Chapter 108 Presurgical Evaluation for Epilepsy Including Intracranial Electrodes
Medically Intractable Epilepsy
As many as 25% to 30% of epileptic patients fail to respond to adequate antiepileptic treatment.1 Medically intractable (or refractory) epilepsy is commonly conceived as that which occurs when satisfactory seizure control cannot be achieved with any of the potentially available effective antiepileptic drugs (AEDs), alone or in combination, at doses or levels not associated with unacceptable side effects.2 On the other hand, surgery has a success rate of about 75% (depending on the site of surgery, the type of surgery performed, and the underlying etiology).3,4 In spite of this, surgical treatment of medically intractable epilepsy is often delayed or withheld. Referral for epilepsy surgery may take 20 to 25 years, resulting in a number of avoidable seizure-related deaths, including drowning, motor vehicle accident, fatal status epilepticus, and sudden unexpected death in eplilepsy (SUDEP).5 In children, appropriate timing for surgical procedures is critical, as lack of seizure control may interfere with consolidation of cognitive and motor functions in the developing brain.6
Other causes of poor response to medical treatment are noncompliance and presence of pseudoepileptic seizures alone or combined with epileptic ones, as well as incorrect classification of seizures and therefore incorrect pharmacologic treatment.7 Therefore, the first step in evaluating surgical candidates is to confirm that the proper diagnosis has been identified and to determine that appropriate medical treatment has been utilized.
Patients’ Perceptions
Patients’ satisfaction with achieved medical treatment results often depends on professional and social circumstances, as well as the type of seizures and the time of day in which they occur.7 Some patients seek surgical treatment for reasons other than medical intractability: intolerable side effects of AEDs, women who desire to get pregnant but are concerned about potential teratogenic medication effects, and avoidance of the social stigmata of the disease are some of the reasons patients express in requesting surgery. Self-assessment of quality of life is an important step in evaluating the patient’s perception of their disease. At our clinic, we use the questionnaires QOLIE-31 for adults8,9 and QOLIE-48-AD for adolescents.10,11
Epilepsy surgery has three main goals: eliminate or decrease epileptic seizures, prevent neurologic deficit due to surgery, and improve the quality of life. In order to achieve these goals, a multidisciplinary team is required to solve the surgical questions: Where do the patient’s seizures start? Are there functional or eloquent areas involved? What is the prognosis after surgery? Answering these questions allows decisions regarding what type of surgery is indicated and how to customize the procedure to meet the patient’s needs. Currently, there are many diagnostic studies that can be performed, but it is important to remember that there is no single test that can be considered the gold standard for making a diagnosis or predicting the outcome. It is the clinician’s ability to correctly interpret and assess the quality and concordance between studies that usually leads to a correct diagnosis and successful surgery.12 The diagnostic workup can be divided into noninvasive and invasive phases.
Phase 1 Testing: Noninvasive Studies
Clinical Diagnosis: Seizure Description
Specific auras have been described in different types of seizures. For example, the manifestations of fear and ascending epigastric sensation suggest a mesial temporal epilepsy; simple visual auras suggest an occipital focus; levitation, somatosensory areas; and sympathetic symptoms such as perspiration, difficulty in swallowing, and salivation to an insular focus.13
The sequence of symptoms that the patient has is also important; epigastric and psychic auras followed by behavioral arrest and automatisms (ocular, oral, hands, ambulatory) indicate a mesial temporal onset of seizures.14 A different sequence or visual, auditory, and somatic auras at the beginning point to extratemporal foci with propagation to the mesial temporal lobe.15 Dystonic positions of the hands as an initial symptom can implicate the contralateral frontal area. If these findings occur late in the seizure event, after behavioral arrest and automatisms, they lose their localizing precision. If a tonic clonic seizure occurs after auras or the symptoms previously mentioned, it is probably the result of propagation of a partial seizure, but if it is not preceded at all by any symptom, then the consideration of a primary generalized seizure should be entertained.
Surface Electroencephalogram
Continuous Video/EEG Monitoring
This technique consists of recording simultaneously EEG as well as the behavior of the patient with a video camera for a period that can last up to several days.17 This method allows for confirmation of the clinical type of seizure and can correlate it with the onset of abnormal EEG activity, particularly ictal activity. It can also allow for the detection of cases with pseudoepileptic seizures.
Neuropsychological Assessment in Surgical Candidates
The overall objective of neuropsychological assessment in candidates for epilepsy surgery is to seek evidence of cognitive deficits and their relationship with other studies (clinical and electrophysiological).18–20
Hemispheric Language Dominance
Determining the lateralization of language can have a significant impact in surgical planning. It has been established that 95% to 99% of right-handed subjects and 15% to 19% of left-handed have a left cerebral hemispheric dominance for language.21,22 Due to the small percentage of subjects who may have a right hemisphere dominance, is very important to clarify each case.23,24 For example, a right-handed patient who presents with difficulties of language might be mistakenly classified as having a left hemisphere dysfunction when in fact he could have dysphasia of the dominant hemisphere, which is not necessarily the left.
For decades, the Wada test has been the gold standard for determining hemispheric language dominance (HLD).21,25–27 Recently, a growing number of publications report that other techniques such as functional magnetic resonance imaging (fMRI) may have the same degree of reliability,27–29 without the disadvantages of cost, risk, and complexity of the technique.30 New noninvasive techniques are evolving to substitute Wada test, however, they still present limitations.21,28,29,31
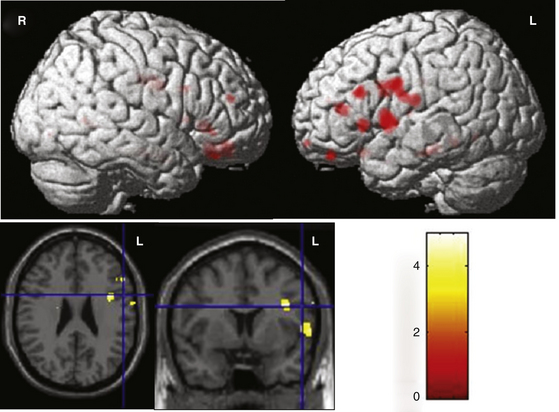
To determine the HLD using fMRI, most authors use the task of evoking words silently, based on a semantic category (e.g., names of animals) to activate temporal regions,32 or a phonologic category (words beginning with the same letter) for activating frontal regions of the dominant hemisphere33 (Fig. 108-1).
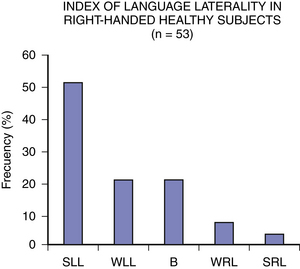
The dichotic listening technique (DLT) has also been proved useful in determining the HLD.34,35 It consists of simultaneous presentation of two words (one in each ear) with the same characteristics in terms of sound, number of syllables and of common use, with the intention to present some competition for processing stimuli between the two hemispheres. With this methodology it has been shown that the majority of right and left-handed subjects have right ear advantages as a reflection of a left HLD.34 Fig. 108-2 shows the variability in the index of HLD according to the dichotic listening test in right-handed healthy subjects. The lateralization index for language (LI) is a predictor of postoperative verbal memory deficit as shown by some studies.36
Lateralization of Memory Deficits
Lateralization of memory deficits (LMD) is another objective of preoperative neuropsychological assessment. Table 108-137 presents some of the available memory tests. At our clinic, we use the Battery of Learning and Memory and the Visuospatial Learning and Memory tests. When evidence is found for a memory deficit in the same hemisphere where the epileptic zone has been localized through clinical, EEG, and imaging data, the decision for surgery is strengthened; otherwise, the complete testing described in Table 108-1 is applied. Evaluation of memory is performed by well-established tests that vary slightly from one center to another.37 In approximately 50% of patients it is not possible to determine the lateralization of memory deficit through neuropsychological studies alone;18 however, that percentage decreases when the information provided by neuropsychological testing is concordant with other studies. A major problem in LMD is that there is a high error rate due to language dominance, as discussed above; that is, verbal memory deficit does not always indicate dysfunction of the left cerebral hemisphere.
| General BatteriesWechsler Intelligence ScaleHasltead-Reitan BatteryHemispheric lateralizationWada TestDichotic ListeningFunctional Magnetic Resonance ImagingAttentionTrail Making TestCancelation TestLanguageBoston Diagnostic Aphasia ExaminationBoston Naming TestToken TestVisuospatial and PerceptualHooper Visual Organization TestConstructional ApraxiaBenton Judgement of Line OrientationMotor and Reaction TimeFinger OscillationHand DynamometerGrooved Pegboard | Problem Solving, FlexibilityWisconsin Card Sorting TestWord FluencyStroop TestBattery of Learning and MemoryWechsler Memory ScaleVerbal Learning and MemoryStory RecallPaired Word LearningRey Auditory Verbal Learning TestCalifornia Verbal Learning TestVisuospatial Learning and MemorySimple Designs RecallRey Osterrieth Complex FigureBenton Visual Retention TestOthersBeck Depression InventoryQuality of Life in Epilepsia (QOLIE-31)International Neuropsyquiatric Interview (MINI) |
Imaging Studies
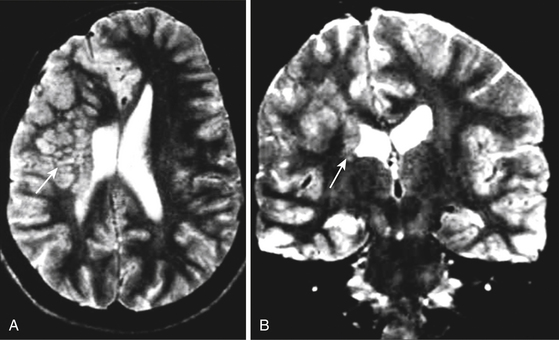
The best imaging method to study intractable epilepsy is magnetic resonance imaging (MRI) because of its excellent spatial resolution employing basic sequences such as T1WI, T2WI, and FLAIR. It has high sensitivity for characterizing signal intensity of normal and pathologic brain tissue and very good specificity for neoplastic, vascular, atrophic, dysplastic, infectious, and degenerative etiologies. MRI allows the differentiation of edema, demyelinating diseases, heterotopic gray matter (Fig. 108-3), and space-occupying lesions involving anatomic structures causing epilepsy.
In a series of 40 patients with refractory focal epilepsy, Knake et al.38 found that studies with 3T phased-array surface coil (PA-MRI) yielded additional diagnostic information in 48% of the studies (19/40), when compared to routine clinical MRI performed at 1.5T. In the subgroup of patients with previous 1.5T MRIs interpreted as normal, 3T PA-MRI resulted in the detection of a new lesion in 65% of patients (15/23). In the subgroup of 15 patients with known lesions, 3T PA-MRI better defined the lesion in 33% (5/15). On the other hand, Zijlmans et al.39 stated that patients studied with 1.5T show loss of cerebral tissue and mesial temporal sclerosis better than at 3T, while those patients with cerebral dysplasias are better studied with 3T. High-resolution 3T MRI (HR 3T MRI) and surface coils applied over the suspected epileptogenic zone are useful to detect lesions in patients suffering refractory epilepsy due to cortical developmental malformations (CDM).40
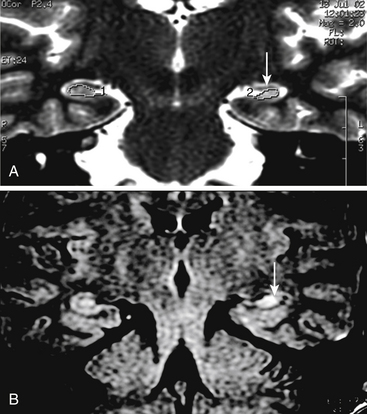
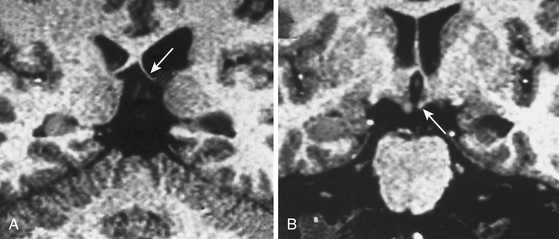
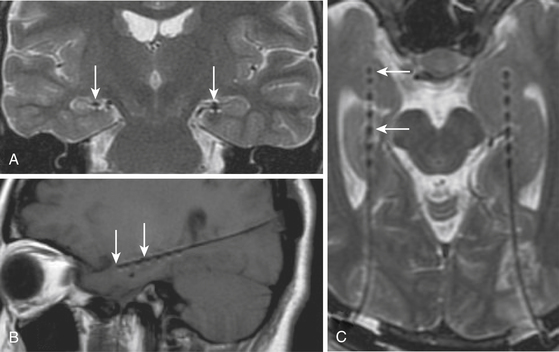
In order to optimize the diagnosis of mesial temporal sclerosis and the severity of hippocampal atrophy, it is important to obtain a precise hippocampal evaluation with axial and coronal images in MRI. Axial images oriented perpendicular to the axis of the clivus, 2-mm thick without any gaps, provide an adequate view of the hippocampal structures. Coronal images are taken perpendicular to axial sections. Hippocampal areas can be evaluated with these regions of interest (ROI) measurement to define possible differences in volumetric areas indicating hippocampal atrophy (Fig. 108-4A). Flair sequences offer an objective method to evaluate hippocampal area signal intensity in order to define hippocampal atrophy and mesial temporal sclerosis (Fig. 108-4B) that may be associated to ipsilateral mammillary body and fornix atrophy (Fig. 108-5). Successful surgery is possible even with normal MRI but it requires compelling clinical and electrophysiologic evidence of seizure onset.
Magnetic Resonance Spectroscopy
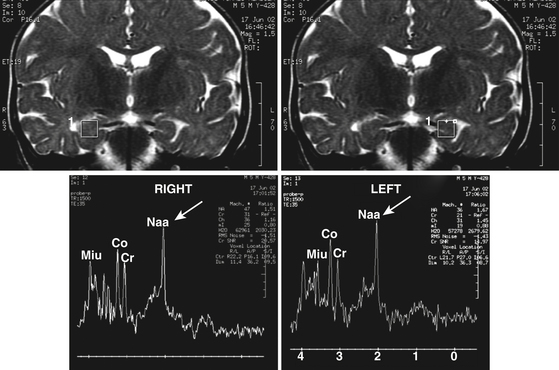
While MRI is primarily employed to obtain structural images of the brain, magnetic resonance spectroscopy (MRS) can assess regional cell loss through determination of the concentrations of intermediate metabolites, including glutamate and glutamine.41 This noninvasive technique can also measure other metabolites of cellular activity such as N-acetyl-aspartate (NAA), creatine (Cr), and choline (Ch) that give indirect information regarding cellularity of the ROI. MRS provides also a broad range of useful functional information such as cerebral concentrations of GABA and glutamate, usually associated with an increase in pH and inorganic phosphate and reduction of phosphate monoesters. Several studies in patients with epilepsy have documented neuronal loss and alterations in energy and lipid metabolism, acid–base homeostasis, and amino acid neurotransmitter metabolism.42 Proton MRS imaging studies consistently demonstrate decreased NAA in the epileptogenic temporal lobe (Fig. 108-6).
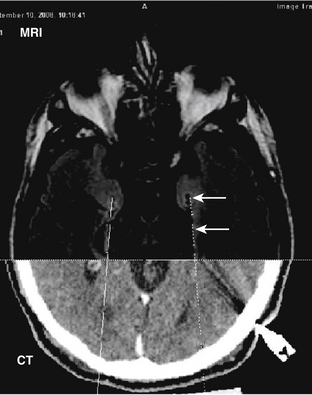
Functional magnetic resonance imaging (fMRI) is a noninvasive functional brain mapping technique assessed on blood oxygen level–dependent (BOLD) signal with echo planar images (EPI) obtained during T1-weighted imaging MRI studies. It offers a map of physiologic and metabolic functions of cerebral activity during ictal and interictal discharges on images with a spatial resolution of a few millimeters and a temporal resolution of a few seconds.43,44 One of the first clinical applications of fMR was presurgical evaluation of cerebral function in patients with epilepsy and neoplastic lesions nearby eloquent areas. This technique detects the localization of the functional areas of primary sensorimotor cortex or language zones prior to surgery. fMRI offers noninvasive preoperative brain mapping with high sensitivity to detect cerebral lesions and defining the border between lesion and normal functional cortex. It may also predict possible deficits in motor, sensory, or language functions due to expansion of the lesion or surgical procedures. Therefore, it offers information to help therapeutic decisions relative to risk–benefit ratio of the treatment. In patients with refractory epilepsy fMR has been used to evaluate the resection feasibility, planning the surgical procedure and better patient selection for invasive mapping45 (Fig. 108-7). Functional MRI holds great promise as a powerful tool in memory evaluation.31 fMRI aids in the localization of language and motor function of candidates for epilepsy surgery, and has up to a 90% concordance with WADA test (Fig. 108-1).46
Diffusion Tensor Imaging
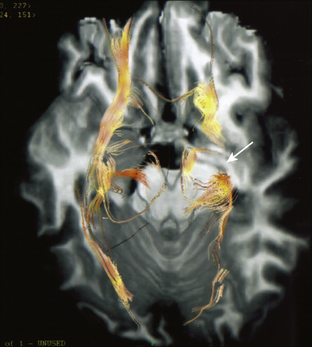
Diffusion tensor imaging (DTI or tractography) allows the detection and examination of the integrity and orientation of discrete white matter fiber bundles not optimally evaluated with conventional MRI. Recently DTI/tractography has been applied to the study of epilepsy and has demonstrated diffusion changes in gray and white matter. DTI gives information on the path of axonic fibers in the white matter as a support to surgical planning.47 It is particularly useful in determining the location of visual pathways in cases proposed for mesial temporal lobe resection and in assessing motor pathways for lesions in the perirolandic area (Fig. 108-8). Intractable TLE is marked by widespread involvement of fiber tracts and asymmetries of white matter fibers, with lower fiber fractional anisotropy (FA) ipsilateral versus contralateral to the seizure focus.
Positron Emission Tomography
The primary use of PET in epilepsy is presurgical localization of the epileptic focus in patients with complex partial seizures. (CPS). It also quantifies cerebral metabolism, blood flow, oxygen extraction, and receptor kinetics in an attempt to understand the pathophysiology of epilepsy.47 The positron-emitting radionuclides used most commonly in PET imaging are oxygen-15 (15O), nitrogen-13 (13N), carbon-11 (11C), and fluorine-18 (18F).48 Assessment of glucose metabolism using 18F-2-deoxyglucose (FDG) and cerebral blood flow using H215O (O15) are the two most common physiologic processes measured by PET. Most PET images are the result of time-dependent integration of metabolic activity. For FDG studies, this varies from 30 to 45 minutes.49 FDG PET has the highest sensitivity and specificity in nonlesional epilepsy by acting as a combined measure of metabolism and anatomy.50
Single-Photon Emission Computed Tomography
Ictal SPECT has been extensively studied in patients with partial epilepsy driven primarily by the need for preoperative localization, the volume of patients affected, and the type of seizure activity. The major limitation to ictal SPECT is the need to inject the tracer within a minute or so of seizure onset. In the temporal lobe there is a pattern of markedly increased perfusion accompanying the ictus, involving the mesial and often to a greater extent the anterolateral neocortical structures.51 Perfusion follows changes in metabolism during seizures. Imaging interictal and ictal brain-blood flow with SPECT is a feasible alternative to PET, requiring less capital outlay, using inexpensive perfusion tracers, and being readily available.52
Phase II Testing: Invasive Studies
1. Patients without structural lesions in imaging studies.
2. Patients with bilateral homologous interictal discharges and/or alternative onset of ictal activity in both hemispheres in video EEG studies.
3. Discordance among clinical seizure type, EEG, and image studies on the localization of the epileptic zone (EZ).
4. EZ located nearby eloquent areas which may compromise surgical resection and/or pose a risk of a neurologic deficit.
Sphenoidal Electrodes
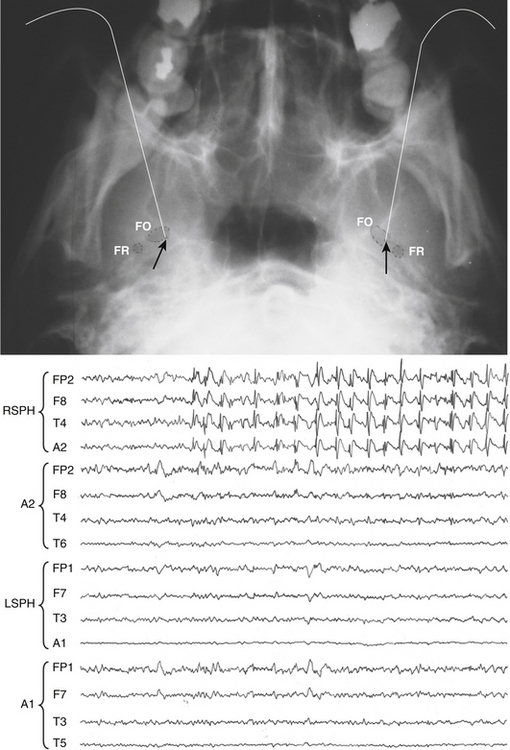
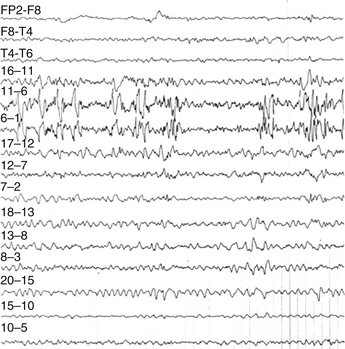
Sphenoidal electrodes help to define the side of the mesiotemporal epileptic foci (MTE) in cases of equivocal scalp EEG recordings. They are sometimes used to rule out MTE in cases with pseudo epileptic seizures (Fig. 108-9). The reason for limiting the use of sphenoidal electrodes is that unfortunately EEG recordings can be contaminated with various artifacts and are uncomfortable for the patient.
Intracranial Electrodes
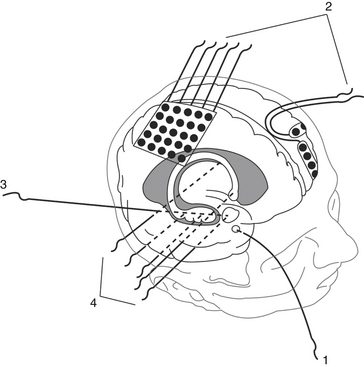
Intracranial electrodes are used to explore cortical and subcortical structures by recording spontaneous and evoked EEG activity and applying electric current to obtain clinical and EEG responses. Intracranial electrodes may be of two types (Fig. 108-10):
Plate Electrodes
We do not recommend burr-holes to introduce strips of electrodes over the cerebral surface since they provide a poor spatial resolution for epileptic foci location and brain mapping.53 For both strips and grids, subdural bridging veins or adhesions could constitute a problem for accurate placement.54
Deep Brain Electrodes
Deep brain electrodes are fine tubular (0.8–1.3 mm in diameter) devices with multiple contacts along their trajectories. They are inserted in different cortical and subcortical anatomic structures using stereotactic techniques, guided by different imaging modalities (x-ray ventriculography, angiography, CT, and MRI), and coupled with anatomic atlases by a fusion imaging software. Imaging fusion allows elaborating virtual trajectories to reach the targets precisely and traversing non vascular territories55 (Fig. 108-11). Deep brain electrodes may be inserted through parasagittal frontal, parietal, and occipital approach, or perpendicular to the sagittal plane (orthogonal approach).
In the parasagittal approach, electrodes usually traverse greater distances to get to the subcortical targets; however, their trajectories may be easily planned to avoid vascular areas. This approach is used to explore longitudinal anatomic areas, such as the amygdala-hippocampal complex when bilateral independent epileptic foci in this region are suspected56 (Fig. 108-12). Frontal and parietal parasagittal approaches are used to explore the insular cortex, avoiding the risk of orthogonal placement traversing the densely vascular Sylvian fissure.13,57
An orthogonal approach allows a better definition of the EZ and areas involved in the propagation of the epileptic seizure, either unilateral or bilateral. It is the most adequate method to be used in cases where multilobar participation in seizure genesis is suspected, such as in frontal-temporal, frontal-parietal, and temporal-occipital foci, as an almost unrestricted number of electrodes may be inserted with low morbidity.58
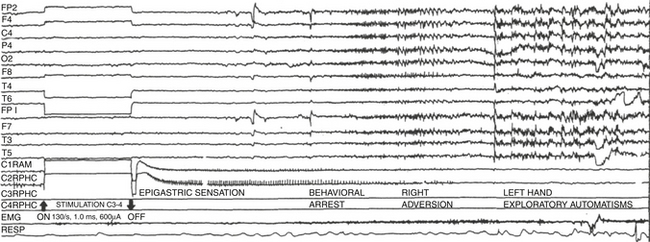
Through intracranial electrodes, we record EEG from different structures of the brain that may be involved in the genesis and propagation of spontaneous seizures.58 The advantage of recording with intracranial electrodes is that they are not subject to physiologic artifacts such as movement and sweating, they are much closer to the seizure origin, and their spatial resolution is high and can show ictal onsets with specificity.4 When EEG recordings with intracranial electrodes are performed with simultaneous videotelemetry, we can determine the relationship between behavioral changes and EEG data with greater accuracy (Fig. 108-13 and Video 108-1).
Interictal Data
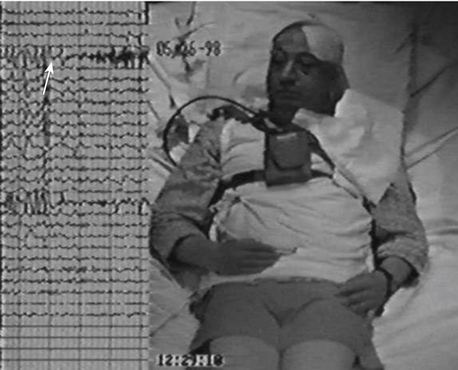
Figure 108-13 shows the interictal spikes that are characteristic of the epileptic tissue. Unlike surface EEG, interictal elements detected with intracranial electrodes are very conspicuous, with high amplitudes. The discharges are acute and fast. It is frequent to find interictal spikes in several contacts and as such, it is difficult to base our focus localization without ictal activity, although sometimes they have high amplitude and show phase reversal which suggests the precise focus location as seen in the figure. Sleep studies are also useful since during REM sleep, the interictal spikes are more localized to the seizure onset.
Ictal activity is the gold standard to define focus location. In the hippocampus, onset of seizures is anticipated by either desynchronized fast activity occurring in the epileptic focus59–62 or a high-voltage slow pattern.63 When ictal onset is found in one or two contacts, it is considered a focal onset; if on the other hand, ictal activity onset is observed in more than three contacts simultaneously, it is not a focal but a regional onset.64 If, as shown in Fig. 108-13 and Video 108-1, the ictal EEG onset is focal and precedes for several seconds the behavioral changes, the postsurgical results regarding seizure reduction are better.12
Epilepsy centers are now including the identification of high frequency oscillations (HFOs), known as ripples (80–250 Hz) and fast ripples (250–500 Hz) as part of the process of identification of the epileptic focus. Total rates of HFOs are significantly higher in the seizure-onset zone than outside, their rate either increases or decreases before the onset of seizures and they extend beyond the epileptic focus, although their pattern has not been described. There appears to be no association between the extent of the area from where HFO are recorded and seizure count but an association has been found between the rate of HFO and seizure count.65–67
On the other hand, electric current applied to different contacts of intracranial electrodes may serve to identify the EZ as the place with lowest threshold to induce an EEG after discharge, possibly accompanied by a clinical seizure similar to the spontaneous seizures the patient presents (Fig. 108-15).68
Intracranial Electrodes and Brain Mapping
Electric current applied through intracranial electrodes is used to identify the location of eloquent areas. BM is carried out with the patient awake and enables the mapping of motor and speech areas.69 The spatial precision is excellent particularly with low intensities.
Two types of pulses are used to map eloquent areas: low frequency (1–2 Hz), high amplitude (1.0–15.0 mA), and long-pulse duration (1.0–3.0 msec) electric current is used to induce focal muscular contractions in contralateral extremities when applied to motor cortex. High-frequency (50–130 Hz), low-amplitude (0.5–10.0 mA), and short pulse-width (0.21–1.0 msec) electric current interferes with spontaneous ongoing activity, such as speech or voluntary movements, or elicit sensory changes in the form of paresthesias.70
Intraoperative versus Extraoperative Electrocorticography and Brain Mapping
Electroencephalogram recording and brain mapping (BM) may be performed just before resection of the EZ or the craniotomy for the electrode implantation can be performed in a first operation and resection of EZ as a separate procedure.71
1. Time available to record electrical cortical activity is limited during surgical procedures, resulting on a limited chance to record a seizure. More often one records only interictal epileptiform activity.72
2. The neurophysiologist must read and interpret the EEG recordings and BM as they are obtained, thus mandating the need for both the equipment and neurophysiologist to be either in the operating room or in an area where close communication with the surgeon is possible.72 If cognitive functions or language has to be explored, the presence of the neuropsychology team is also required. This often results in crowded operating areas where the sterility of the procedure is compromised and accidents are likely to occur (e.g., tripping over one of the many cables).
3. Spontaneous seizures seldom occur during intraoperative ECoG, so important decisions have to be taken based on interictal recordings or on seizures induced by a convulsing drug. Seizures induced with pentilenetetrazol or other methods do not necessarily originate at the epileptic focus. Besides, spontaneous seizures that may occur during the intraoperative electrocorticography are not necessarily the ones that the patient presents most frequently.
4. Intraoperative ECoG recording is usually done with an awake patient because of concerns of interference of anesthetic agents with the recordings. It is noteworthy that sufentanil, fentanyl, alfentanil, propofol, and methohexital have been reported to produce epileptiform changes on ECoG, whereas propofol, halothane, barbiturates, and benzodiazepines may suppress the epileptic activity.72,73
5. Awake craniotomies are particularly complicated in children and in developmentally disabled, anxious, or even tired patients. The surgical experience can generate unnecessary stress and poor cooperation that is only augmented by the cortical mapping phase of the procedure.
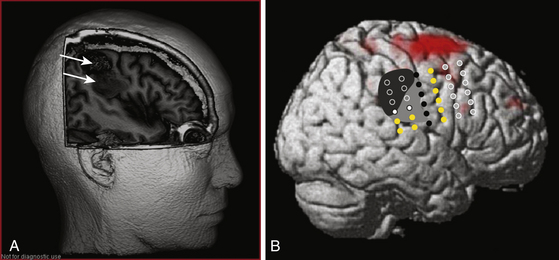
Surgical Technique for Subdural Electrode Placement
Standard surgical techniques are used; general anesthesia is undertaken. Bony surfaces should be padded as necessary; microsurgical techniques are seldom necessary so head fixation is not mandatory. The skin flap is reflected and a bone flap created, making sure that thorough hemostasis is obtained; placement of an epidural hemostatic agent below the craniotomy’s edges and dural tack-up sutures should not be missed and once the dura has been opened the surgeon must look for and coagulate bleeding points on the dural edge. All of these precautions tend to prevent a subdural hematoma that could interpose itself between the arachnoid surface and the electrode grids resulting in movement or faulty recording from the latter.
Surgical Technique for Intracerebral Electrodes
Orthogonally implanted electrodes require a much more sophisticated technique. CT and MRI images should be fused with an angiography to prevent injury of vascular structures. Each electrode has four to eight contacts and at least three of them are used to explore the hippocampus and amygdala. They offer the advantage of recording neocortical and paleocortical structures simultaneously, thus allowing the detection of extramesial foci. Other areas of the brain, anatomically related to the suspected EZ, can be covered. This is the case of orbitofrontal and motor areas. Insular electrodes can be implanted but, as mentioned previously, due to the high risk of injuring the Sylvian vessels, orthogonal electrodes have been substituted by parasagittal, frontally, or parietal inserted electrodes. Each orthogonal electrode requires a stereotactically planned burr hole and a separate stab wound. Moreover, when extraoperative recording is to be undertaken, implantation of a screw in the cranial vault is used to hold the electrodes in place. Hollow macroelectrodes allow the implantation of microelectrodes in the same trajectory for unit recordings, and are used mainly for research purposes. In some cases restricted EZ or lesions causing epileptogenesis may be destroyed by radiofrequency lesions through the same orthogonal electrode used for recording.74
Focal Epilepsy Detected Near Eloquent Areas
Cortical stimulation is usually indicated when the planned resection will be in or adjacent to eloquent cortex. ECoG and BM allow the identification of epileptic foci in or near eloquent cortical areas and permit tailoring the resection with satisfactory safety and seizure relief, this has been proved for pure insular lesions and those extending to adjacent lobes.75 In sensory-motor cortices, an ECoG that demonstrates few postresectional spikes and no distant spikes predicts a better seizure control.
Complications
Infection rate has been reported as 3.9% and epidural hematomas and brain edema rates in 2% and 8%, respectively.76 It has been said that almost all patients subject to subdural grid placement for extraoperative recordings develop a subdural collection,77 but it has also been reported that only 7.8% of them require surgical drainage.76 Most require only conservative management, since volume, midline shift, and maximal thickness do not predict the clinical course of this patients, so sound clinical judgment must be exercised to guide these patient’s care and need for evacuation.77 The artifact caused by electrodes on CT makes evaluation of electrode placement and complications difficult and MRI is the preferred method to evaluate them.78
In a series of 67 pediatric patients, a CSF leak was seen in 21 patients, 10 had positive subdural cultures but only 1 of these had a positive lumbar CSF culture and none developed clinical meningitis, and 1 patient developed transient visual field loss after placement of grids over the occipital lobes.79 Another series of 112 children (122 procedures) revealed that placement of additional electrodes was necessary in 5.7% of patients; wound infection occurred in 2.4% of these patients; cerebrospinal fluid leak in 1.6%; and subdural hematoma, symptomatic pneumocephalus, bone flap osteomyelitis, or strip electrode fracture occurred in 0.8% each. There were four cases of transient neurologic deficit (3.3%) and no permanent deficit or death.80
Paradigm of Surgical Treatment
1. When the EZ is not in an eloquent area and seizures have a lesional origin, besides performing a lesionectomy, information derived from the ECoG is used to tailor the resection of the EZ, which usually extends beyond the lesion margins. ECoG also guides the resection of EZ in epilepsies not associated with lesions.
2. When EZ is near an eloquent area, fMRI and BM are imperative. If EZ is not in an eloquent area, carefully restricted cortical resection according to the information obtained from BM is performed. If the EZ is within an eloquent area, alternative techniques such as subpial transection or chronic electrical stimulation of the epileptic focus are indicated. Preliminary results with this latter technique are very promising.81
3. In cases of bilateral homologous foci, such as bilateral independent temporal lobe foci with unilateral structural lesion (temporal sclerosis), one can operate on the side of the lesion causing the preponderance of seizures and use vagus nerve or hippocampal stimulation in case of residual seizures. If there is no lesion, bilateral neuromodulation of epileptic foci is highly efficient.56
4. In cases of multiple bilateral EZ, centromedian thalamic bilateral stimulation provides 79% to 83% overall improvement,82,83 extended corpus callosum section up to 80%,84 and vagus nerve stimulation up to 56%.85
Abou-Khalil B. An update on determination of language dominance in screening for epilepsy surgery: the Wada test and newer noninvasive alternatives. Epilepsia. 2007;48(3):442-455.
Afif A., Chabardes S., Minotti L., et al. Safety and usefulness of insular depth electrodes implanted via an oblique approach in patients with epilepsy. Neurosurgery. 2008;62(5 suppl 2):ONS471-ONS479.
Ahmadi M.E., Hagler D.J.Jr., McDonald C.R., et al. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. Am J Neuroradiol. 2009;30(9):1740-1747.
Chauvel P., Landré E., Trottier S., et al. Electrical stimulation with intracerebral electrodes to evoke seizures. Adv Neurol. 1993;63:115-121.
Cossu M., Chabardès S., Hoffmann D., et al. Presurgical evaluation of intractable epilepsy using stereo-electro-encephalography methodology: principles, technique and morbidity. Neurochirurgie. 2008;54(3):367-373.
Delgado-Escueta A.V., Walsh G.O. Type I complex partial seizures of hippocampal origin: excellent results of anterior temporal lobectomy. Neurology. 1985;35(2):143-154.
Fontoura D., Branco D., Anés M., et al. Language brain dominance in patients with refractory temporal lobe epilepsy: a comparative study between functional magnetic resonance imaging and dichotic listening test. Arq Neuropsiquiatr. 2008;66(1):34-39.
Herscovitch P. Evaluation of the brain by positron emission tomography. Rheum Dis Clin North Am. 1993;19(4):765-794.
Johnston J.M.J., Mangano F.T., Ojemann J.G., et al. Complications of invasive subdural electrode monitoring at St. Louis Children’s Hospital, 1994-2005. J Neurosurg. 2006;105(suppl 5):343-347.
Kwan P., Sperling M.R. Refractory seizures: try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? Epilepsia. 2009;50(suppl 8):57-62.
Lee W.S., Lee J.K., Lee S.A., et al. Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol. 2005;54(5):346-351.
Matthews P.M., Jezzard P. Functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2004;75(1):6-12.
Park Y.D., Murro A.M., King D.W., et al. The significance of ictal depth EEG patterns in patients with temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1996;99(5):412-415.
Ryvlin P., Rheims S. Epilepsy surgery: eligibility criteria and presurgical evaluation. Dialogues Clin Neurosci. 2008;10(1):91-103.
Spencer S.S. When should temporal-lobe epilepsy be treated surgically? Lancet Neurol. 2002;1(6):375-382.
Spencer S.S., Guimaraes P., Katz A., et al. Morphological patterns of seizures recorded intracranially. Epilepsia. 1992;33(3):537-545.
Springer J.A., Binder J.R., Hammeke T.A., et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122(Pt 11):2033-2046.
Velasco A.L., Boleaga B., Brito F., et al. Absolute and relative predictor values of some non-invasive and invasive studies for the outcome of anterior temporal lobectomy. Arch Med Res. 2000;31(1):62-74.
Velasco A.L., Velasco F., Velasco M., et al. Neuromodulation of epileptic foci in patients with non-lesional refractory motor epilepsy. Int J Neural Syst. 2009;19(3):139-147.
Velasco A.L., Wilson C.L., Babb T.L., et al. Functional and anatomic correlates of two frequently observed temporal lobe seizure-onset patterns. Neural Plast. 2000;7(1-2):49-63.
Walsh G.O., Delgado-Escueta A.V. Type II complex partial seizures: poor results of anterior temporal lobectomy. Neurology. 1984;34(1):1-13.
Wellmer J., Weber B., Weis S., et al. Strongly lateralized activation in language fMRI of atypical dominant patients-implications for presurgical work-up. Epilepsy Res. 2008;80(1):67-76.
Williamson P.D., French J.A., Thadani V.M., et al. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol. 1993;34(6):781-787.
Zijlmans M., Kort G.d., Witkamp T.D., et al. 3T versus 1.5T phased-array MRI in the presurgical work-up of patients with partial epilepsy of uncertain focus. J Magn Reson Imaging. 2009;30(2):256-262.
Zumsteg D., Wieser H.G. Presurgical evaluation: current role of invasive EEG. Epilepsia. 2004;41(suppl 3):S55-S560.
1. Kwan P., Sperling M.R. Refractory seizures: try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? Epilepsia. 2009;50(suppl 8):57-62.
2. Bourgeois B. General concepts of medical intractability. In: Lüders H.O., editor. Epilepsy Surgery. New York: Raven Press, 1991.
3. Devaux B., Chassoux F., Landré E., et al. Surgical resections in functional areas: report of 89 cases. Neurochirurgie. 2008;54(3):409-417. 2008
4. Spencer S.S. When should temporal-lobe epilepsy be treated surgically? Lancet Neurol. 2002;1(6):375-382.
5. Ryvlin P., Rheims S. Epilepsy surgery: eligibility criteria and presurgical evaluation. Dialogues Clin Neurosci. 2008;10(1):91-103.
6. Dulac O. Indications for surgery in childhood epilepsies: when and how should children be operated? Neurochirurgie. 2008;54(3):340-341.
7. European Concerted Action on Research in Epilepsy: refractory Epilepsy. Epilepsia. 2003;S6(44):81-82.
8. Cramer J.A., Perrine K., Devinsky O., et al. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia. 1998;39(1):81-88.
9. Torres X., Arroyo S., Araya S., et al. The Spanish Version of the Quality-of-Life in Epilepsy Inventory (QOLIE-31): translation, validity, and reliability. Epilepsia. 1999;40(9):1299-1304.
10. Benavente-Aguilar I., Morales-Blánquez C., Rubio-Calvo E. Cross-cultural adaptation of the quality of life inventory (Qolie-AD-48) for epileptic adolescents. Psiquis. 2002;23(6):226-237.
11. Cramer J.A., Westbrook L.E., Devinsky O., et al. Development of the Quality of Life in Epilepsy Inventory for Adolescents: the QOLIE-AD-48. Epilepsia. 1999;40(8):1114-1121.
12. Velasco A.L., Boleaga B., Brito F., et al. Absolute and relative predictor values of some non-invasive and invasive studies for the outcome of anterior temporal lobectomy. Arch Med Res. 2000;31(1):62-74.
13. Isnard J., Guénot M., Sindou M., et al. Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia. 2004;45(9):1079-1090.
14. Delgado-Escueta A.V., Walsh G.O. Type I complex partial seizures of hippocampal origin: excellent results of anterior temporal lobectomy. Neurology. 1985;35(2):143-154.
15. Walsh G.O., Delgado-Escueta A.V. Type II complex partial seizures: poor results of anterior temporal lobectomy. Neurology. 1984;34(1):1-13.
16. Williamson P.D., French J.A., Thadani V.M., et al. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol. 1993;34(6):781-787.
17. American Clinical Neurophysiology Society. Long-Term Monitoring for Epilepsy. ACNS, 2008 www.acns.org/pdfs/Guideline Twelve – LTME – 2008.pdf
18. Dodrill C.B.. Overview: presurgical neuropsychological evaluation, Lüders H.O., Comair Y.G. Epilepsy Surgery, 2nd ed, Philadelphia: Lippincott, Williams & Wilkins, 2001.
19. Kneebone C.. Presurgical neuropsychological evaluation for localization and lateralization of the epileptogenic zone, Lüders H.O., Comair Y.G. Epilepsy Surgery, 2nd ed, Philadelphia: Lippincott, Williams & Wilkins, 2001.
20. Loring D.W., Chelune G.D.. Neuropsychological evaluation in epilepsy, Lüders H.O., Comair Y.G. Epilepsy Surgery, 2nd ed, Philadelphia: Lippincott, Williams & Wilkins, 2001.
21. Baxendale S. The Wada test. Curr Opin Neurol. 2009;22(2):185-189.
22. Medina L.S., Aguirre E., Bernal B., et al. Functional MR imaging versus Wada test for evaluation of language lateralization: cost analysis. Radiology. 2004;230(1):49-54.
23. Springer J.A., Binder J.R., Hammeke T.A., et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122(Pt 11):2033-2046.
24. Wellmer J., Weber B., Urbach H., et al. Cerebral lesions can impair fMRI-based language lateralization. Epilepsia. 2009;50(10):2213-2224.
25. Arora J., Pugh K., Westerveld M. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50(10):2225-2241.
26. Kanner A. Can fMRI replace the Wada test in predicting postsurgical deterioration of verbal memory? Nat Rev Neurol. 2008;4:364-365.
27. Paolicchi J.M. Is the Wada test still relevant? Yes. Arch Neurol. 2008;65(6):838-840.
28. Abou-Khalil B. An update on determination of language dominance in screening for epilepsy surgery: the Wada test and newer noninvasive alternatives. Epilepsia. 2007;48(3):442-455.
29. Pelletier I., Sauerwein H.C., Lepore F. Non-invasive alternatives to the Wada test in the presurgical evaluation of language and memory functions in epilepsy patients. Epileptic Disord. 2007;9(2):111-126.
30. Wada J., Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of speech dominante. J Neurosurg. 1960;17:266-282.
31. Kesavadas C., Thomas B. Clinical applications of functional MRI in epilepsy. Indian J Radiol Imaging. 2008;18(3):210-217.
32. Alvarez-Linera J., Martín-Plasencia P., Maestú-Unturbe F., et al. Hemispheric dominance for language and functional magnetic resonance: a comparison of three tasks. Rev Neurol. 2002;35(2):115-118.
33. Wellmer J., Weber B., Weis S., et al. Strongly lateralized activation in language fMRI of atypical dominant patients-implications for presurgical work-up. Epilepsy Res. 2008;80(1):67-76.
34. Caner-Cukiert A.R., Cukiert A. Dichotic words listening test. Technical aspects and results in normal right-handed individuals. Arq Neuropsiquiatr. 1994;52(2):204-209.
35. Fontoura D., Branco D., Anés M., et al. Language brain dominance in patients with refractory temporal lobe epilepsy: a comparative study between functional magnetic resonance imaging and dichotic listening test. Arq Neuropsiquiatr. 2008;66(1):34-39.
36. Binder J.R., Sabsevitz D.S., Swanson S.J., et al. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49(8):1377-1394.
37. Jones-Gotman M., Lou-Smith M.. Neuropsychological testing for localizing and lateralizing the epileptogenic region, EngelJr J., editor. Surgical Treatment of the Epilepsies, 2nd ed, New York: Raven Press, 1993.
38. Knake S., Triantafyllou C., Wald L.L., et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology. 2005;65(7):1026-1031.
39. Zijlmans M., Kort G.D., Witkamp T.D., et al. 3T versus 1.5T phased-array MRI in the presurgical work-up of patients with partial epilepsy of uncertain focus. J Magn Reson Imaging. 2009;30(2):256-262.
40. Strandberg M., Larsson E., Backman S., et al. Pre-surgical epilepsy evaluation using 3T MRI. Do surface coils provide additional information? Epileptic Disord. 2008;10(2):83-92.
41. Prichard JW: Nuclear magnetic resonance spectroscopy of seizure states. Epilepsia. 35(suppl 6):S14-S20, 1004.
42. Hugg J.W., Laxer K.D., Matson G.B., et al. Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol. 1993;34(6):788-794.
43. Matthews P.M., Jezzard P. Functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2004;75(1):6-12.
44. Ricci G.B., De Carli D., Colonnese C., et al. Hemodynamic response (BOLD/fMRI) in focal epilepsy with reference to benzodiazepine effect. Magn Reson Imaging. 2004;22(10):1487-1492.
45. Lee C.C., Ward H.A., Sharbrough F.W., et al. Assessment of functional MR imaging in neurosurgical planning. Am J Neuroradiol. 1999;20(8):1511-1519.
46. Sell E. Functional magnetic resonance. Medicina (Buenos Aires). 2007;67(6):661-664.
47. Ahmadi M.E., Hagler D.J.Jr., McDonald C.R., et al. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. Am J Neuroradiol. 2009;30(9):1740-1747.
48. Herscovitch P. Evaluation of the brain by positron emission tomography. Rheum Dis Clin North Am. 1993;19(4):765-794.
49. Kumar A., Braun A., Schapiro M., et al. Cerebral glucose metabolic rates after 30 and 45 minute acquisitions: a comparative study. J Nucl Med. 1992;33(12):2103-2105.
50. Theodore W.H. Neuroimaging in evaluation of patients for surgery. In: Wyler A., Hermann B. The Surgical Management of Epilepsy. Woburn, MA: Butterworth-Heinemann, 1994.
51. Stefan H., Bauer J., Feistel H., et al. Regional cerebral blood flow during focal seizures of temporal and frontocentral onset. Ann Neurol. 1990;27(2):162-166.
52. Stefan H., Pawlik G., Bocher-Schwarz H.G., et al. Functional and morphological abnormalities in temporal lobe epilepsy: a comparison of interictal and ictal EEG, CT, MRI, SPECT and PET. J Neurol. 1987;234(6):377-384.
53. Steven D.A., Andrade-Souza Y.M., Burneo J.G., et al. Insertion of subdural strip electrodes for the investigation of temporal lobe epilepsy. Technical note. J Neurosurg. 2007;106(6):1102-1106.
54. Zumsteg D., Wieser H.G. Presurgical evaluation: current role of invasive EEG. Epilepsia. 2004;41(suppl 3):S55-S60.
55. Kelly P.J. Stereotactic navigation, Jean Talairach, and I. Neurosurgery. 2004;54(2):454-463. discussion 463-464
56. Velasco A.L., Velasco F., Velasco M., et al. Electrical stimulation of the hippocampal epileptic foci for seizure control: a double-blind, long-term follow-up study. Epilepsia. 2007;48(10):1895-1903.
57. Afif A., Chabardes S., Minotti L., et al. Safety and usefulness of insular depth electrodes implanted via an oblique approach in patients with epilepsy. Neurosurgery. 2008;62(suppl 2):ONS471-ONS479.
58. Cossu M., Chabardès S., Hoffmann D., et al. Presurgical evaluation of intractable epilepsy using stereo-electro-encephalography methodology: principles, technique and morbidity. Neurochirurgie. 2008;54(3):367-373.
59. Park Y.D., Murro A.M., King D.W., et al. The significance of ictal depth EEG patterns in patients with temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1996;99(5):412-415.
60. Spanedda F., Cendes F., Gotman J. Relations between EEG seizure morphology, interhemispheric spread, and mesial temporal atrophy in bitemporal epilepsy. Epilepsia. 1997;38(12):1300-1314.
61. Spencer S.S., Guimaraes P., Katz A., et al. Morphological patterns of seizures recorded intracranially. Epilepsia. 1992;33(3):537-545.
62. Schiller Y, Cascino GD, Busacker NE, et al. Characterization and comparison of local onset and remote propagated electrographic seizures recorded with intracranial electrodes. Epilepsia. 39(4):380-388, 198.
63. Velasco A.L., Wilson C.L., Babb T.L., et al. Functional and anatomic correlates of two frequently observed temporal lobe seizure-onset patterns. Neural Plast. 2000;7(1-2):49-63.
64. Engel J.Jr. Inhibitory mechanisms of epileptic seizure generation. Adv Neurol. 1995;67:157-171.
65. Ogren J.A., Wilson C.L., Bragin A., et al. Three-dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol. 2009;66(6):783-791.
66. Zijlmans M., Jacobs J., Zelmann R., et al. High frequency oscillations and seizure frequency in patients with focal epilepsy. Epilepsy Res. 2009;85(2-3):287-292.
67. Jacobs J., Zelmann R., Jirsch J., et al. High frequency oscillations (80-500 Hz) in the preictal period in patients with focal seizures. Epilepsia. 2009;50(7):1780-1792.
68. Chauvel P., Landré E., Trottier S., et al. Electrical stimulation with intracerebral electrodes to evoke seizures. Adv Neurol. 1993;63:115-121.
69. Berger M.S., Cohen W.A., Ojemann G.A. Correlation of motor cortex brain mapping data with magnetic resonance imaging. J Neurosurg. 1990;72(3):383-387.
70. Gregorie E.M., Goldring S. Localization of function in the excision of lesions from the sensorimotor region. J Neurosurg. 1984;61(6):1047-1054.
71. Bancaud J., Chauvel P.. Commentary: acute and chronic intracranial recording and stimulation with deep electrodes, EngelJr J., editor. Surgical Treatment of the Epilepsies, 2nd ed, New York: Raven Press, 1986.
72. Keene D.L., Whiting S., Ventureyra E.C. Electrocorticography. Epileptic Disord. 2000;2(1):57-63.
73. Al-Ghanem S.S.M., Al-Oweidi A.S., Tamimi A.F., et al. Anesthesia and electrocorticography for epilepsy surgery: a Jordanian experience. Middle East J Anesthesiol. 2009;20(1):31-37.
74. Guénot M., Isnard J., Ryvlin P., et al. SEEG-guided RF thermocoagulation of epileptic foci: feasibility, safety, and preliminary results. Epilepsia. 2004;45(11):1368-1374.
75. von Lehe M., Wellmer J., Urbach H., et al. Epilepsy surgery for insular lesions. Rev Neurol (Paris). 2009;165(10):755-761.
76. Lee W.S., Lee J.K., Lee S.A., et al. Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol. 2005;54(5):346-351.
77. Mocco J., Komotar R.J., Ladouceur A.K., et al. Radiographic characteristics fail to predict clinical course after subdural electrode placement. Neurosurgery. 2006;58(1):120-125.
78. Silberbusch M.A., Rothman M.I., Bergey G.K., et al. Subdural grid implantation for intracranial EEG recording: CT and MR appearance. Am J Neuroradiol. 1998;19(6):1089-1093.
79. Simon S.L., Telfeian A., Duhaime A. Complications of invasive monitoring used in intractable pediatric epilepsy. Pediatr Neurosurg. 2003;38(1):47-52.
80. Johnston J.M.J., Mangano F.T., Ojemann J.G., et al. Complications of invasive subdural electrode monitoring at St. Louis Children’s Hospital, 1994-2005. J Neurosurg. 2006;105(suppl 5):343-347.
81. Velasco A.L., Velasco F., Velasco M., et al. Neuromodulation of epileptic foci in patients with non-lesional refractory motor epilepsy. Int J Neural Syst. 2009;19(3):139-147.
82. Cukiert A., Burattini J.A., Cukiert C.M., et al. Centro-median stimulation yields additional seizure frequency and attention improvement in patients previously submitted to callosotomy. Seizure. 2009;18(8):588-592.
83. Velasco F., Velasco A.L., Velasco M., et al. Centromedian thalamic stimulation for epilepsy, Lozano A.M., Gildenberg P.L., Tasker R.R. Textbook of Stereotactic and Functional Neurosurgery, 2nd ed, Berlin: Springer-Verlag, 2009.
84. Cukiert A., Burattini J.A., Mariani P.P., et al. Extended, one-stage callosal section for treatment of refractory secondarily generalized epilepsy in patients with Lennox-Gastaut and Lennox-like syndromes. Epilepsia. 2006;47(2):371-374.
85. Karceski S. Vagus nerve stimulation and Lennox-Gastaut syndrome: a review of the literature and data from the VNS patient registry. CNS Spectr. 2001;6(9):766-770.