrophy may affect the ability to regain normal knee motion and maintain knee extension. Chronic swelling may also cause a limitation of knee motion by inhibiting the function of the quadriceps muscle.29,110,140,145,146 It is important that a knee joint effusion be treated to lessen its deleterious effect on quadriceps function.
Infection may result in loss of knee motion after ACL reconstruction. The rule followed in the authors’ clinical practice is to always consider first that an exaggerated inflammatory response with joint swelling, synovitis, and early limitation of joint motion is caused by a joint infection until proved otherwise. Even when an infectious process appears to have been excluded, a knee joint that does not respond to the gentle modalities to regain knee motion, or that has continued pain or lack of patellar mobility, should undergo repeat aspiration, cell count, culture, and diagnostic studies. In the authors’ experience, the most severe cases of arthrofibrosis that initially are presumed to be on a genetic basis are subsequently proved to have an occult unrecognized infectious etiology. In patients with an established infectious process, the principles of treatment have been discussed (see Chapter 7, Anterior Cruciate Ligament Primary and Revision Reconstruction: Diagnosis, Operative Techniques, and Clinical Outcomes), and it is important that gentle motion and overpressure programs continue to maintain the joint motion. Even in knees with swelling of soft tissues and the initial host response to an infection, it is possible within days after instituting arthroscopic lavage, débridement, and appropriate antibiotics to initiate motion exercises and prevent a flexion contracture or patella infera. Although aggressive management of this complication usually leads to resolution of the infection, studies report permanent loss of extension and flexion in the majority of patients treated, which may be avoidable.80,125,148,153
Complex regional pain syndromes and reflex sympathetic dystrophy may cause knee motion problems as a result of quadriceps atrophy, chronic swelling, and an increased sensitivity to pain.49,107 These issues are discussed in detail in Chapter 43, Diagnosis and Treatment of Complex Regional Pain Syndrome. Appropriate management using a variety of medical specialists is essential to diagnose and treat these problems.
PATHOPHYSIOLOGY
In 1972, Enneking and Horowitz36 published one of the first studies on the pathophysiology of a joint contracture after immobilization in humans. The investigators described in a series of case reports the presence of fibrofatty connective tissue in the infrapatellar fat pad, suprapatellar pouch, and posterior recesses of the knee joint with eventual obliteration of the joint cavity. Over time, this tissue was replaced with mature fibrous connective tissue.
Tissue homeostasis is maintained by the normal level of cell growth and proliferation along with the production and turnover of the extracellular matrix.13,15 Polypeptides known as cytokines or growth factors are responsible for the constant signaling that occurs both between local cells (paracrine) and within cells (autocrine). Cytokines exist as small proteins that signal cells in response to injury and infection. One cytokine, interleukin-1, has been found to stimulate platelets and macrophages to release a variety of growth factors, including transforming growth factor–β (TGF-β). TGF-β is thought to be one of the factors (among others19,68,124) mediating Dupuytren’s Contracture.3 Cytokines regulate all aspects of tissue remodeling and may act positively or negatively on tissue damage. This variability results in a wide range of potential responses to injury between patients.
Critical Points PATHOPHYSIOLOGY
ASMA, α-smooth muscle actin; TGF-β, transforming growth factor–β.
TGF-β, released by platelets, tendon fibroblasts,137,138 and the joint capsule,52,53 has been identified as a key cytokine that both initiates and ends the process of tissue repair. Although other growth factors are involved in tissue remodeling, TGF-β is unique in its actions that enhance the deposition of extracellular matrix and regulate the actions of other cytokines.118,138 An overexpression of TGF-β1 results in progressive accumulation of matrix in tissues and an elevated number of myofibroblasts, leading to fibrosis.13,15,52,57,58,87 Border and Noble13 believe that repeated injury or trauma may also cause autoinduction of TGF-β beyond normal levels, creating a “chronic, vicious circle of TGF-β overproduction,” resulting in tissue fibrosis that may also occur in organ systems such as the kidney, liver, and lung. Investigators have documented that elevated levels of myofibroblasts and TGF-β occur rapidly, within 2 weeks after injury in experimental models, and that these changes are similar to those observed in humans with chronic elbow joint contractures.52,57,58
There is evidence that during the exaggerated fibrotic response that occurs in knee arthrofibrosis, a certain amount of tissue contraction or shrinkage occurs.16,111,131 Specific α-smooth muscle actin (ASMA)–expressing myofibroblasts generate tissue contraction during wound healing and are responsible for collagen overproduction during fibrotic diseases.28,34,38,46 TGF-β is capable of up-regulating ASMA and collagen in fibroblasts.
Unterhauser and associates147 measured the amount of ASMA-containing fibroblastic cells in arthrofibrotic tissue to determine whether the number of these cells was increased over that found in normal tissue. Tissue biopsies were obtained from 9 patients who underwent surgery for arthrofibrosis that had developed after ACL reconstruction. The patients all had primary arthrofibrosis and underwent the débridement procedure between 4 and 12 months after the ACL reconstruction. Tissue samples were also taken from 5 patients who underwent ACL reconstruction for chronic ligament deficiency and from 8 patients who had follow-up arthroscopy after ACL reconstruction for meniscus pathology; all 13 of these control patients showed no signs of arthrofibrosis. A significantly higher expression of ASMA-positive fibroblastic cells were found in the collagen bundles and remaining fatty tissue in the study group compared with both control groups (23.4% of the total cell count vs. 2.3% and 10.8%, respectively; P < .001). The authors concluded that this cell type is involved in scar formation and scar tissue contraction during the course of primary arthrofibrosis. They speculated that the expression of ASMA is most likely variable according to the time from the onset of the disease to tissue biopsy and probably decreases as the disease progresses.
Unterhauser and associates147 also reported that the fatty tissues in the arthrofibrotic knees were replaced by a dense collageneous network with parallel fiber orientation. A significantly higher total cell count and lower vessel density were measured in the arthrofibrotic knees compared with the control group who underwent ACL reconstruction. Similar findings were noted by Mariani and colleagues76 who biopsied 17 knees with arthrofibrosis that occurred after a variety of surgical procedures. The tissue samples were obtained less than 6 months from the onset of stiffness in 9 patients, from 6 to 12 months in 5 patients, and more than 12 months in 4 patients. The histologic evaluations demonstrated collagen-producing fibroblastic tissue with differing amounts of cellularity and vascularization. This study supports the hypothesis that arthrofibrotic tissue undergoes progressive remodeling and acquires the characteristics of mature collagen, all within the first 6 months after the onset of joint stiffness. There was no association between the amount of knee motion and the time from surgery or the degree of tissue maturation. The severity of loss of motion was associated only with the location and amount of adhesive tissue in the knee joint.
Unterhauser and associates147 concluded that the ratio of myofibroblasts to the total cells in connective tissue is a factor in the onset of arthrofibrosis. The authors suggested that future antifibrosis agents (such as decorin) be evaluated, because they inhibit the signaling pathway of TGF-β. Decorin is a human proteoglycan that inactivates the effect of TGF-β and may have a beneficial effect in arthrofibrotic tissues. Fukushima and coworkers45 reported that injection of decorin improved both the muscle structure and the function of lacerated muscle to near-complete recovery in mice. Several other experimental models have demonstrated the effectiveness of decorin in inhibiting adhesion formation in intra-articular adhesions,44 kidneys,14,64 and lungs.48 However, the effectiveness of this agent in humans with early or later-stage arthrofibrosis has not been investigated to date.58
In an in vivo small animal model, Bedair and associates8 showed that an antiotension II systemic receptor blocker decreased fibrosis and enhanced muscle regeneration in acutely injured skeletal muscle and hypothesized that the blocker modulated TGF-β1. Hagiwara and colleagues53 reported that the joint capsule has the potential to produce TGF-β1 and connective tissue growth factor, both of which play a role in causing fibrosis.
Zeichen and coworkers155 compared tissue samples of 13 patients with arthrofibrosis, obtained a mean of 16 months after the inciting knee ligament surgery, with those of 8 patients undergoing primary ACL surgery. Histologic examination from the patients with arthrofibrosis showed marked synovial hyperplasia, infiltration of inflammatory cells, and vascular proliferation with an increased level of collagen types VI and III. None of the control subjects demonstrated these findings. The authors noted that this confirmed the observations of Murakami and associates88 regarding the association between an inflammatory process and the proliferation of fibroblasts. The suggestion was made that collagen type VI could play a contributory role in the deposition of extracellular matrix that leads to arthrofibrosis.
Bosch and colleagues17 advanced the hypothesis that primary arthrofibrosis is the result of an immune response. Tissue samples obtained from 18 patients between 4 and 48 months from the triggering event to surgical débridement demonstrated synovial hyperplasia with fibrotic enlargement of the subintima and infiltration of inflammatory cells. The authors believed their findings supported an immune response as the cause of capsulitis leading to formation of diffuse scar tissue. The clinical implication was to avoid further surgery or aggressive manipulation while patients are suffering from acute capsulitis and to wait 3 to 6 months for the inflammatory response to resolve before débridement is considered. As is discussed, too often, there is a delay in treatment that results in a permanent limitation of knee motion. Unfortunately, even though a delay in treatment may allow tissues to “quiet down,” the resulting fibrotic tissue that forms becomes well organized and dense and treatment becomes more difficult. It appears that in the future, chemical agents will become available to treat fibrotic response to injury by controlling elevated levels of myofibroblast up-regulators and fibrogenic growth factors.8,22,42,45,52,57,58,70,90
PREVENTION
Preoperative
After an acute ACL tear and before reconstruction, the surgeon-therapist team must manage pain, effusion, quadriceps weakness, knee motion limitations, and gait abnormalities. Effusion and hemarthrosis are treated with appropriate nonsteroidal anti-inflammatory drugs (NSAIDs), cryotherapy, compression, and limb elevation. Patients work with physical therapists to learn safe and effective muscle-strengthening exercises (such as straight leg raises, bicycling, mini-squats, wall-sits, calf raises, knee extensions from 90°–30°, hamstring curls, and swimming) and must demonstrate good quadriceps control without an extensor lag before consideration for surgery. Regaining normal or nearly normal knee motion is essential and may take several weeks after an ACL rupture. The exceptions are knees with a mechanical block to motion, such as a displaced meniscus tear, in which early surgical intervention is warranted to preserve and repair the meniscus. A decision regarding future ACL reconstruction is based on the patient’s activity goals and other factors (see Chapter 7, Anterior Cruciate Ligament Primary and Revision Reconstruction: Diagnosis, Operative Techniques, and Clinical Outcomes). Other exceptions are knees with concomitant posterolateral ligament ruptures who are candidates for surgical intervention within 7 to 10 days of the injury. Knees with concomitant MCL tears are treated conservatively for at least 4 to 8 weeks to allow the medial side structures to heal and regain motion and muscle strength before proceeding to ACL reconstruction (see Chapter 24, Medial and Posteromedial Ligament Injuries: Diagnosis, Operative Techniques, and Clinical Outcomes).
Intraoperative
Intraoperative
The principles of correct graft placement, tensioning, and fixation are paramount to avoid a limitation of knee motion postoperatively (see Chapter 7, Anterior Cruciate Ligament Primary and Revision Reconstruction: Diagnosis, Operative Techniques, and Clinical Outcomes). The ACL graft is placed within the femoral and tibial footprint, occupying the central two thirds or more of the footprint, to achieve an anatomic position that allows the graft to control the pivot shift and anterior tibial translation. To achieve an anatomic graft placement in the center of the tibial attachment may require a limited notchplasty to avoid a limitation of knee extension. For the femoral tunnel, an anteromedial portal drilling technique or a two-incision procedure is favored to obtain a central anatomic femoral footprint placement. An endoscopic transtibial drilling technique is not recommended.55 In most chronic ACL-deficient knees, there is an overgrowth of cartilage and spur formation in the femoral notch, which necessitates a notchplasty to prevent ACL graft impingement.
In knees that undergo concomitant meniscus repair, the sutures are tied with the knee at 30° of flexion to prevent capsular shortening or increased pain with postoperative extension exercises. If an associated lateral extra-articular procedure is required, care is taken to avoiding overtensioning the iliotibial band graft and to place the graft in the correct position on the femur to allow restoration of normal knee flexion (see Chapter 7, Anterior Cruciate Ligament Primary and Revision Reconstruction: Diagnosis, Operative Techniques, and Clinical Outcomes). Posteromedial and posterolateral capsular plication procedures are performed in a manner that will not result in a limitation of knee extension, which is tested at the operative table.
Postoperative
The 1st week after ACL or other knee ligament reconstruction represents a critical time period for patients to control knee joint pain and swelling, regain adequate quadriceps muscle contraction, initiate immediate knee motion exercises, and maintain adequate limb elevation (see Chapter 13, Rehabilitation of Primary and Revision Anterior Cruciate Ligament Reconstructions). A bulky compression dressing (cotton-Ace Brand [Becton, Dickinson and Company, Franklin Lakes, NY] elastic bandage–cotton–Ace Brand elastic bandage) is used for 48 hours, followed by antiembolic compression stockings and supplemental Ace bandage. Cryotherapy is begun in the recovery room after surgery. NSAIDs are used for 5 days along with pain medication to allow the immediate exercise protocol to be successfully implemented. Electrogalvanic stimulation or hi-volt electrical muscle stimulation may be used along with ice, compression, and elevation to control swelling.109
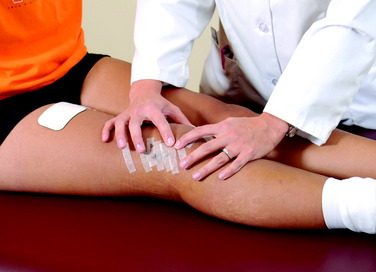
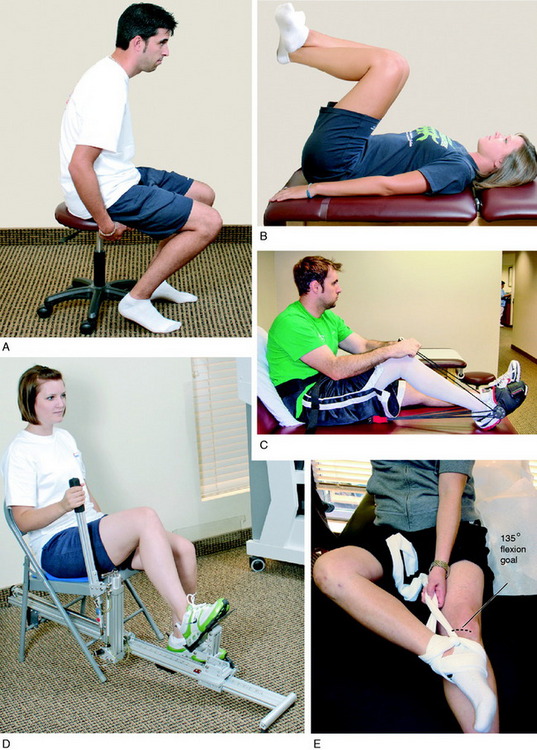
On the 1st postoperative day, patients perform passive and active range of motion exercises in a seated position for 10 minutes a session, approximately four to six times per day. This is accompanied by patellar glides (mobilization), which are performed in all four planes (superior, inferior, medial, and lateral) with sustained pressure applied to the appropriate patellar border for at least 10 seconds (Fig. 41-5). The goal is to achieve 0° to 90° of knee motion by the 7th postoperative day. Patients who are unable to achieve this amount of extension and flexion are immediately placed into an overpressure motion program, described in detail later in this chapter. In the authors’ experience, the exercises and modalities in the overpressure program resolve the majority of knee motion limitations when initiated early postoperatively. In 650 knees that underwent ACL reconstruction, described elsewhere in this chapter, only 18 (3%) required a gentle manipulation under anesthesia and 6 (<1%) underwent surgical débridement and lysis of adhesions after the overpressure program failed to resolve knee motion limitations.
Hamstring and gastrocnemius-soleus flexibility exercises are begun immediately, as are straight leg raises in all four planes of hip movement. Partial weight-bearing is permitted as long as pain and swelling are controlled and a voluntary quadriceps contraction demonstrated. Isometric quadriceps contractions are also begun the 1st postoperative day and are performed on an hourly basis following the repetition rules of 10-second holds, 10 repetitions, 10 times per day. The remainder of the rehabilitation program is detailed in Chapter 13, Rehabilitation of Primary and Revision Anterior Cruciate Ligament Reconstructions.
DIAGNOSIS AND CLINICAL PRESENTATION
The early detection of a limitation of knee motion after ACL reconstruction is paramount for resolving the problem and preventing permanent arthrofibrosis. The establishment of specific knee flexion and extension goals according to the amount of time that has elapsed postoperatively and concurrent major operative procedures performed is required. In all knees, full passive knee extension must be obtained immediately to avoid excessive fibrous tissue proliferation in the intercondylar notch and posterior capsular contracture. In patients with physiologic bilateral knee hyperextension, the authors recommend the gradual return of 3° to 5° of hyperextension in the reconstructed knee and not more than 5° of hyperextension owing to potentially deleterious forces on the healing graft. Specific range of knee motion goals after ACL reconstruction (Table 41-5) are communicated to the patient before surgery and during postoperative assessments. Knee motion is carefully documented by the therapist in a closely supervised postoperative rehabilitation program so that detection of a knee motion problem occurs in a timely manner. Failure to meet these goals prompts entering the patient in an overpressure program using appropriate medical and therapeutic measures, to be described.
TABLE 41-5 Authors’ Goals for Restoration of Knee Motion after Anterior Cruciate Ligament Reconstruction
| Operative Procedure Details | Weeks Postoperative | Range of Knee Motion Goal* |
|---|---|---|
| ACL B-PT-B autograft reconstruction, isolated or with meniscus repair, patient desires to return to strenuous athletics as soon as possible | 1–2 | 0°–110° |
| 3–4 | 0°–120° | |
| 5–6 | 0°–135° | |
ACL, anterior cruciate ligament; B-PT-B, bone–patellar tendon–bone.
* In patients with physiologic bilateral knee hyperextension, the authors recommend the gradual return of 3°–5° of hyperextension in the reconstructed knee. Greater than 5° of hyperextension is not recommended owing to potentially deleterious forces placed on the healing graft.
Critical Points DIAGNOSIS AND CLINICAL PRESENTATION
ACL, anterior cruciate ligament; MRI, magnetic resonance imaging.
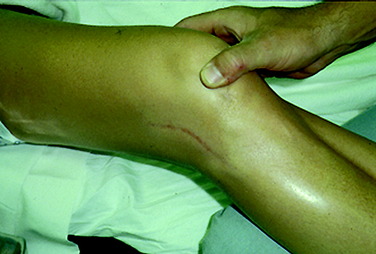
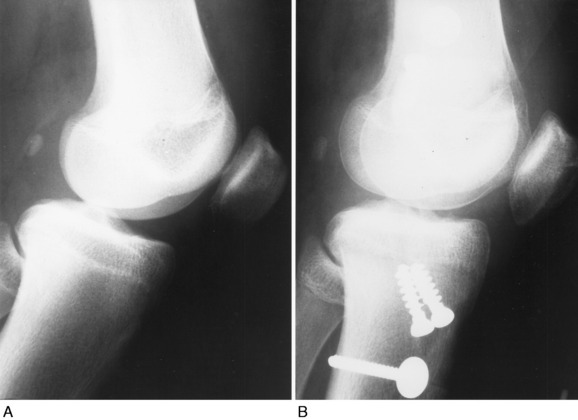
Patients who present with an established arthrofibrosis condition require a comprehensive evaluation, including appropriate radiographs (Fig. 41-6), bone scans, and magnetic resonance imaging (MRI) to determine whether the condition is primary or secondary, as previously discussed. The examination may demonstrate diffuse edema, increased warmth, constant pain, limited patellar mobility (Fig. 41-7), contracture of peripatellar and infrapatellar soft tissues, tenderness in the fat pad, and limits in both extension and flexion compared with the contralateral normal knee (Fig. 41-8). Anterior knee pain, stiffness, pain with attempted knee motion, a flexed-knee gait, and severe quadriceps atrophy are common findings.
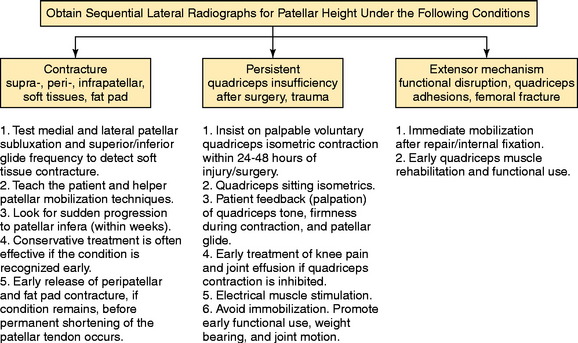
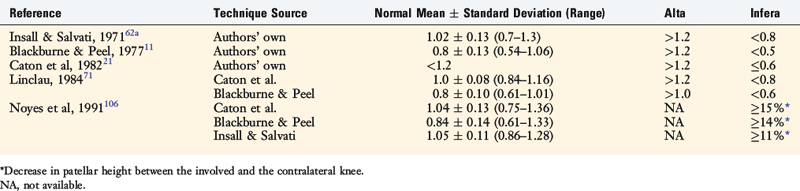
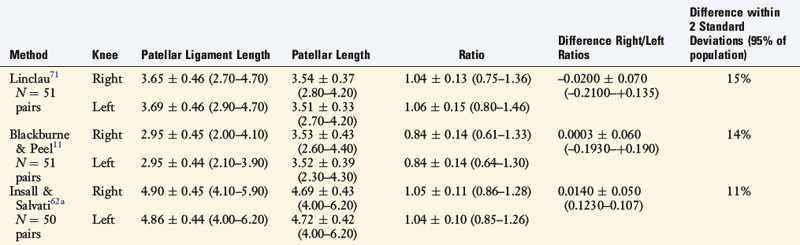
Patellar vertical height is determined using the radiographic method described by Caton and coworkers21 and Linclau.71 In Figure 41-9, Left, the patellar vertical-height ratio is calculated by dividing line segment A, the distance between the most ventral rim of the tibial plateau and the lowest end of the patellar articular surface, by line segment B, the maximum length of the patellar articular surface. An alternative method described by Blackburne and Peel11 locates the tibial reference point at an anterior central location on the tibial plateau (shown in Fig. 41-9 as line segment C). Using this method, the patellar vertical-height ratio equals C/B. The senior author conducted a study to determine normal right to left patellar vertical height ratios within the same individual in a group of 51 patients (102 knees).106 The difference in the vertical-height ratio of the patella between these two methods ranged from 0 to 9%, with an average difference of 3%. Large variations existed in the ratios between individual patients (range, 0.75–1.46). Therefore, the diagnosis of patellar infera requires analysis of patellar height ratios compared with those of the opposite knee and subsequent radiographs compared with the same knee at different time periods (Fig. 41-10). A decrease in the vertical-height ratio of 14% to 15%, depending on the method used (Table 41-6), indicates a patella infera condition because this amount of difference is greater than 2 standard deviations of the normal difference between knees. An alternative method for the calculation of patellar height is the patellotrochlear index,10 expressed as a percentage at knee extension (Fig. 41-9, Right).
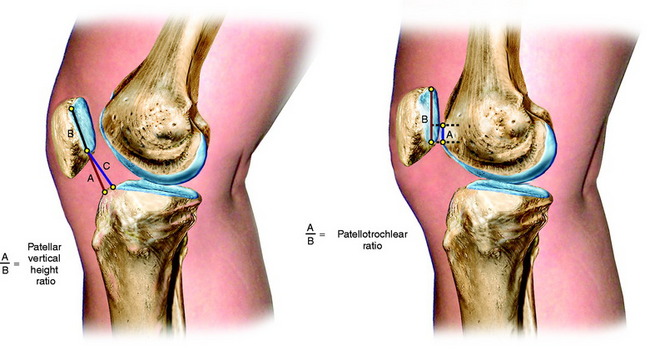
FIGURE 41-9 Methods used to determine patellar vertical-height ratio on a lateral radiograph. Left, The numerator (line segment A) is the distance between the most ventral (anterosuperior) rim of the tibial plateau and the lowest end of the patellar articular surface. The denominator (line segment B) is the maximum length of the patellar articular surface. An alternative numerator (line segment C) locates the tibial reference point on the middle of the tibial plateau. The patellar vertical-height ratio equals A/B or C/B. Right, The numerator (A) is the superiormost aspect of the trochlear cartilage that is in contact with line segment B. The denominator (line segment B) is the maximum length of the articular patellar surface. Using this method, the mean index is 32 ± 12%, >50% is a patellar infera, and <12% is a patella alta.10
TABLE 41-6 Differences between Three Radiographic Methods of Computing Patellar Vertical-Height Ratios
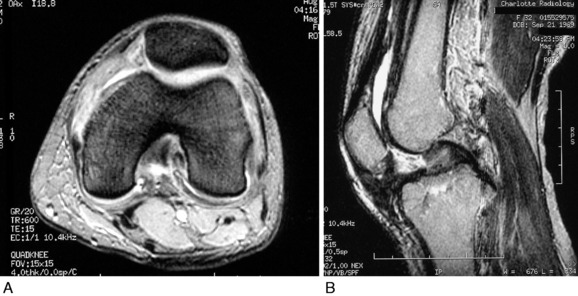
MRI is useful in assessing the extent and location of scar tissue formation (Fig. 41-11). The infrapatellar band of scar tissue frequently found in the patellar infera state is just anterior to the fat pad and attaches from the inferior patellar pole to the anterior aspect of the tibia. Obliteration of the fat pad, with the patellar tendon attaching directly to the anterior tibia, is a poor prognostic sign, because the patella may adhere to the proximal tibia, which markedly restricts medial-to-lateral mobility. Thick peripatellar tissues are frequently observed. The location of prior ACL or posterior cruciate ligament (PCL) grafts must be determined. Important signs of an infectious process or unexplained edema and fluid in soft tissues or bone or about fixation implants are specifically evaluated to exclude an infectious etiology.
Patients who present with arthrofibrosis secondary to a cyclops lesion or an impinged or improperly placed ACL graft require surgical débridement of the lesion or graft and scar tissue, followed by several months of physical therapy to regain normal knee motion and muscle function. The decision may then be made regarding the necessity for revision ACL reconstruction. In addition, patients with established primary arthrofibrosis require surgical management of the condition, described in detail elsewhere. These two groups of patients will not be successfully managed with conservative treatment measures. However, patients who fail to meet the knee motion goals established after ACL reconstruction (see Table 41-5) and in whom the arthrofibrosis is in the early, transient stage may usually be effectively treated with the exercises and modalities described later. The key is the early detection of the limitation of knee motion, as soon as the 7th postoperative day if 0° to 90° is not demonstrated.
CONSERVATIVE MANAGEMENT OF LIMITATION OF KNEE EXTENSION
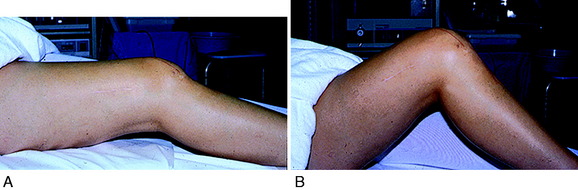
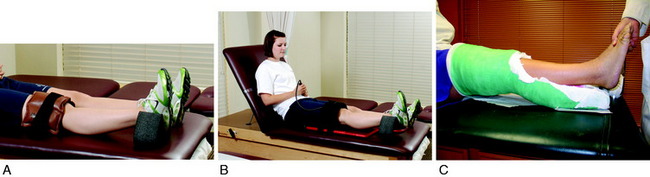
If the patient has not achieved at least 0° by the 7th postoperative day, she or he is placed into an overpressure extension program. The goal is to produce a gradual stretching of posterior capsular tissues, but not to induce soft tissue tearing and further injury because this could lead to an inflammatory response and worsen the condition. One effective exercise involves hanging weights, in which the foot and ankle are propped on a towel or other device to elevate the hamstrings and gastrocnemius, which allows the knee to drop into full extension (Fig. 41-12A). This position is maintained for 10 to 15 minutes and repeated at least eight times per day. Initially a 10-pound weight is used, which may be progressed up to 25 pounds to the distal thigh and knee to provide overpressure to stretch the posterior contracted tissues. Full knee extension is usually obtained by the 2nd to 3rd postoperative week. A commercial extension appliance (see Fig. 41-12B) is also an option.
If these treatment measures are not effective, a drop-out (bivalved) cast (see Fig. 41-12C) is implemented for continuous extension overpressure. The advantage of this technique is that the patient is in control of the process and can apply or delete wedge material as tolerated and may bathe. A hyperextension splint may be used in the same manner; however, the authors believe that the bivalved cast offers superior benefits. Casting is not recommended in knees that have greater than a –12° extension deficit with a hard block to terminal extension. These knees are usually 3 or more months postoperative, and dense scar tissue has formed posteriorly with posterior capsular contracture requiring surgical releases and débridement. In knees with a rigid block to extension, extension casts have the potential to produce high tibiofemoral compressive forces and articular cartilage damage. Extension casts are also not effective when there is proliferative ingrowth of fibrous tissue in the femoral notch. These cases require arthroscopic débridement.
Critical Points CONSERVATIVE MANAGEMENT OF LIMITATION OF KNEE EXTENSION
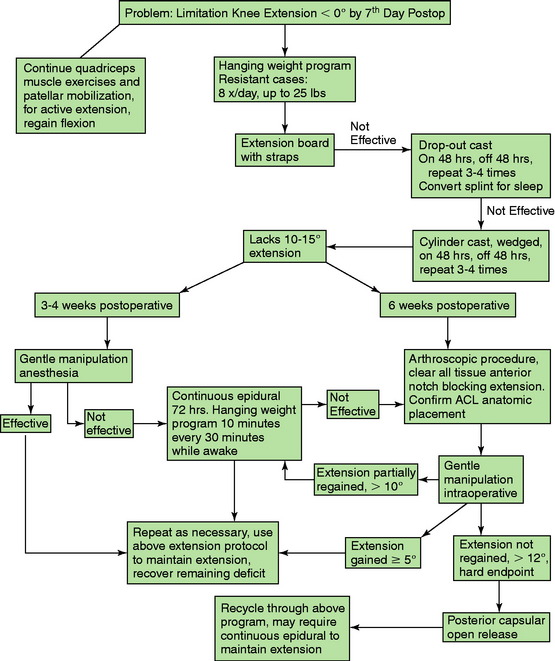
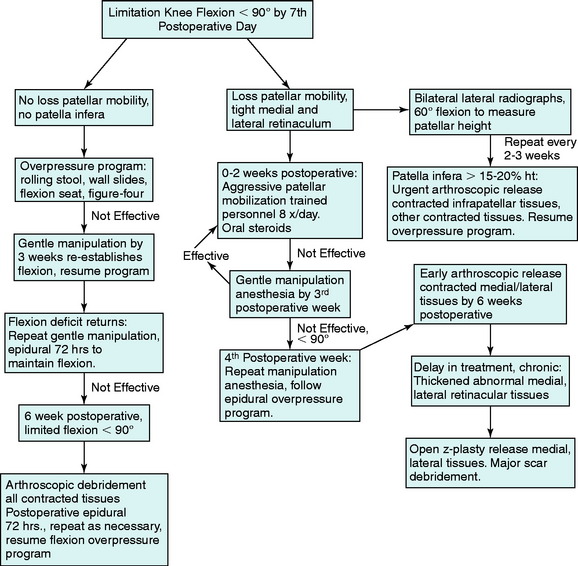
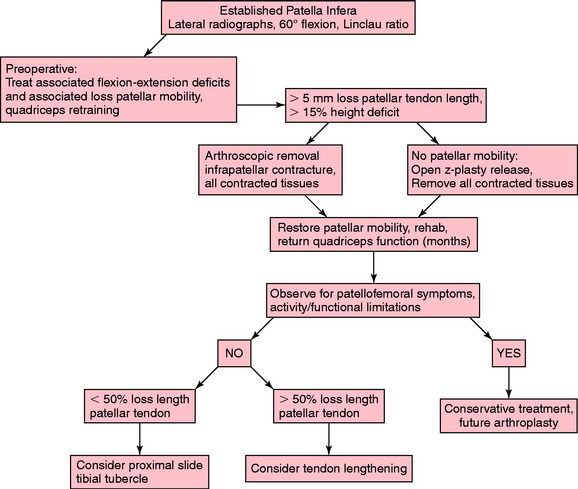
The treatment algorithm for limitations of knee extension is shown in Figure 41-13. The goal is to resolve the loss of knee extension by 4 weeks, using the modalities outlined. Frequently, it is necessary to repeat steps in the treatment protocol, because the return in a knee extension deficit may occur. It is rare that manipulation or continuous epidural is necessary when the program is followed; however, these steps are provided for the most resistant cases. The most difficult problem is the knee joint that does not respond and, despite the overpressure program, continues to show a lack of full extension. If the lack of knee extension is 10° or less, the overpressure program will usually be successful. However, it is important to identify the knee in which there is a rigid hard block to extension and the deficit is greater than 10°. In these knees, it is better to proceed with a posterior capsulotomy through a limited medial and lateral approach, to be described.
CONSERVATIVE MANAGEMENT OF LIMITATION OF KNEE FLEXION
Because full knee flexion is present at the end of the ACL reconstruction, any limitation in the early postoperative period is due to intra-articular adhesions and contracture of peripatellar tissues and muscle spasm or pain. The early detection of a limitation of flexion (<90° by the 7th postoperative day) necessitates an overpressure flexion program. A rolling stool exercise (Fig. 41-14A) may be done in which the patient sits on a small stool close to the ground, the knee is flexed to the maximum position possible and held in that position for approximately 1 minute or as long as the patient is able to tolerate mild discomfort. Then, the patient rolls the stool forward without moving the foot position on the floor to achieve a few more degrees of flexion. The procedure is performed for 10 to 12 minutes and repeated six to eight times a day. Wall-slides are another effective exercise to achieve flexion. The patient lies on his or her back and places the foot of the reconstructed knee on a wall as shown in Figure 41-14B. The foot of the opposite leg is used to gently slide the opposite foot and flex the reconstructed knee in a gradual manner. Commercial knee flexion devices (see Fig. 41-14C and D) may also be used as available to further promote overpressure. In addition, a figure-four overpressure exercise is effective (see Fig. 41-14E). A 4-inch tubular stocking is used, double-wrapped around the foot and ankle, that allows the patient under his or her own power to flex the knee. The patient shown in Figure 41-14E had 90° of flexion, and an arthroscopic release was successful in achieving 135°. After the débridement and placement of an epidural, the surgeon sets a mark on the thigh by gentle pressure to establish the flexion goal for that day. The mark on the thigh in the photograph demonstrates the 135° flexion position, which is the overall goal. The mark on the distal portion of the patella in the photograph demonstrates the starting position, which in this case was 90°. The goal of these exercises and modalities is to gradually and passively stretch tissues in a controlled manner, while not inducing pain or tearing of tissues. Patients who have difficulty achieving 90° by the 3rd to 4th week require a gentle ranging of the knee under anesthesia (not a forceful manipulation) in which full flexion is easily obtained with only light loads applied. This stretches out early joint adhesions, markedly reduces postoperative pain, and allows the patient to regain motion.
Critical Points CONSERVATIVE MANAGEMENT OF LIMITATION OF KNEE FLEXION
The treatment algorithm for a limitation of knee flexion is shown in Figure 41-15. The first step is to determine whether there is associated contracture of patellar tissues, which makes the treatment more difficult. The loss of knee flexion when there is no peripatellar contracture is more easily resolved. With the treatment protocol shown, it is rare to progress to the necessity for an arthroscopic débridement and release of peripatellar tissues. When there is an associated peripatellar contracture, it is important that the condition be immediately treated because this can result in a patella infera. A conservative patellar mobilization program with oral steroids is performed for a week. If the limitation of motion and peripatellar contracture persists, a gentle manipulation under anesthesia is performed; however, no more than “two to three fingers of pressure” is placed at the distal tibia to achieve knee flexion. If more force is necessary, it indicates that the peripatellar contracture is composed of more dense, resistant scar that requires arthroscopic débridement. An aggressive manipulation under higher forces risks damaging the patellofemoral articular cartilage, because the tight contracted tissues increase patellofemoral contact forces with knee flexion. Knee flexion is gained, but at the expense of possibly damaging the patellofemoral joint. This is also true for pushing knee flexion in the clinic when there is no patellar mobility. Again, an operative release of tight contracture peripatellar tissues is indicated instead of overzealous flexion exercises or a knee manipulation with high flexion loads.
ROLE OF ORAL CORTICOSTEROIDS
Critical Points ROLE OF ORAL CORTICOSTEROIDS
GENTLE MANIPULATION UNDER ANESTHESIA
As already described, a forceful manipulation of a knee with extensive scar tissue is harmful and ineffective in treating the limitation of knee motion. A forced manipulation may tear intra-articular and extra-articular tissues and cause excessive tibiofemoral and patellofemoral compression with the risk of fracture, damage to the articular cartilage, rupture of the patellar tendon, and even femoral fracture.72 Therefore, any patient who has a hard-stop to knee motion (which represents a joint contracture) is treated with an arthroscopic débridement and lysis of adhesions.
OPERATIVE MANAGEMENT OF SEVERE RESTRICTION OF KNEE MOTION
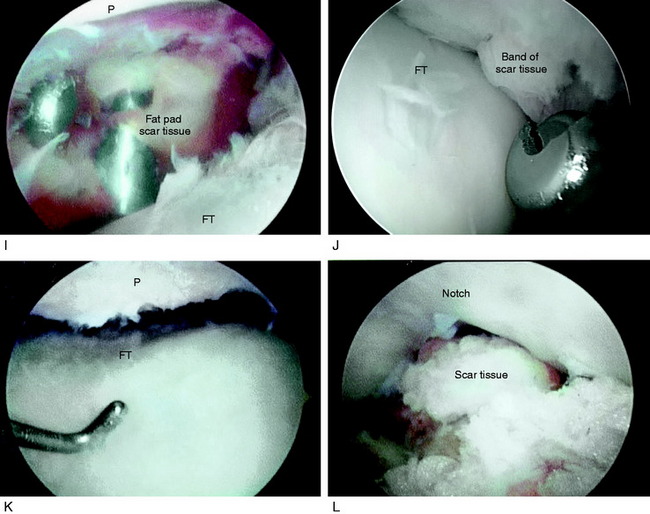
Arthroscopic Soft Tissue Releases
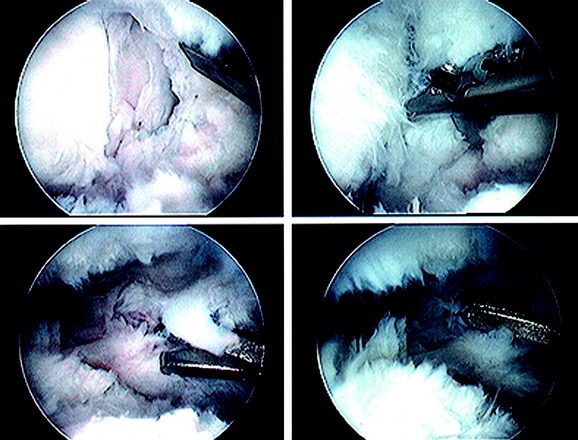
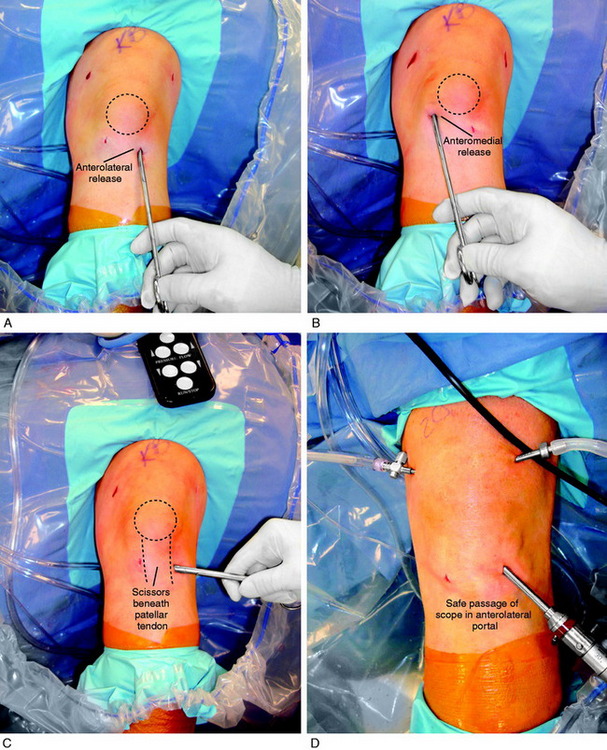
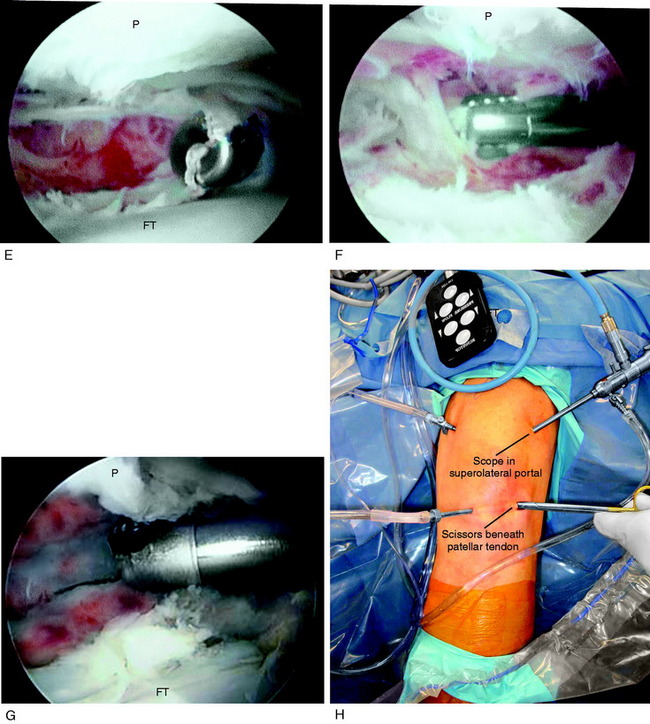
A demonstration of an arthroscopic technique for débridement of contracted tissues in a knee with arthrofibrosis is shown in Figure 41-16. In this patient, there was a restriction in medial-to-lateral translation (glide) of the patellofemoral joint, with a contracture of the medial and lateral retinaculum. However, approximately 50% of normal patellar mobility was still present, indicating that an arthroscopic technique could be used and an open extensive technique was not necessary. There was a limitation of motion at 0°/5°/90°. The procedure was performed after tourniquet inflation.
Critical Points GENTLE MANIPULATION UNDER ANESTHESIA
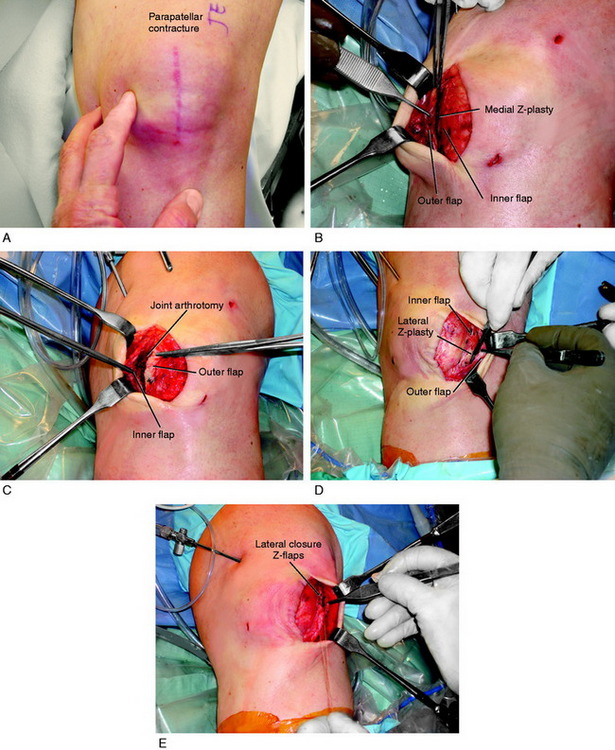
Open Z-Plasty, Medial-Lateral Retinacular Tissues
Figure 41-17 demonstrates the operative technique for an open Z-plasty release of the medial and lateral retinacular tissues. An arthroscopic release alone is contraindicated in this case, because the release is so extensive (extending above the proximal patella and releasing all medial and lateral soft tissues) that there would be open communication of the joint into the subcutaneous tissues. The principle shown is to preserve the function of the medial and lateral retinacular soft tissues and, as well, débride the tissues to a normal thickness. The Z-plasty open release allows an arthrotomy to thoroughly débride infrapatellar contracted tissues, protect the patellar tendon, and lengthen contracted medial and lateral patellar tissues.
Posterior Medial-Lateral Capsulotomy
The technique for a posterior medial-lateral capsulotomy involves the same exposure as that used for a medial or lateral meniscus repair (Fig. 41-18). On the medial side, the posterior capsule is sectioned at its femoral attachment proximally. On the lateral side, the capsule is sectioned in its proximal aspect just below the attachment of the lateral gastrocnemius to the posterior capsule. The dissection and posterior capsule excision are carried directly over the posterior femoral condyles and not into the posterior intercondylar region, because scar tissue may extend to the neurovascular structures. It is often impressive how dense the posterior capsular structures are, and with their release, further gains in knee extension are possible on the operating table. The remaining posterior intercondylar tissues are easily stretched with the overpressure program. The posterior capsule releases are also accompanied with an arthroscopic procedure to remove any tissue that has grown into the notch producing an anterior impingement that would also block extension.
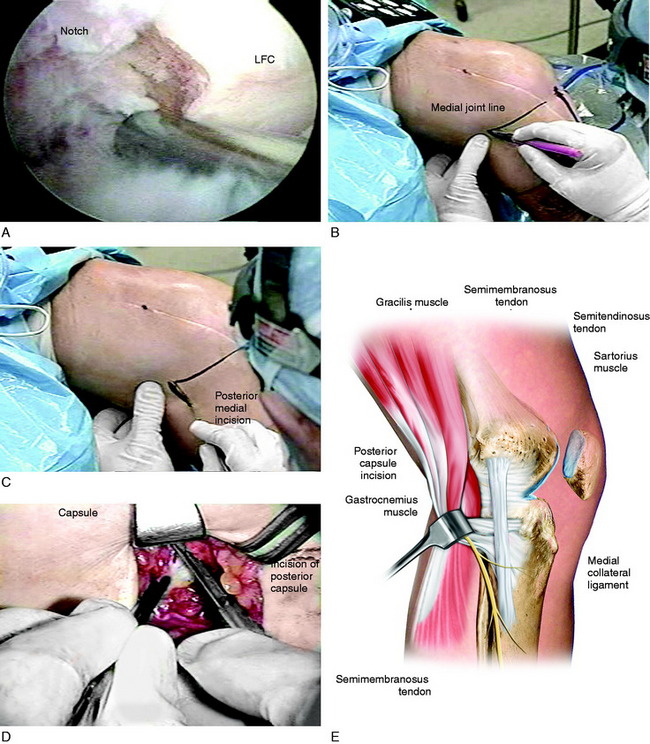
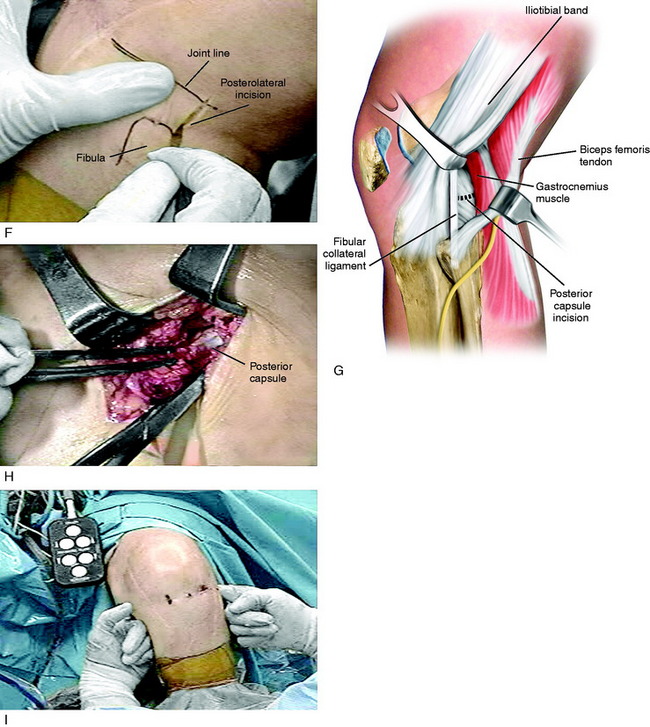
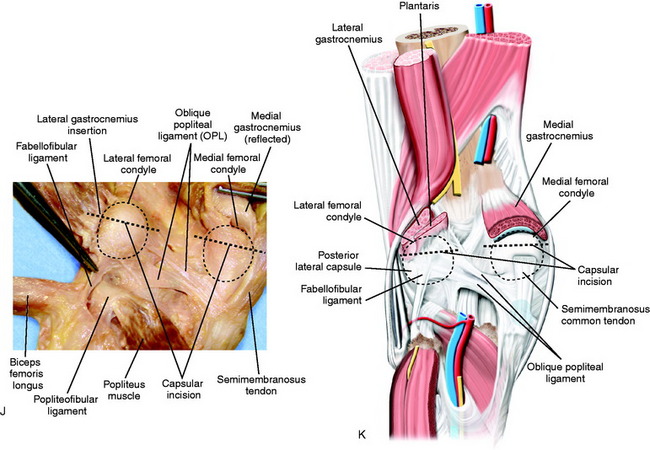
FIGURE 41-18 Operative photographs of a patient with arthrofibrosis (0°/15°/80°) requiring an arthroscopic lysis of adhesions, parapatellar releases, and posterior medial and lateral capsulotomy for the 15° flexion contracture. A, An arthroscopic lysis of adhesions, removal of scar tissue, and parapatellar releases is performed, as already described. All scar tissue from the notch region is removed and a notchplasty performed as required so that these tissues do not block extension. B, The medial joint line is marked. C, A posteromedial incision is made. D, The approach reflecting the sartorius is used as described for an inside-out medial meniscus repair (see Chapter 28, Meniscus Tears: Diagnosis, Repair Techniques, and Clinical Outcomes). E, A vertical posteromedial capsulotomy is made directly behind the superficial medial collateral ligament (MCL). Operative photograph: The surgeon uses a headlamp and the femoral insertion of the posteromedial capsule is identified and transected millimeter by millimeter to the edge of the medial femoral condyle, but not beyond to avoid the neurovascular structures. F, The posterolateral approach for a capsular release uses the same approach as that for a lateral meniscus repair (see Chapter 28, Meniscus Tears: Diagnosis, Repair Techniques, and Clinical Outcomes) with a skin incision just distal to the joint line as shown. G, The posterolateral capsule anterior to the gastrocnemius tendon is identified. H, The posterolateral capsule is carefully incised under direct visualization to the edge of the lateral femoral condyle, avoiding the neurovascular structures. I, A finger is placed into the posteromedial and posterolateral incisions and the central neurovascular structures can be palpated. The central posterior capsule anterior to the neurovascular structures is not released. The finger palpation confirms that the capsule directly posterior to the medial and lateral femoral condyles has been incised, removing this block to extension as the knee is extended. J and K, The posterior capsular releases. The remaining posterior capsular tissues are gently stretched with the overpressure program to achieve extension. This is a limited cosmetic approach that does not disturb other posterior soft tissues and removes only the contracted posterior capsule as shown by the dotted line.
IN-PATIENT PHYSICAL THERAPY PROGRAM
The overpressure extension (Fig. 41-19) and flexion (Fig. 41-20) exercises are described in Table 41-7. The authors recommend an active range of motion program performed by the patient instead of use of a continuous passive motion machine. As well, the patient should continue with leg lifts and isometric exercises while in the hospital. It is imperative to control soft tissue inflammation and avoid a hemarthrosis. If soft tissue swelling persists, or thigh muscle pain occurs owing to excessive stretching, an NSAID or steroidal dose pack should be considered. After the epidural, it is routine to obtain a venous ultrasound to exclude venous clot disease.
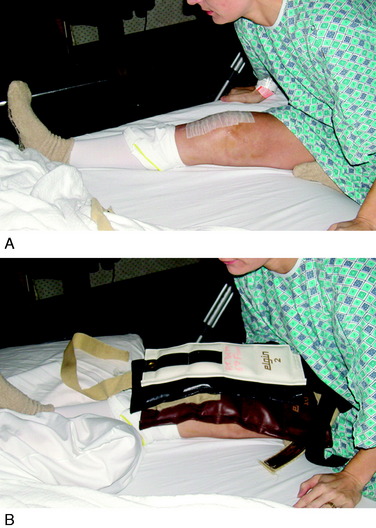
FIGURE 41-19 In-patient extension overpressure exercises include hamstring stretching (A) and hanging weights (B).
TABLE 41–7 In-Patient Overpressure Extension and Flexion Program
| Exercises | |
|---|---|
| Extension (perform first, before flexion) |
Critical Points IN-PATIENT PHYSICAL THERAPY PROGRAM
DEVELOPMENTAL PATELLA INFERA
Critical Points DEVELOPMENTAL PATELLA INFERA
The senior author has reported on the complication of developmental patella infera (Fig. 41-21) that occurs after major knee injury or surgery, secondary to contracture of peripatellar and infrapatellar scar tissues and quadriceps weakness.105,106 This condition may result in permanent shortening of the patellar tendon, patellofemoral arthritis, and severe functional limitations. To prevent this problem, the steps previously described to prevent arthrofibrosis are taken before and after surgery. In addition, the patient is instructed preoperatively on the importance of achieving a voluntary quadriceps contraction as soon as possible after surgery, which is expected to occur by the 2nd postoperative day. Patellar mobility is assessed weekly by the therapist to detect any early contracture. Serial lateral radiographs are taken to detect any decrease in patellar height for patients demonstrating limited patellar mobility or in whom an early arthrofibrotic response is detected (Fig. 41-22). The differences in criteria for determining the presence of patella infera according to published techniques are shown in Table 41-8.
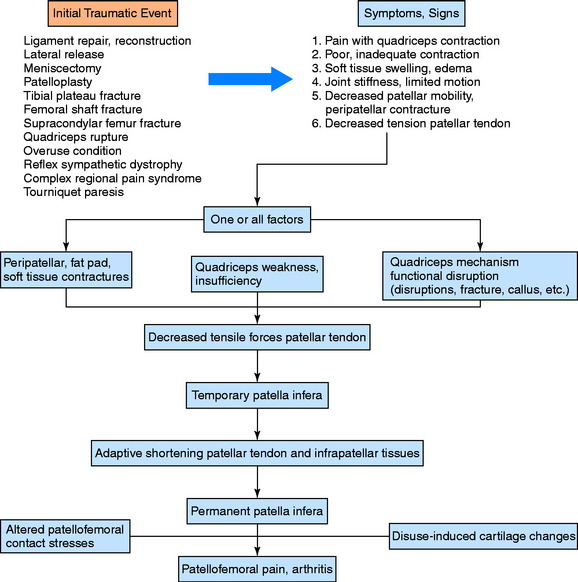
FIGURE 41-21 Pathomechanics for developmental patella infera, quadriceps insufficiency, patellar tendon shortening, and arthritis.
(From Noyes, F. R.; Wojtys, E. M.; Marshall, M. T.: The early diagnosis and treatment of developmental patella infera syndrome. Clin Orthop 265:241–252, 1991.)
For patients who present with an established patella infera, the treatment algorithm is shown in Figure 41-23. The first principle of treatment is the preoperative preparation of the patient to establish quadriceps muscle function, rehabilitation, and training to avoid a quadriceps shutdown after the surgical procedure. Second, to remove the contracted and scarred tissues by either arthroscopic or open surgery, as already discussed. A number of months of intensive therapy are then required. The final functional limitations are based on residual motion deficits and joint arthritis that are present. A majority of patients with established patella infera have functional limitations.106 The patients do not desire any further surgery and are willing to live with these limitations, understanding that an eventual arthroplasty procedure may be required. A small percentage of patients with a remaining patella infera and minimal patellofemoral arthritis who have symptoms of anterior knee pain and stiffness may be candidates for restoration of a normal patellotrochlear relationship.
AUTHORS’ CLINICAL STUDIES
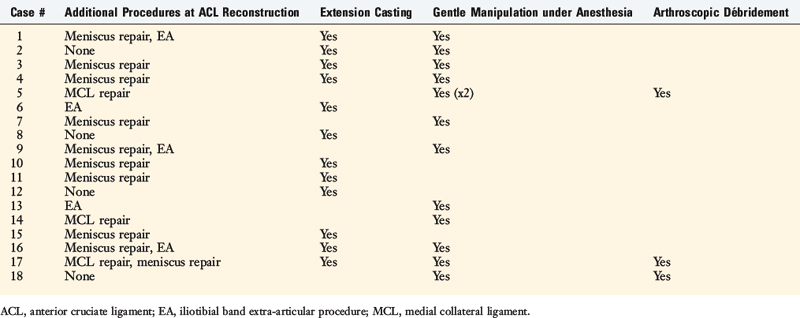
Two studies were conducted by the authors to determine the effectiveness of the postoperative rehabilitation program and early diagnosis and treatment of knee motion limitations after ACL reconstruction in preventing arthrofibrosis. In the first study,102 a group of 207 knees that underwent an ACL bone–patellar tendon–bone (B-PT-B) allograft reconstruction were followed for at least 1 year postoperatively to determine the final amount of knee flexion and extension obtained and document the necessity of treatment interventions. Sixty-nine patients received the operation for an acute ACL rupture, and 138 for chronic ACL deficiency. Of the 207 knees, 90 had the ACL reconstruction alone, 52 had a concurrent iliotibial band extra-articular procedure, 52 had a concurrent meniscus repair, and 13 had a concurrent MCL repair.
The investigation found that 189 of the knees (91%) regained normal motion without requiring additional treatment intervention. The remaining 18 required extension casting, gentle manipulation under anesthesia, or (in only 3 knees) arthroscopic débridement and lysis of adhesions (Table 41-9). There was a correlation between concurrent procedures and the incidence of patients requiring addition treatment, because 4% in the ACL alone subgroup, 10% in the iliotibial band extra-articular subgroup, 12% in the meniscus repair subgroup, and 23% in the MCL subgroup required intervention. The timing of surgery had no effect on the necessity for treatment intervention.
TABLE 41-9 Patients Requiring Treatment Intervention for Knee Motion Limitations after Anterior Cruciate Ligament Allograft Reconstruction
Critical Points AUTHORS’ CLINICAL STUDIES
Study #1
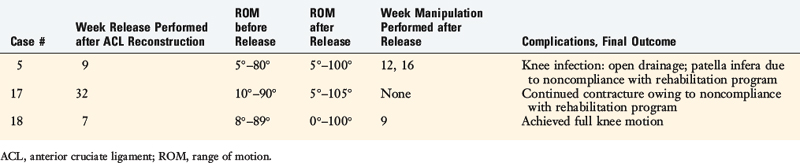
Extension casts were applied to 6 knees between 4 and 12 weeks postoperatively and all regained normal extension. A gentle manipulation under anesthesia was performed in 9 knees between 3 and 13 weeks postoperatively. All achieved full flexion and all but 2 achieved full extension; two knees lacked 5° of extension at follow-up. Arthroscopic débridement was done in 3 knees; the details and results are shown in Table 41-10. At follow-up, full knee motion was present in 98%, 1% had a 5° limitation of extension, and 1% had a permanent contracture.
TABLE 41-10 Results of Arthroscopic Débridement for Arthrofibrosis after ACL Allograft Reconstruction
The authors conducted a second study101 comprising 443 knees that underwent ACL B-PT-B autograft reconstruction. The operations were performed for acute/subacute ACL ruptures in 161 patients and for chronic ruptures in 283 patients. All were followed an average of 25 months (range, 12–86 mo) postoperatively. Concurrent procedures done in 224 knees included meniscus repairs in 194 knees, proximal advancement of the posterolateral structures in 32 knees, an autograft reconstruction of the fibular collateral ligament (FCL) in 4 knees, a repair or reconstruction of the MCL in 9 knees, and a proximal patellar realignment in 17 knees.
The investigation found that 98% of the knees regained normal motion and 2% had mild losses of extension of 5°. In 23 knees, treatment intervention was required (Table 41-11), which was successful in treating the limitation of knee flexion or extension (Table 41-12). The 2% (7 knees) who had mild losses of extension refused treatment intervention.
OTHER AUTHORS’ CLINICAL STUDIES
Several authors have reported on the outcome of the treatment of arthrofibrosis after ACL reconstruction (Table 41-13).* These studies help identify risk factors for a limitation of knee motion, discussed previously. In general, the surgical débridement procedures were done an average of 1 year after the reconstruction, which in the opinion of authors of this chapter, is too late. In many of the series, patients were referred from other centers and the treating surgeon was unable to perform the débridement earlier. Although many authors reported that significant gains were obtained in knee motion, most found that the majority of patients had permanent residual losses of flexion and extension and more complaints of pain and limitations compared with patients who did not suffer from this complication. Table 41-13 also shows other studies of the treatment of arthrofibrosis after fractures, infection, meniscectomy, and other knee ligament reconstructions.1,73,149,151 These studies report even poorer results in terms of restoration of normal knee motion and function.
Illustrative Cases
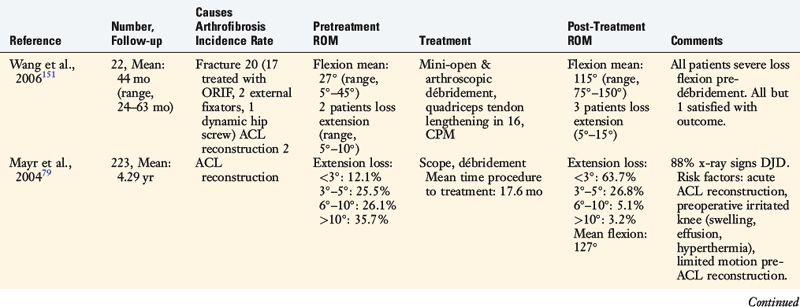
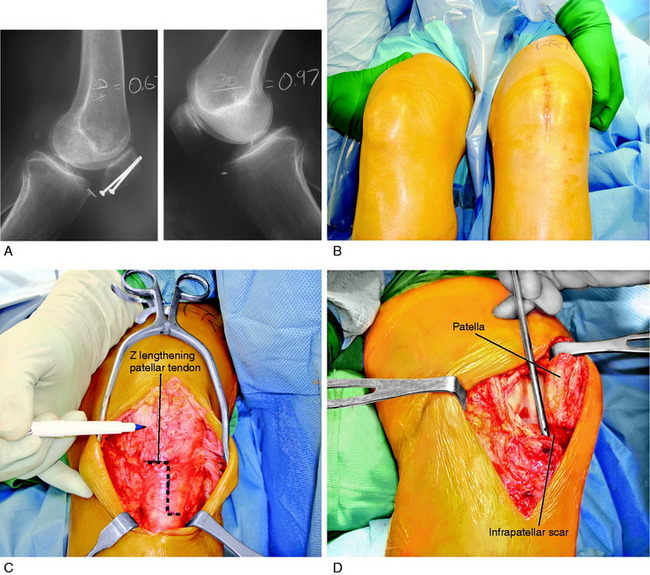
Case 1
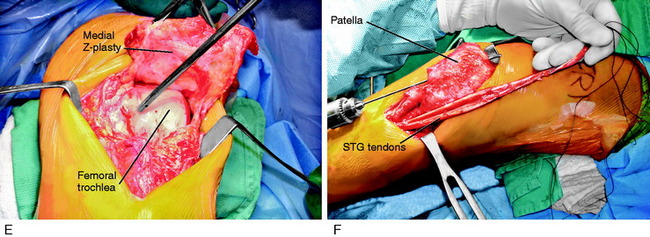
A 42-year-old woman presented 7 months after an open reduction internal fixation of a patellar fracture and 4 weeks of immobilization with a severe patella infera, marked by a Linclau ratio of 60% of the left knee (compared with 97% on the opposite knee; Fig. 41-24A). She complained of muscle weakness, pain, and limitations with activities of daily living. She had a range of motion of 0°/10°/95° and a significant decrease in patellar mobility in both medial-lateral and superior-inferior directions. Physical examination showed an obvious patella infera, with mild patellofemoral crepitus. An isokinetic evaluation revealed a 75% deficit in quadriceps peak torque compared with the contralateral side. She underwent 2 months of rehabilitation owing to this severe quadriceps muscle weakness.
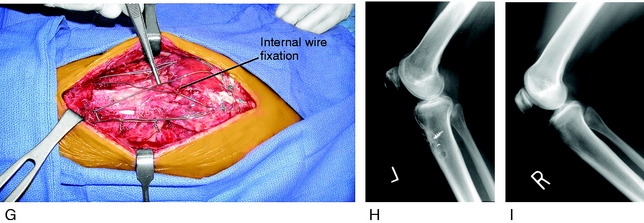
Two months later, the patient continued to have symptoms and minimal improvement. Therefore, the decision was reached to perform a patellar lengthening procedure. Figure 41-24B-G demonstrates the tendon lengthening. A Z-plasty lengthening of the patient’s patellar tendon was performed along with an augmentation using a four-strand semitendinosus-gracilis autograft. Internal fixation with wire was used to initiate immediate knee motion. The procedure was successful in restoring a normal patellar height (see Fig. 41-24H and I). The goal is to buy time until the patient eventually requires a patellofemoral arthroplasty, which is possible with normal patellar height.
Case 2
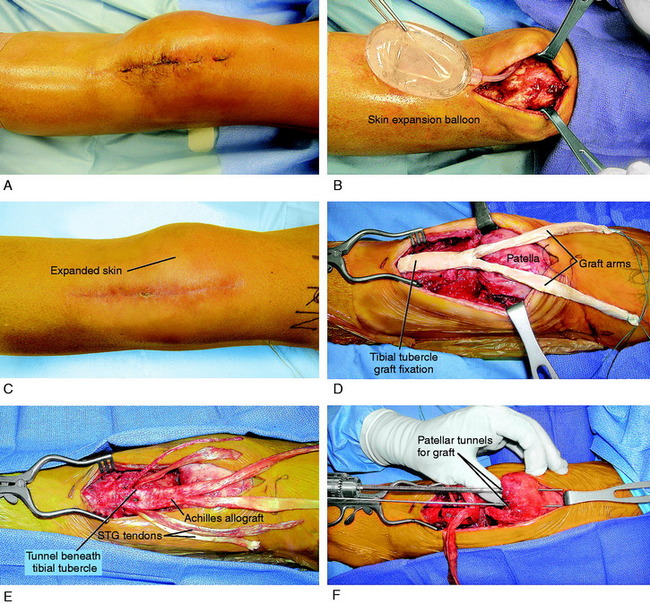
A 33-year-old woman presented with severe patellar infera (Linclau ratio, 29%) 4 months after resection of a ganglion cyst, which was growing under her patellar tendon. A portion of the patellar tendon was damaged in the procedure and the patient was treated with 5 weeks of plaster immobilization, which resulted in severe quadriceps atrophy and severely contracted peripatellar and joint soft tissues. This represented a salvage knee condition because the patient had significant loss of function of the lower extremity. The range of motion was 0°/10°/75°. She underwent 6 weeks of intense preoperative rehabilitation for muscle strengthening and then a skin expansion procedure (Fig. 41-25A and B). Because this patient had no remaining patellar tendon to use in a Z-plasty fashion to incorporate into the reconstruction, she underwent a combined allograft-autograft patellar tendon lengthening procedure (see Fig. 41-25C-G). The patient required intensive rehabilitation for 6 months.
At the most recent follow-up evaluation, 2 years postoperative, the patient had equal Linclau ratios of 85% (see Fig. 41-25H and I) and a range of motion of 0 to 120°. She has no symptoms with activities of daily living or swimming and, while pleased with her condition, realizes that in the future a patellofemoral arthroplasty may be required.
Authors’ comment: The salvage procedure of lengthening the patellar tendon was performed in this young patient to buy time before an anticipated total knee replacement. The benefit of reestablishing the normal patellar height (see Fig. 41-25H and I) allowed a return of moderate lower limb function, with the added benefit at the time of the total joint procedure of retaining patellofemoral joint function.
Case 3
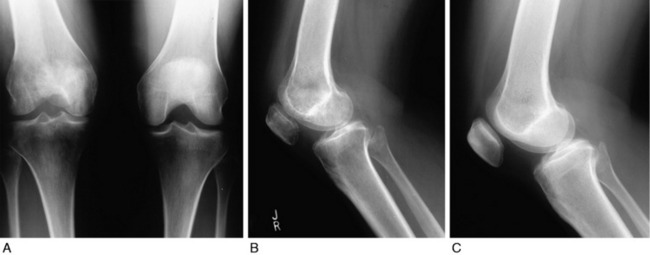
Standing posteroanterior radiographs showed a decrease in the height of the patella and osteopenia (Fig. 41-26A). Lateral radiographs with the knee flexed showed an obvious patella infera, with a 68% Linclau ratio on the involved side (see Fig. 41-26B) compared with the opposite side of 87% (see Fig. 41-26C). The patient underwent Z-plasty lengthening of the medial and lateral retinacular structures to restore patellar mobility, an arthroscopic suprapatellar débridement to open the synovial pouch, and removal of infrapatellar contracture. The patellar tendon shortening was permanent. The patient underwent 3 months of intensive rehabilitation, muscle strengthening, and gait retraining and achieved 0°/0°/125° of motion, with restoration of patellar mobility.
1 Achalandabaso J., Albillos J. Stiffness of the knee—mixed arthroscopic and subcutaneous technique: results of 67 cases. Arthroscopy. 1993;9:685-690.
2 Aglietti P., Buzzi R., De Felice R., et al. Results of surgical treatment of arthrofibrosis after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 1995;3:83-88.
3 Al-Qattan M.M. Factors in the pathogenesis of Dupuytren’s contracture. J Hand Surg [Am]. 2006;31:1527-1534.
4 Alm A., Liljedahl S.-O., Stromberg B. Clinical and experimental experience in reconstruction of the anterior cruciate ligament. Orthop Clin North Am. 1976;7:181-189.
5 Austin J.C., Phornphutkul C., Wojtys E.M. Loss of knee extension after anterior cruciate ligament reconstruction: effects of knee position and graft tensioning. J Bone Joint Surg Am. 2007;89:1565-1574.
6 Bach B.R., Jones G.T., Sweet F.A., Hager C.A. Arthroscopy-assisted anterior cruciate ligament reconstruction using patellar tendon substitution. Two- to four-year follow-up results. Am J Sports Med. 1994;22:758-767.
7 Barber-Westin S.D., Noyes F.R., Andrews M. A rigorous comparison between the sexes of results and complications after anterior cruciate ligament reconstruction. Am J Sports Med. 1997;25:514-526.
8 Bedair H.S., Karthikeyan T., Quintero A., et al. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med. 2008;36:1548-1554.
9 Berns G.S., Howell S.M. Roofplasty requirements in vitro for different tibial hole placements in anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:292-298.
10 Biedert R.M., Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14:707-712.
11 Blackburne J., Peel T. A new method for measuring patellar height. J Bone Joint Surg Br. 1977;58:241-242.
12 Blauth W., Jaeger T. [Arthrolysis of the knee joint]. Orthopade. 1990;19:388-399.
13 Border W.A., Noble N.A. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286-1292.
14 Border W.A., Noble N.A., Yamamoto T., et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361-364.
15 Border W.A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1-7.
16 Bosch U. [Etiology of arthrofibrosis]. Arthroskopie. 1999;12:215-221.
17 Bosch U., Zeichen J., Skutek M., et al. Arthrofibrosis is the result of a T-cell–mediated immune response. Knee Surg Sports Traumatol Arthrosc. 2001;9:282-289.
18 Bottoni C.R., Liddell T.R., Trainor T.J., et al. Postoperative range of motion following anterior cruciate ligament reconstruction using autograft hamstrings: a prospective, randomized clinical trial of early versus delayed reconstructions. Am J Sports Med. 2008;36:656-662.
19 Bowley E., O’Gorman D.B., Gan B.S. Beta-catenin signaling in fibroproliferative disease. J Surg Res. 2007;138:141-150.
20 Bylski-Austrow D.I., Grood E.S., Hefzy M.S., et al. Anterior cruciate ligament replacements: a mechanical study of femoral attachment location, flexion angle at tensioning, and initial tension. J Orthop Res. 1990;8:522-531.
21 Caton J., Deschamps G., Chambat P., et al. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68:317-325.
22 Chan Y.S., Li Y., Foster W., et al. Antifibrotic effects of suramin in injured skeletal muscle after laceration. J Appl Physiol. 2003;95:771-780.
23 Cole B.J., Harner C.D. The multiple ligament injured knee. Clin Sports Med. 1999;18:241-262.
24 Cosgarea A.J., DeHaven K.E., Lovelock J.E. The surgical treatment of arthrofibrosis of the knee. Am J Sports Med. 1994;22:184-191.
25 Cosgarea A.J., Sebastianelli W.J., DeHaven K.E. Prevention of arthrofibrosis after anterior cruciate ligament reconstruction using the central third patellar tendon autograft. Am J Sports Med. 1995;23:87-92.
26 Coutts R., Rothe C., Kaita J. The role of continuous passive motion in the rehabilitation of the total knee patient. Clin Orthop. 1981;159:126-132.
27 Creighton R., Bach B.R.Jr. Arthrofibrosis: evaluation, prevention, and treatment. Tech Knee Surg. 2005;4:163-172.
28 Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21-29.
29 deAndrade J.R., Grant C., Dixon A.S.J. Joint distension and reflex muscle inhibition in the knee. J Bone Joint Surg Am. 1963;47:313-322.
30 DeCarlo M.S., Sell K. Normative data for range of motion and single leg hop in high school athletes. J Sports Rehabil. 1997;6:246-255.
31 DeHaven K.E., Cosgarea A.J., Sebastianelli W.J. Arthrofibrosis of the knee following ligament surgery. Instr Course Lect. 2003;52:369-381.
32 Dehne E., Torp R.P. Treatment of joint injuries by immediate mobilization. Based upon the spinal adaptation concept. Clin Orthop Relat Res. 1971;77:218-232.
33 Del Pizzo W., Fox J.M., Friedman M.J. Operative arthroscopy for the treatment of arthrofibrosis of the knee. Contemp Orthop. 1985;10:67-72.
34 Eddy R.J., Petro J.A., Tomasek J.J. Evidence for the nonmuscle nature of the “myofibroblast” of granulation tissue and hypertropic scar. An immunofluorescence study. Am J Pathol. 1988;130:252-260.
35 Ekeland A., Vikne J. Treatment of acute combined knee instabilities and subsequent sport performance. Knee Surg Sports Traumatol Arthrosc. 1995;3:180-183.
36 Enneking W.F., Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54:973-985.
37 Eriksson E. Rehabilitation of muscle function after sport injury—major problem in sports medicine. Int J Sports Med. 1981;2:1-6.
38 Estes J.M., Vande Berg J.S., Adzick N.S., et al. Phenotypic and functional features of myofibroblasts in sheep fetal wounds. Differentiation. 1994;56:173-181.
39 Feller J.A., Webster K.E. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31:564-573.
40 Feller J.A., Webster K.E., Gavin B. Early post-operative morbidity following anterior cruciate ligament reconstruction: patellar tendon versus hamstring graft. Knee Surg Sports Traumatol Arthrosc. 2001;9:260-266.
41 Fisher S.E., Shelbourne K.D. Arthroscopic treatment of symptomatic extension block complicating anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:558-564.
42 Foster W., Li Y., Usas A., et al. Gamma interferon as an antifibrosis agent in skeletal muscle. J Orthop Res. 2003;21:798-804.
43 Fu F.H., Bennett C.H., Ma C.B., et al. Current trends in anterior cruciate ligament reconstruction. Part II. Operative procedures and clinical correlations. Am J Sports Med. 2000;28:124-130.
44 Fukui N., Fukuda A., Kojima K., et al. Suppression of fibrous adhesion by proteoglycan decorin. J Orthop Res. 2001;19:456-462.
45 Fukushima K., Badlani N., Usas A., et al. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29:394-402.
46 Gabbiani G., Hirschel B.J., Ryan G.B., et al. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972;135:719-734.
47 Gertel T.H., Lew W.D., Lewis J.L., et al. Effect of anterior cruciate ligament graft tensioning direction, magnitude, and flexion angle on knee biomechanics. Am J Sports Med. 1993;21:572-579.
48 Giri S.N., Hyde D.M., Braun R.K., et al. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 1997;54:1205-1216.
49 Graf B., Uhr F. Complications of intra-articular anterior cruciate reconstruction. Clin Sports Med. 1988;7:835-848.
50 Grant J.A., Mohtadi N.G., Maitland M.E., Zernicke R.F. Comparison of home versus physical therapy–supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33:1288-1297.
51 Haggmark T., Eriksson E. Cylinder or mobile cast brace after knee ligament surgery: a clinical analysis and morphologic and enzymatic studies of changes in the quadriceps muscle. Am J Sports Med. 1979;7:48-56.
52 Hagiwara Y., Chimoto E., Ando A., et al. Expression of type I collagen in the capsule of a contracture knee in a rat model. Ups J Med Sci. 2007;112:356-365.
53 Hagiwara Y., Chimoto E., Takahashi I., et al. Expression of transforming growth factor–beta1 and connective tissue growth factor in the capsule in a rat immobilized knee model. Ups J Med Sci. 2008;113:221-234.
54 Harner C.D., Irrgang J.J., Paul J., et al. Loss of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20:499-506.
55 Heming J.F., Rand J., Steiner M.E. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35:1708-1715.
56 Henriksson M., Rockborn P., Good L. Range of motion training in brace vs. plaster immobilization after anterior cruciate ligament reconstruction: a prospective randomized comparison with a 2-year follow-up. Scand J Med Sci Sports. 2002;12:73-80.
57 Hildebrand K.A., Zhang M., Germscheid N.M., et al. Cellular, matrix, and growth factor components of the joint capsule are modified early in the process of posttraumatic contracture formation in a rabbit model. Acta Orthop. 2008;79:116-125.
58 Hildebrand K.A., Zhang M., Hart D.A. Joint capsule matrix turnover in a rabbit model of chronic joint contractures: correlation with human contractures. J Orthop Res. 2006;24:1036-1043.
59 Howell S.M., Clark J.A. Tibial tunnel placement in anterior cruciate ligament reconstructions and graft impingement. Clin Orthop Relat Res. 1992;283:187-195.
60 Howell S.M., Clark J.A., Farley T.E. A rationale for predicting anterior cruciate graft impingement by the intercondylar roof. A magnetic resonance imaging study. Am J Sports Med. 1991;19:276-282.
61 Howell S.M., Taylor M.A. Failure of reconstruction of the anterior cruciate ligament due to impingement by the intercondylar roof. J Bone Joint Surg Am. 1993;75:1044-1055.
62 Hunter R.E., Mastrangelo J., Freeman J.R., et al. The impact of surgical timing on postoperative motion and stability following anterior cruciate ligament reconstruction. Arthroscopy. 1996;12:667-674.
62a Insall J., Salvati E. Patella position in the normal knee joint. Radiology. 1971;101:101-104.
63 Irrgang J.J., Harner C.D. Loss of motion following knee ligament reconstruction. Sports Med. 1995;19:150-159.
64 Isaka Y., Brees D.K., Ikegaya K., et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418-423.
65 Jackson D.W., Schaefer R.K. Cyclops syndrome: loss of extension following intra-articular anterior cruciate ligament reconstruction. Arthroscopy. 1990;6:171-178.
66 Johnson K.L., Fu F.H. Anterior cruciate ligament reconstruction: why do failures occur? In: Jackson D.W., editor. Instructional Course Lectures. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:391-406.
67 Johnson R.J., Eriksson E., Haggmark T., Pope M.H. Five- to ten-year follow-up evaluation after reconstruction of the anterior cruciate ligament. Clin Orthop Relat Res. 1984;183:122-140.
68 Johnston P., Chojnowski A.J., Davidson R.K., et al. A complete expression profile of matrix-degrading metalloproteinases in Dupuytren’s disease. J Hand Surg [Am]. 2007;32:343-351.
69 Klein W., Shah N., Gassen A. Arthroscopic management of postoperative arthrofibrosis of the knee joint: indication, technique, and results. Arthroscopy. 1994;10:591-597.
70 Li Y., Negishi S., Sakamoto M., et al. The use of relaxin improves healing in injured muscle. Ann N Y Acad Sci. 2005;1041:395-397.
71 Linclau L. Measuring patellar height. Acta Orthop Belg. 1984;50:70-74.
72 Lindenfeld T.N., Wojtys E.M., Husain A. Operative treatment of arthrofibrosis of the knee. J Bone Joint Surg Am. 1999;81:1772-1784.
73 Lobenhoffer H.P., Bosch U., Gerich T.G. Role of posterior capsulotomy for the treatment of extension deficits of the knee. Knee Surg Sports Traumatol Arthrosc. 1996;4:237-241.
74 Magit D., Wolff A., Sutton K., Medvecky M.J. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15:682-694.
75 Majors R.A., Woodfin B. Achieving full range of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1996;24:350-355.
76 Mariani P.P., Santori N., Rovere P., et al. Histological and structural study of the adhesive tissue in knee fibroarthrosis: a clinical-pathological correlation. Arthroscopy. 1997;13:313-318.
77 Markolf K.L., Hame S., Hunter D.M., et al. Effects of femoral tunnel placement on knee laxity and forces in an anterior cruciate ligament graft. J Orthop Res. 2002;20:1016-1024.
78 Marzo J.M., Bowen M.K., Warren R.F., et al. Intra-articular fibrous nodule as a cause of loss of extension following anterior cruciate ligament reconstruction. Arthroscopy. 1992;8:10-18.
79 Mayr H.O., Weig T.G., Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124:518-522.
80 McAllister D.R., Parker R.D., Cooper A.E., et al. Outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. Am J Sports Med. 1999;27:562-570.
81 Meighan A.A., Keating J.F., Will E. Outcome after reconstruction of the anterior cruciate ligament in athletic patients. A comparison of early versus delayed surgery. J Bone Joint Surg Br. 2003;85:521-524.
82 Melby A.3rd, Noble J.S., Askew M.J., et al. The effects of graft tensioning on the laxity and kinematics of the anterior cruciate ligament reconstructed knee. Arthroscopy. 1991;7:257-266.
83 Millett P.J., Wickiewicz T.L., Warren R.F. Motion loss after ligament injuries to the knee. Part II: prevention and treatment. Am J Sports Med. 2001;29:822-828.
84 Millett P.J., Wickiewicz T.L., Warren R.F. Motion loss after ligament injuries to the knee. Part I: causes. Am J Sports Med. 2001;29:664-675.
85 Millett P.J., Williams R.J., Wickiewicz T.L. Open debridement and soft tissue release as a salvage procedure for the severely arthrofibrotic knee. Am J Sports Med. 1999;27:552-561.
86 Mohtadi N.G., Webster-Bogaert S., Fowler P.J. Limitation of motion following anterior cruciate ligament reconstruction. A case-control study. Am J Sports Med. 1991;19:620-624. discussion 624–625
87 Murakami S., Muneta T., Ezura Y., et al. Quantitative analysis of synovial fibrosis in the infrapatellar fat pad before and after anterior cruciate ligament reconstruction. Am J Sports Med. 1997;25:29-34.
88 Murakami S., Muneta T., Furuya K., et al. Immunohistologic analysis of synovium in infrapatellar fat pad after anterior cruciate ligament injury. Am J Sports Med. 1995;23:763-768.
89 Nabors E.D., Richmond J.C., Vannah W.M., McConville O.R. Anterior cruciate ligament graft tensioning in full extension. Am J Sports Med. 1995;23:488-492.
90 Negishi S., Li Y., Usas A., et al. The effect of relaxin treatment on skeletal muscle injuries. Am J Sports Med. 2005;33:1816-1824.
91 Noyes F.R., Barber S.D. The effect of a ligament-augmentation device on allograft reconstructions for chronic ruptures of the anterior cruciate ligament. J Bone Joint Surg Am. 1992;74:960-973.
92 Noyes F.R., Barber S.D. The effect of an extra-articular procedure on allograft reconstructions for chronic ruptures of the anterior cruciate ligament. J Bone Joint Surg Am. 1991;73:882-892.
93 Noyes F.R., Barber S.D., Mangine R.E. Bone-patellar ligament-bone and fascia lata allografts for reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1990;72:1125-1136.
94 Noyes F.R., Barber-Westin S. Posterior cruciate ligament replacement with a two-strand quadriceps tendon–patellar bone autograft and a tibial inlay technique. J Bone Joint Surg Am. 2005;87:1241-1252.
95 Noyes F.R., Barber-Westin S.D. A comparison of results in acute and chronic anterior cruciate ligament ruptures of arthroscopically assisted autogenous patellar tendon reconstruction. Am J Sports Med. 1997;25:460-471.
96 Noyes F.R., Barber-Westin S.D. Posterolateral knee reconstruction with an anatomical bone–patellar tendon–bone reconstruction of the fibular collateral ligament. Am J Sports Med. 2007;35:259-273.
97 Noyes F.R., Barber-Westin S.D. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25:769-778.
98 Noyes F.R., Barber-Westin S.D. The treatment of acute combined ruptures of the anterior cruciate and medial ligaments of the knee. Am J Sports Med. 1995;23:380-389.
99 Noyes F.R., Barber-Westin S.D., Hewett T.E. High tibial osteotomy and ligament reconstruction for varus angulated anterior cruciate ligament–deficient knees. Am J Sports Med. 2000;28:282-296.
100 Noyes F.R., Barber-Westin S.D., Rankin M. Meniscal transplantation in symptomatic patients less than fifty years old. J Bone Joint Surg Am. 2004;86:1392-1404.
101 Noyes F.R., Berrios-Torres S., Barber-Westin S.D., Heckmann T.P. Prevention of permanent arthrofibrosis after anterior cruciate ligament reconstruction alone or combined with associated procedures: a prospective study in 443 knees. Knee Surg Sports Traumatol Arthrosc. 2000;8:196-206.
102 Noyes F.R., Mangine R.E., Barber S.D. The early treatment of motion complications after reconstruction of the anterior cruciate ligament. Clin Orthop. 1992;277:217-228.
103 Noyes F.R., Mayfield W., Barber-Westin S.D., et al. Opening wedge high tibial osteotomy: an operative technique and rehabilitation program to decrease complications and promote early union and function. Am J Sports Med. 2006;34:1262-1273.
104 Noyes F.R., Torvik P.J., Hyde W.B., DeLucas J.L. Biomechanics of ligament failure. II. An analysis of immobilization, exercise, and reconditioning effects in primates. J Bone Joint Surg Am. 1974;56:1406-1418.
105 Noyes F.R., Wojtys E.M. The early recognition, diagnosis and treatment of the patella infera syndrome. In: Tullos H.S., editor. Instructional Course Lectures. Rosemont, IL: AAOS; 1991:233-247.
106 Noyes F.R., Wojtys E.M., Marshall M.T. The early diagnosis and treatment of developmental patella infera syndrome. Clin Orthop. 1991;265:241-252.
107 O’Brien S.J., Ngeow J., Gibney M.A., et al. Reflex sympathetic dystrophy of the knee. Causes, diagnosis, and treatment. Am J Sports Med. 1995;23:655-659.
108 O’Driscoll S.W., Kumar A., Salter R.B. The effect of continuous passive motion on the clearance of a hemarthrosis from a synovial joint. An experimental investigation in the rabbit. Clin Orthop Relat Res. 1983;176:305-311.
109 Paillard T. Combined application of neuromuscular electrical stimulation and voluntary muscular contractions. Sports Med. 2008;38:161-177.
110 Palmieri-Smith R.M., Kreinbrink J., Ashton-Miller J.A., Wojtys E.M. Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med. 2007;35:1269-1275.
111 Paulos L.E., Rosenberg T.D., Drawbert J., et al. Infrapatellar contracture syndrome. An unrecognized cause of knee stiffness with patella entrapment and patella infera. Am J Sports Med. 1987;15:331-341.
112 Paulos L.E., Wnorowski D.C., Greenwald A.E. Infrapatellar contracture syndrome. Diagnosis, treatment, and long-term follow-up. Am J Sports Med. 1994;22:440-449.
113 Perkins G. Rest and movement. J Bone Joint Surg Br. 1953;35:521-539.
114 Perry J., Antonelli D., Ford W. Analysis of knee joint forces during flexed-knee stance. J Bone Joint Surg Am. 1975;57:961-967.
115 Petsche T.S., Hutchinson M.R. Loss of extension after reconstruction of the anterior cruciate ligament. J Am Acad Orthop Surg. 1999;7:119-127.
116 Reider B., Belniak R.M., Preiskorn D. Arthroscopic arthrolysis for flexion contracture following intra-articular reconstruction of the anterior cruciate ligament. Arthroscopy. 1996;12:165-173.
117 Risberg M.A., Holm I., Steen H., et al. The effect of knee bracing after anterior cruciate ligament reconstruction. A prospective, randomized study with two years’ follow-up. Am J Sports Med. 1999;27:76-83.
118 Roberts A.B., Sporn M.B. The transforming growth factors–β. Sporn M.B., Roberts A.B., editors. Peptide Growth Factors and Their Receptors. New York: Springer-Verlag; 1990;Vol. 95:419-472. of Handbook of Experimental Pharmacology
119 Romano V.M., Graf B.K., Keene J.S., Lange R.H. Anterior cruciate ligament reconstruction. The effect of tibial tunnel placement on range of motion. Am J Sports Med. 1993;21:415-418.
120 Rowe P.J., Myles C.M., Walker C., Nutton R. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life? Gait Posture. 2000;12:143-155.
121 Sachs R.A., Daniel D.M., Stone M.L., Garfein R.F. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17:760-765.
122 Salter R.B. The biologic concept of continuous passive motion of synovial joints. The first 18 years of basic research and its clinical application. Clin Orthop Relat Res. 1989;242:12-25.
123 Salter R.B., Simmonds D.F., Malcolm B.W., et al. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980;62:1232-1251.
124 Satish L., Laframboise W.W., O’Gorman D.B., et al. Identification of differentially expressed genes in fibroblasts derived from patients with Dupuytren’s Contracture. BMC Med Genomics. 2008;1:10.
125 Schulz A.P., Gotze S., Schmidt H.G., et al. Septic arthritis of the knee after anterior cruciate ligament surgery: a stage-adapted treatment regimen. Am J Sports Med. 2007;35:1064-1069.
126 Shanker V.S., Gadikoppula S., Loeffler M.D. Post-traumatic osteoma of tibial insertion of medial collateral ligament of knee joint. Br J Sports Med. 1998;32:73-74.
127 Shapiro M.S., Freedman E.L. Allograft reconstruction of the anterior and posterior cruciate ligaments after traumatic knee dislocation. Am J Sports Med. 1995;23:580-587.
128 Shelbourne K.D., Baele J.R. Treatment of combined anterior cruciate ligament and medial collateral ligament injuries. Am J Knee Surg. 1988;1:56-58.
129 Shelbourne K.D., Foulk D.A. Timing of surgery in acute anterior cruciate ligament tears on the return of quadriceps muscle strength after reconstruction using an autogenous patellar tendon graft. Am J Sports Med. 1995;23:686-689.
130 Shelbourne K.D., Johnson G.E. Outpatient surgical management of arthrofibrosis after anterior cruciate ligament surgery. Am J Sports Med. 1994;22:192-197.
131 Shelbourne K.D., Patel D.V. Treatment of limited motion after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1999;7:85-92.
132 Shelbourne K.D., Patel D.V., Martini D.J. Classification and management of arthrofibrosis of the knee after anterior cruciate ligament reconstruction. Am J Sports Med. 1996;24:857-862.
133 Shelbourne K.D., Porter D.A. Anterior cruciate ligament–medial collateral ligament injury: nonoperative management of medial collateral ligament tears with anterior cruciate ligament reconstruction. A preliminary report. Am J Sports Med. 1992;20:283-286.
134 Shelbourne K.D., Wilckens J.H., Mollabashy A., DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19:332-336.
135 Simmons R., Howell S.M., Hull M.L. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. J Bone Joint Surg Am. 2003;85:1018-1029.
136 Sisto D.J., Warren R.F. Complete knee dislocation. A follow-up study of operative treatment. Clin Orthop Relat Res. 1985;198:94-101.
137 Skutek M., van Griensven M., Zeichen J., et al. Cyclic mechanical stretching enhances secretion of interleukin 6 in human tendon fibroblasts. Knee Surg Sports Traumatol Arthrosc. 2001;9:322-326.
138 Skutek M., van Griensven M., Zeichen J., et al. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur J Appl Physiol. 2001;86:48-52.
139 Skyhar M.J., Danzig L.A., Hargens A.R., Akeson W.H. Nutrition of the anterior cruciate ligament. Effects of continuous passive motion. Am J Sports Med. 1985;3:415-418.
140 Spencer J.D., Hayes K.C., Alexander I.J. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65:171-177.
141 Sprague N.F., O’Connor R.L., Fox J.M. Arthroscopic treatment of postoperative knee fibroarthrosis. Clin Orthop Relat Res. 1982;166:165-172.
142 Steadman J.R., Burns T.P., Peloza J., et al. Surgical treatment of arthrofibrosis of the knee. J Orthop Tech. 1993;1:119-128.
143 Sterett W.I., Hutton K.S., Briggs K.K., Steadman J.R. Decreased range of motion following acute versus chronic anterior cruciate ligament reconstruction. Orthopedics. 2003;26:151-154.
144 Strum G.M., Friedman M.J., Fox J.M., et al. Acute anterior cruciate ligament reconstruction. Analysis of complications. Clin Orthop Relat Res. 1990;253:184-189.
145 Torry M.R., Decker M.J., Millett P.J., et al. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4:1-8.
146 Torry M.R., Decker M.J., Viola R.W., et al. Intra-articular knee joint effusion induces quadriceps avoidance gait patterns. Clin Biomech (Bristol, Avon). 2000;15:147-159.
147 Unterhauser F.N., Bosch U., Zeichen J., Weiler A. Alpha-smooth muscle actin containing contractile fibroblastic cells in human knee arthrofibrosis tissue. Winner of the AGA-DonJoy Award 2003. Arch Orthop Trauma Surg. 2004;124:585-591.
148 Van Tongel A., Stuyck J., Bellemans J., Vandenneucker H. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: a retrospective analysis of incidence, management and outcome. Am J Sports Med. 2007;35:1059-1063.
149 Vaquero J., Vidal C., Medina E., Baena J. Arthroscopic lysis in knee arthrofibrosis. Arthroscopy. 1993;9:691-694.
150 Wang J.C., Shapiro M.S. Pellegrini-Stieda syndrome. Am J Orthop. 1995;24:493-497.
151 Wang J.H., Zhao J.Z., He Y.H. A new treatment strategy for severe arthrofibrosis of the knee. A review of twenty-two cases. J Bone Joint Surg Am. 2006;88:1245-1250.
152 Wasilewski S.A., Covall D.J., Cohen S. Effect of surgical timing on recovery and associated injuries after anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:338-342.
153 Williams R.J.3rd, Laurencin C.T., Warren R.F., et al. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Diagnosis and management. Am J Sports Med. 1997;25:261-267.
154 Yaru N.C., Daniel D.M., Penner D. The effect of tibial attachment site on graft impingement in an anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20:217-220.
155 Zeichen J., van Griensven M., Albers I., et al. Immunohistochemical localization of collagen VI in arthrofibrosis. Arch Orthop Trauma Surg. 1999;119:315-318.