Chapter 11 Preparation of the Patient for Awake Intubation
I Background
A History
The preceding quotation is from Dr. Macewen’s 1880 account in the British Medical Journal of the first awake endotracheal intubation and describes a patient suffering from glottic edema. The patient underwent an awake manual endotracheal intubation using a metallic endotracheal tube (ETT). This technique was performed without benefit of anesthesia and without topical or regional blocks, sedatives, or analgesics. The ETT was kept in place with the patient in an awake state for 36 hours.1 Although one may perceive this as brutal, Dr. Macewen was aware, more than 130 years ago, that despite the patient’s discomfort, the safest method for securing the airway was to perform an awake intubation (AI) rather than to provide comfort at the risk of further compromising the airway. Since that time, there have been countless reports of successful AI for management of the difficult airway (DA).2–5
B Awake Intubation in Management of the Difficult Airway
The DA is defined as “the clinical situation in which a conventionally trained anesthesiologist experiences difficulty with mask ventilation, difficulty with endotracheal intubation, or both.”6,7 The American Society of Anesthesiologists (ASA) Closed Claims Study in 1990 found that 34% of injury cases in the Closed Claims database involved respiratory events.8 The ASA formed the Task Force on Management of the Difficult Airway (TFMDA) in 1992 and sought to establish guidelines to facilitate the management of the DA and reduce the likelihood of adverse outcomes.6 The task force constructed an algorithm to assist the anesthesiologist in devising a strategy for management of the DA (see Chapter 10). In this algorithm, one of the primary management choices for the anesthesiologist is the decision whether to perform AI or to attempt intubation after induction of general anesthesia.6 In 2002, the practice guidelines were updated and the choice of AI remained an important component of the recommendations.7
C Indications for Awake Intubation
The ASA algorithm stresses the concept that formulation of a strategy for intubation should include the feasibility of three basic options: (1) AI versus intubation after induction of general anesthesia, (2) noninvasive versus invasive (surgical) techniques, and (3) preservation versus ablation of spontaneous ventilation.7 It is the opinion of most of the consultants of the TFMDA, and has been expressed in the literature,2,3,5–7,9–11 that the safest method for a patient who requires endotracheal intubation and has a DA is for that patient to undergo AI for the following reasons:
1. Patency of the airway is maintained by upper pharyngeal muscle tone.
2. Spontaneous ventilation is maintained.
3. A patient who is awake and well topicalized is easier to intubate, because the larynx moves to a more anterior position after induction of anesthesia compared with the awake state.
4. The patient can still protect his or her airway from aspiration.
5. The patient is able to monitor his or her own neurologic symptoms (e.g., the patient with potential cervical pathology).2,5,9
General indications for AI are compiled in Box 11-1. There are no absolute contraindications to AI other than patient refusal, a patient who is unable to cooperate (such as a child, a mentally retarded patient, or an intoxicated, combative patient), or a patient with a documented true allergy to all local anesthetics.9
Box 11-1
Indications for Awake Intubation
1. Previous history of difficult intubation
2. Anticipated difficult airway based on physical examination:
4. Trauma to the face or upper airway
5. Anticipated difficult mask ventilation
From Kopman AF, Wollman SB, Ross K, et al: Awake endotracheal intubation: A review of 267 cases. Anesth Analg 54:323–327, 1975; Thomas JL: Awake intubation: Indications, techniques and a review of 25 patients. Anaesthesia 24:28–35, 1969; Bailenson G, Turbin J, Berman R: Awake intubation: Indications and technique. Anesth Prog 14:272–278, 1967.
II the Preoperative Visit
In this section, the focus is placed on elective patients, for whom there is time for airway evaluation and meaningful communication. In the setting of an emergency—which in itself should increase the probability of airway difficulty, especially with a patient in extremis9—the physician may not have time, nor can he or she be expected to be able to perform the detailed investigation of the airway described here.
A Reviewing Old Charts
Whenever possible, previous anesthesia records should be examined because they may provide useful information.7,12 Obviously, the most important records are those involving intubation, especially the most recent ones. Other records documenting ease of mask ventilation and tolerance of drugs are also valuable. One should be alert for evidence of reactions to local anesthetics and of apnea with minimal doses of opioids. Another reason for checking as many anesthesia records as possible, including the surgical procedure involved, is that the last intubation may have been routine but the three previous ones may have been difficult, or the last intubation may have been routine but the operation then performed may have rendered the airway difficult.
When reading through a chart, one should focus on four important features:
1. Degree of difficulty of the endotracheal intubation (the difficulty encountered and the method used)
2. Positioning of the patient during laryngoscopy (sniffing position, use of a ramp)
3. Equipment used (even if the intubation was performed routinely in one attempt, a Bullard blade or a fiberoptic bronchoscope [FOB], neither of which requires alignment of the three axes, may have been used)
4. Whether the technique that was used previously is a familiar one (one should not attempt to learn a new technique on a DA)
B The Patient Interview
Dorland’s Medical Dictionary defines “empathy” as the intellectual and emotional awareness and understanding of another person’s thoughts, feelings, and behaviors, even those that are distressing and disturbing. Although the anesthesiologist may participate in 1000 operations per year, few patients undergo more than five in a lifetime.13 The patient’s perception of empathy from the physician is the cornerstone of the patient’s acceptance of an AI. Empathy helps the interviewer establish effective communication, which is important for accurate diagnosis and management.14 Two facets of medical education limit the clinician’s development of empathy: the traditional format of interviewing training and the social ethos of medical training and medical practice, which stresses clinical detachment.14,15 With empathy and the ability to communicate it, the physician can perform the interview in a more patient-oriented rather than disease-oriented fashion, resulting in better data gathering and better patient compliance.16
After the anesthesia practitioner has made the decision that AI is necessary, communication with the patient and psychological preparation is of the utmost importance to maximize the odds for a successful AI.9 One should in a careful, unhurried manner describe to the patient conventional intubation contrasted with AI. Focusing on the fact that the former is easier and less time-consuming but that the latter is safer in light of the patient’s own anatomy or condition, one must communicate to the patient that the knowledgeable, caring physician is willing to take extra measures to ensure the patient’s safety. Recommendations should be presented to the patient with conviction but at the same time allowing the patient the option of the conventional method of intubation as a last resort.10
Complications of AI should also be presented, including local anesthetic toxicity, airway trauma, discomfort, recall, and failure to secure the airway. Patients’ recall after AI using different methods of sedation, analgesia, or local anesthetics has not been studied in a controlled fashion. Although episodes of explicit awareness during general anesthesia are rare, the incidence of recall during AI with minimal levels of sedation is likely to be higher.13 In a review of 443 cases of AI (four studies) in which various combinations of sedation and analgesia were used (Table 11-1), a mean of 17% of the patients had partial recall and 6% had recall with unpleasant memories.2,5,17,18
III Preoperative Preparations
A Transport
The acuteness and urgency of the case must be considered when arranging transport to the operating room (OR). In some cases, the airway needs to be secured at once (e.g., the patient in extremis who warrants a bedside emergency airway procedure). In other cases, there is an urgent need for intubation but with sufficient time to transport the patient to the OR with supplemental oxygen (O2) and appropriate monitors (electrocardiogram [ECG], pulse oximeter, and noninvasive blood pressure monitoring), accompanied by the anesthesiologist, surgeon, or both. Finally, there may be no requirement for immediate attention, and the patient can be transported routinely to the OR. In the elective scenario, supplemental O2 should be provided, if appropriate (high-dose O2 may be detrimental in some patients, such as those who rely on hypoxic respiratory drive),13 and position should be considered (e.g., the patient who is morbidly obese may experience dramatic physiologic changes when supine and should be transported in a wheelchair or on a gurney in a semirecumbent position.).19,20
B Staff
According to the TFMDA guidelines, there should be “at least one additional individual who is immediately available to serve as an assistant in difficult airway management.”7 Whenever possible, it is preferable to have a second member of the anesthesia care team who can assist in the monitoring, ventilation, and pharmacotherapy of the patient, as well as providing an extra set of hands during fiberoptic intubation (FOI). For patients in extremis and those who refuse AI, a surgeon trained in performing a surgical airway should be available with a tracheostomy/cricothyrotomy tray, ready to perform an emergency surgical airway, if necessary.
D Supplemental Oxygen
Administration of supplemental O2 should be considered throughout the process of DA management.7 Arterial hypoxemia has been well documented during bronchoscopy, with an average decrease in arterial oxygen tension (PaO2) of 20 to 30 mm Hg in patients breathing room air, and has been associated with cardiac dysrhythmias.21 In addition, sedation administered to supplement topicalization for AI may result in unintended respiratory depression or apnea.
Studies have shown that either traditional preoxygenation (≥3 minutes of tidal volume [VT] ventilation) or fast-track preoxygenation (i.e., four vital-capacity breaths in 30 seconds) is effective in delaying arterial desaturation during subsequent apnea.7 Daos and colleagues showed that the use of supplemental O2 delayed circulatory arrest resulting from local anesthetic toxic effects in animals.22
In the interest of improving patient safety, therefore, adequate preoxygenation and the use of supplemental O2 throughout airway management (including sedation, topicalization, intubation, and extubation) is encouraged in all patients undergoing AI.7
In addition to the standard methods of supplemental O2 delivery (nasal cannula or face mask), other opportunities include, but are not limited to, delivering O2 through the suction port of a flexible fiberoptic bronchoscope (FFB),9 delivering O2 through an atomizer or nebulizer during topicalization, and elective transtracheal jet ventilation (TTJV) in a patient in extremis.9,23,24
E Airway Equipment
Consultants of the TFMDA strongly agreed that “preparatory efforts enhance success and minimize risk to the patient” (i.e., lead to fewer adverse outcomes).7 The concept of preassembled carts for emergency situations is not new; examples include “crash carts” for cardiac arrest and malignant hyperthermia carts. The task force recommends that every anesthetizing location should have readily available a portable storage unit that contains specialized equipment for DA management. Suggested contents of this portable unit are listed in Box 11-2.
Box 11-2
Suggested Contents of the Portable Unit for Difficult Airway Management
1. Rigid laryngoscope blades of alternative designs and sizes from those routinely used; this may include a rigid fiberoptic laryngoscope
2. Endotracheal tubes (ETTs) of assorted sizes and styles, such as the Parker FlexTip tube (Parker Medical, Highlands Ranch, CO) or the Endotrol tube (Covidien-Nellcor, Boulder, CO)
3. ETT guides, such as semirigid stylets, ventilating tube changer, light wands, and forceps designed to manipulate the distal portion of the ETT
4. Laryngeal mask airways of assorted sizes and styles, such as the intubating laryngeal mask airway and the LMA-Proseal (LMA North America, Inc., San Diego, CA)
5. Fiberoptic intubation equipment
6. Retrograde intubation equipment
7. At least one device for emergency nonsurgical airway ventilation, such as a transtracheal jet ventilation stylet or the esophageal-tracheal Combitube (Kendall-Sheridan Catheter Corporation, Argyle, NY)
8. Equipment suitable for emergency surgical airway access (e.g., cricothyrotomy)
Modified from Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway: Practice guidelines for management of the difficult airway. Anesthesiology 98:1269–1277, 2003.
There are many techniques that can be used to secure the airway in the awake patient. Direct laryngoscopy, video laryngoscopy, intubating laryngeal mask airways (iLMAs), FFB, rigid fiberoptic laryngoscopy, retrograde intubation, lighted stylets, and blind nasal intubation have all been used successfully to perform AI.2,25–30 No matter which technique is selected, all of the necessary equipment should be prepared ahead of time and readily available when needed. The practitioner should also have several backup modalities in mind, and the required equipment available, in case the initial technique used is ineffective.
IV Premedication and Sedation
A Antisialagogues
The medications most often used for their antisialagogic properties are the anticholinergics.31 These drugs inhibit salivary and bronchial secretions by their antimuscarinic effects. Because they only prevent formation of new secretions and do not eliminate secretions that are already present, anticholinergics should be administered at least 30 minutes before topicalization. The anticholinergics used in clinical practice are atropine, glycopyrrolate, and scopolamine. A summary of their pharmacologic properties is presented in Table 11-2.
B Nasal Mucosal Vasoconstrictors
The nasal mucosa and nasopharynx are highly vascular. When a patient requires nasal AI, adequate vasoconstriction is essential, because bleeding can make FOI extremely difficult. One agent commonly used for this purpose is 4% cocaine, which has vasoconstrictive as well as local anesthetic effects (see later discussion). An alternative to cocaine is a 3 : 1 mixture of 4% lidocaine and 1% phenylephrine, which yields a solution of 3% lidocaine with 0.25% phenylephrine.32 When these agents are applied with cotton swabs or pledgets to the nasal area, adequate anesthesia and vasoconstriction can be achieved in 10 to 15 minutes, facilitating nasal AI. Alternatively, vasoconstrictive nose sprays may be administered to the patient before local anesthetic topicalization. Phenylephrine 0.5% and oxymetazoline 0.05% sprays are available. They should be administered 15 minutes before attempted nasal intubation.
C Aspiration Prophylaxis
A percentage of patients requiring AI may need prophylaxis against aspiration pneumonitis because of the presence of risk factors such as a full stomach, symptomatic gastroesophageal reflux disease, hiatal hernia, presence of a nasogastric tube, morbid obesity, diabetic gastroparesis, or pregnancy.33,34
Preoperative administration of a nonparticulate antacid, such as sodium citrate, provides effective buffering of gastric acid pH.35 Total gastric volume is increased, but this effect is offset by an increase in the pH of gastric fluid so that, if aspiration occurs, morbidity and mortality are significantly lower.36 One disadvantage of sodium citrate is the potential to cause emesis due to its unpleasant taste.
1 Histamine Receptor Blockers
Histamine (H2) receptor blockers are selective and competitive antagonists that block secretion of hydrogen ion (H+) by gastric parietal cells and also decrease the secretion of gastric fluid. With IV administration of cimetidine 300 mg, famotidine 20 mg, or ranitidine 50 mg, peak effects are achieved within 30 to 60 minutes, increasing gastric pH and decreasing gastric volume.37,38 Of the three, ranitidine is probably the drug of choice because it has fewer adverse effects, greater efficacy, and a longer duration of action.39,40
2 Proton Pump Inhibitors
Proton pump inhibitors (PPIs), such as pantoprazole and omeprazole, have not been shown to be as effective as H2 blockers at increasing gastric pH and decreasing gastric volume.41,42 PPIs may have a role in aspiration prophylaxis for the patient on chronic H2 blocker therapy.43
3 Metoclopramide
Metoclopramide is a dopamine antagonist that stimulates motility of the upper gastrointestinal tract and increases lower esophageal sphincter tone. The net effect is accelerated gastric emptying with no effect on gastric pH. The standard adult dose is 10 mg IV. Metoclopramide can precipitate extrapyramidal symptoms and should be avoided in patients with Parkinson’s disease.34 For patients at high risk for aspiration, antacids, H2 blockers, and metoclopramide may be used alone or in combination.
D Sedatives/Hypnotics
Depending on the clinical circumstance, IV sedation may be useful in allowing the patient to tolerate AI by providing anxiolysis, amnesia, and analgesia. Benzodiazepines, opioids, hypnotics, α2-agonists, and neuroleptics can be used alone or in combination.25 It is important that these agents be carefully titrated to effect, because oversedation can render a patient uncooperative and make AI more difficult.9 Spontaneous respiration with adequate oxygenation and ventilation should always be maintained.25 Care should be taken in the presence of critical airway obstruction, because awake muscle tone is sometimes necessary in these patients to maintain airway patency.44 Avoidance of oversedation is also important in the patient with a full stomach, because an awake patient can protect his or her own airway in the chance of regurgitation.9
1 Benzodiazepines
Benzodiazepines, via their action at the γ-aminobutyric acid (GABA)–benzodiazepine receptor complex, have hypnotic, sedative, anxiolytic, and amnestic properties.45 They have also been shown to depress upper airway reflex sensitivity,46 a property that is desirable for AI. Benzodiazepines are frequently used to achieve sedation for AI in combination with opioids,47 and they are used for their amnestic and anxiolytic effects when other sedatives (e.g., dexmedetomidine, ketamine, remifentanil) are chosen as the primary agent.48,49 Three benzodiazepine receptor agonists are commonly used in anesthesia practice: midazolam, diazepam, and lorazepam.45
a Midazolam
Because of its more rapid onset and relatively short duration, midazolam is the most commonly used agent. Sedation with midazolam is achieved with doses of 0.5 to 1 mg IV repeated until the desired level of sedation is achieved. The IM dose is 0.07 to 0.1 mg/kg. Onset is rapid, with peak effect usually achieved within 2 to 3 minutes of IV administration. The duration of action is 20 to 30 minutes, with termination of effect primarily a result of redistribution. Although recovery is rapid, the elimination half-life is 1.7 to 3.6 hours, with increases noted in patients with cirrhosis, congestive heart failure, or morbid obesity; in the elderly; and in patients with renal failure. It is extensively metabolized by the liver and renally eliminated as glucuronide conjugates.45,50
b Diazepam and Lorazepam
Diazepam has a slightly slower onset and longer duration of action than midazolam and has been shown to be a less potent amnestic.45,50,51 It can cause pain on IV injection and has the added risk of thrombophlebitis.25 Lorazepam provides the most profound sedating and amnestic properties; however, it is more difficult to use, because these effects are slower in onset and longer lasting than with either midazolam or diazepam.25,45
c Precautions
Care must be used when using benzodiazepines in combination with other sedative drugs. The pharmacologic effects of benzodiazepines are augmented synergistically by other medications used for sedation, including opioids and α2-agonists.52 Propofol has been shown to increase the plasma concentration of midazolam by decreasing distribution and clearance.53 Systemic absorption of local anesthetics used for airway topicalization may also lead to potentiation of the sedative/hypnotic effects of midazolam.54
The primary adverse effect of oversedation with benzodiazepines is respiratory depression, which may lead to hypoxemia or apnea.45 Flumazenil, a specific benzodiazepine antagonist, may be used to reverse the sedative and respiratory effects of benzodiazepines if a patient becomes too heavily sedated. It is given in incremental IV doses of 0.2 mg, repeated as needed to a maximum dose of 3 mg. Because it has a half-life of 0.7 to 1.8 hours, resedation can be a problem if flumazenil is being used to reverse high doses or longer-acting benzodiazepines, and patients should be monitored carefully in those circumstances. Flumazenil is generally safe and devoid of major side effects.55,56
2 Opioids
Opioids, by way of their agonist effect on opioid receptors in the brain and spinal cord, provide analgesia, depress airway reflexes, and prevent hyperventilation associated with pain or anxiety. These properties make them a useful addition to the sedating regimen for AI. Although any opioid receptor agonist could theoretically be used for this purpose, the synthetic phenylpiperidine class of opioids—fentanyl, sufentanil, alfentanil, and remifentanil—are best suited to the task. These drugs are particularly useful due to their rapid onset, relatively short duration of action, and ease of titration.57
a Fentanyl
Analgesic doses range from 0.5 to 2 µg/kg IV. Onset is rapid, within 2 to 3 minutes. Duration of a single bolus dose is roughly 30 to 60 minutes. The duration is relatively short because fentanyl is redistributed to a large peripheral compartment rather than rapidly eliminated. Hence, the duration of effect after cessation of a prolonged infusion is markedly longer, due to redistribution to the central compartment from the peripheral compartment. Fentanyl is widely used in anesthesia practice, and it seems to be the most commonly used opioid for AI.47,58
b Sufentanil
Sufentanil is 7 to 10 times more potent than fentanyl and has a similar pharmacokinetic profile after a single bolus dose. The primary difference is a significantly faster recovery, compared with fentanyl, after prolonged infusion.59 Sedative doses are 5 to 20 µg IV in adult patients. Its use in combination with midazolam and droperidol for AI has been reported.60
c Alfentanil
Compared with fentanyl and sufentanil, alfentanil has an even quicker onset (1.5 to 2 minutes). It is approximately 1/70 as potent as fentanyl; however, because of rapid plasma to effect-site equilibration, comparatively smaller doses are needed to achieve a similar peak effect. Because smaller doses are needed relative to its potency, recovery from a single bolus of alfentanil is faster than with the other agents of this class, potentially making alfentanil the drug of choice when a transient peak effect after a single bolus is desired, as in AI.59 Sedative doses range from 10 to 30 µg/kg IV.57 In patients premedicated with oral diazepam, alfentanil 20 µg/kg IV significantly improved fiberoscopic conditions and attenuated the hemodynamic effects of awake nasal FOI. Moderate respiratory depression was noted without overt apnea or hypoxia.61
d Remifentanil
Remifentanil is an ultrashort-acting opioid that is unique compared with the other short-acting agents in that it is metabolized by nonspecific plasma esterases, with a half-life of 3 minutes. It undergoes no redistribution and therefore has no context sensitivity. Its potency approximates that of fentanyl.62 Several studies have shown the effectiveness and safety of remifentanil sedation for AI as a single agent,48,49,63 as well as in combination with midazolam or propofol.64–68 Dosing is usually weight-based.
Several different remifentanil dosing strategies have been described in the literature for AI, with infusion rates ranging between 0.06 and 0.5 µg/kg/min, with or without an initial bolus of 0.5 to 1.5 µg/kg.48,63,64,67 Studies using target-controlled infusions for AI have shown that the mean effect-site concentration of remifentanil needed for AI is 2 to 3 ng/mL.49,65,66,68 The dosing strategy described by Atkins and Mirza,62 a bolus of 0.5 µg/kg followed by an infusion of 0.1 µg/kg/min, uses the Minto pharmacokinetic model and rapidly achieves a target site concentration of 2 to 2.5 ng/mL. The infusion can subsequently be titrated by 0.025 to 0.05 µg/kg/min in 5-minute intervals to achieve adequate sedation.
e Precautions
Naloxone, an opioid antagonist, can be used to restore spontaneous ventilation in patients after an opioid overdose. Onset after IV administration is rapid, within 1 to 2 minutes, and the duration of action is 30 to 60 minutes. Naloxone should be administered in boluses of 0.04 to 0.08 µg IV boluses every 2 to 3 minutes. Doses of 1 to 2 µg/kg will restore adequate spontaneous ventilation in most cases while preserving adequate analgesia. Potential complications of naloxone administration are reversal of analgesia, tachycardia, hypertension, and, in severe cases, pulmonary edema or myocardial ischemia. Because of the relatively short duration of action of naloxone, one should carefully monitor for recurrence of respiratory depression, especially when it is used to reverse longer-acting opioids such as morphine or hydromorphone. In those situations, an intramuscular dose of 2 times the required IV dose or a continuous IV infusion (2.5 to 5 µg/kg/hr) should be considered.57
Chest wall rigidity leading to ineffectual bag-mask ventilation is commonly cited as a potential adverse effect of opioids, particularly fentanyl, sufentanil, alfentanil, and remifentanil. Opioids do have the potential to cause muscle rigidity, but clinically significant rigidity usually occurs only after an opioid dose sufficient to cause apnea, because the patient loses consciousness.57 Studies in intubated patients and patients with tracheostomies have shown that decreases in pulmonary compliance due to chest wall rigidity are not sufficient to explain an inability to maintain bag-mask ventilation after a large dose of opioid,69,70 and fiberoscopic examination of the vocal cords during induction with sufentanil has shown that vocal cord closure is the primary cause of difficult ventilation after opioid-induced anesthesia.71 Careful titration to prevent overdose is perhaps the best way to prevent rigidity-associated difficult ventilation. Should it occur, treatment with naloxone or neuromuscular blocking agents is effective.72,73
3 Intravenous Anesthetics
a Propofol
Propofol (2,6-diisopropylphenol) is the most frequently used IV anesthetic today.45 Its primary effect is hypnosis, which results from an unclear mechanism; however, there is evidence that a significant portion of this hypnotic effect is mediated by interaction with GABA receptors. Propofol has a rapid onset of approximately 90 seconds, with rapid recovery (4 to 5 minutes after an induction dose) as a result of both elimination and redistribution. It attenuates airway responses in induction doses via an unclear mechanism and provides a smooth induction with few excitatory effects. Although it is frequently used as an induction agent in doses of 1.5 to 2.5 mg/kg IV, intermittent doses of approximately 0.25 mg/kg IV or a continuous IV infusion of 25 to 75 µg/kg/min provides an easily titratable level of sedation with rapid recovery.
The use of propofol in AI is well described both as a single agent and in combination with remifentanil.26,65,68 Target-controlled infusion studies have shown that the effective plasma concentration of propofol to facilitate AI is 1 to 2 µg/mL.26,65,68 Care should be taken if the patient has a critical airway, because propofol causes reduction of Vt and an increase in respiratory frequency at sedative doses. At sufficiently elevated plasma concentrations, propofol can lead to apnea. Other common adverse effects are a decrease in arterial blood pressure and pain on injection. An additional benefit of propofol is its antiemetic properties.
b Dexmedetomidine
Dexmedetomidine is a centrally acting, highly selective α2-adrenoreceptor agonist with several properties that make it well suited for use in AI.74–77 It has sedative, analgesic, anxiolytic, antitussive, and antisialagogue effects while causing minimal respiratory impairment, even at high doses. Compared with clonidine, it is 8 to 10 times more specific for the α2– versus the α1-adrenergic receptor and has a shorter half-life (2 to 3 hours). Dexmedetomidine sedation provides unique conditions in which the patient is asleep but is easily arousable and cooperative when stimulated. It is approved for continuous IV sedation in intubated and mechanically ventilated patients and for sedation in nonintubated patients undergoing surgical or other procedures.
There are several reports of dexmedetomidine sedation in awake FOI,77–80 including a phase IIIb Food and Drug Administration (FDA) study specifically for this indication.81 Dosing for AI is a 1 µg/kg load over 10 minutes, followed by a continuous infusion of 0.2 to 0.7 µg/kg/hr. Some patients may require higher maintenance doses.82,83 A reduced loading dose of 0.5 µg/kg and a reduction in the maintenance infusion should be considered in elderly patients (age >65 years) and in those with hepatic or renal impairment.84 For AI, dexmedetomidine is usually combined with airway topicalization, although its successful use as a sole agent without local anesthetics has been reported.78 Although lowering of bispectral index scores (BIS) and partial amnesia have been reported with its use,85,86 dexmedetomidine is not a reliable amnestic and is frequently combined with midazolam to decrease the incidence of recall.79,87
Dexmedetomidine can cause adverse hemodynamic effects including bradycardia, hypotension, and hypertension. During the loading dose, hypertension and bradycardia can occur due to stimulation of peripheral postsynaptic α2B-adrenergic receptors, resulting in vasoconstriction. Central α2A-mediated sympatholysis eventually leads to bradycardia, hypotension, and decreased cardiac output.88 The bradycardic effect can be mitigated by pretreatment with an anticholinergic (e.g., glycopyrrolate) or by combining the dexmedetomidine with ketamine for its cardiostimulatory properties.79,89 Caution should be used in patients with depressed systolic function.84
c Ketamine
Ketamine45is a phencyclidine derivative and N-methyl-D-aspartate (NMDA) antagonist that produces dissociative anesthesia, which manifests clinically as a cataleptic state with eyes open and many reflexes intact, including the corneal, cough, and swallow reflexes. Ketamine-induced anesthesia is associated with amnesia, nystagmus, and the potential for hallucinations and other undesirable psychological reactions. Benzodiazepines are commonly administered to attenuate or treat these reactions; dexmedetomidine has also been shown to reduce the incidence and severity of ketamine-induced postanesthesia delirium.90 Ketamine has an opioid-sparing effect and produces analgesia that extends well into the postoperative period, because plasma levels required for analgesia are considerably lower than those required for loss of consciousness. Usual doses for sedation range from 0.2 to 0.8 mg/kg IV, with peak plasma levels occurring after less than 1 minute and a duration of hypnosis of 5 to 10 minutes. At these doses, minute ventilation, Vt, FRC, and the minute ventilation response to CO2 are maintained. Although airway reflexes and upper airway skeletal muscle tone are preserved, airway protection remains necessary in patients who are at risk for aspiration.
Ketamine increases blood pressure, heart rate, cardiac output, and myocardial O2 consumption via a centrally mediated stimulation of the sympathetic nervous system. Ketamine is a direct myocardial depressant, however, and in patients with depleted catecholamine stores (e.g., the patient in shock), it may cause hypotension.91 Its use in AI has been described in combination with benzodiazepines and dexmedetomidine.2,89,92 Patients receiving ketamine sedation should always be pretreated with an antisialagogue, because ketamine causes increased airway secretions that can lead to upper airway obstruction or make FOI difficult.
d Droperidol
Droperidol, a butyrophenone, is a neuroleptic medication occasionally used in anesthesia practice for its sedative and antiemetic properties.10,45 Its mechanism of action is antagonism of dopamine receptors in the central nervous system; it also interferes with GABA-, norepinephrine-, and serotonin-mediated neuronal activity. In combination with fentanyl, it produces a state of hypnosis, analgesia, and immobility classically referred to as neuroleptanalgesia. In combination with a hypnotic or nitrous oxide, droperidol and fentanyl produce general anesthesia (neuroleptanesthesia), not unlike the dissociative state produced by ketamine. Neuroleptanalgesia has been used for AI with favorable results.2,93,94 In this state, patients may experience extreme fear and apprehension despite appearing outwardly calm; droperidol is also a poor amnestic. For this reason, benzodiazepines should be administered for anxiolysis and amnesia.
Doses for neuroleptanalgesia range from 2.5 to 5 mg IV; the antiemetic dose is 0.625 to 1.25 mg. Onset is in 20 minutes, with a half-life of about 2 hours. Side effects include mild hypotension due to peripheral α-adrenergic blockade, dysphoria, and extrapyramidal symptoms. Droperidol can also cause QT prolongation, especially in larger doses. This has led to an FDA “black box” warning concerning the risk for potentially fatal torsades de pointes. As a result, droperidol should not be administered to patients with a prolonged QT interval (>440 msec for males, >450 msec for females), and ECG monitoring should be performed during and for 2 to 3 hours after treatment.95
V Topicalization
Topicalization of the airway with local anesthetics should, in most cases, be the primary anesthetic for AI; many times, it is all that is needed.9 When using local anesthetics, it is important to be familiar with the speed of onset, duration of action, optimal concentration, signs and symptoms of toxicity, and maximum recommended dosage of the drug chosen.96 The rate and amount of topical local anesthetic absorption vary depending on the site of application, the concentration and total dose of local anesthetic applied, the hemodynamic status of the patient, and individual patient variation.97 Local anesthetic absorption is more rapid from the alveoli than from the tracheobronchial tree, and more rapid from the tracheobronchial tree than from the pharynx. The most commonly used agents for topical anesthesia of the airway are lidocaine and cocaine.
Although AI with airway topicalization is considered the safest method of airway management for the patient with a DA, it is not without risk. Local anesthetic toxicity is a real concern; death due to lidocaine toxicity of a healthy volunteer undergoing bronchoscopy has occurred.98,99 In addition, total airway obstruction during topical anesthesia in a nonsedated patient with a critical airway has been reported. This was postulated to have been caused by dynamic airway obstruction related to loss of upper airway tone as a result of topicalization.100
A Lidocaine
Lidocaine, an amide local anesthetic, is the most commonly used agent for airway topicalization.101,102 It is available in various concentrations (1% to 10%) and in preparations including aqueous and viscous solutions, ointments, gels, and creams. For infiltration and minor nerve blocks, 1% to 2% lidocaine is commonly used; for topical anesthesia, the concentration used is 2% to 4%. Lidocaine is an excellent choice for airway anesthesia because of its reasonably rapid onset of 2 to 5 minutes and its high therapeutic index. Its duration of action is 30 to 60 minutes after topical application or infiltration; addition of epinephrine extends the duration to 2 to 3 hours when used for infiltration. It is hepatically metabolized with a half-life of 90 minutes; care should be taken in patients with hepatic failure.
The maximum recommended dosage for infiltration of lidocaine without epinephrine is 5 mg/kg of lean body mass. For topicalization of the airway, the maximum dose is less well established. The British Thoracic Society recommends a maximum dose of 8.2 mg/kg,103 based on a study by Langmack and associates.104 Studies of plasma concentration after nebulization of 6 mg/kg of lidocaine showed peak plasma levels an order of magnitude lower than the toxic plasma level of 5 µg/mL.105 Other studies using total doses as high as 10.5 mg/kg in obese patients failed to demonstrate signs or symptoms of lidocaine toxicity.106 Early symptoms of lidocaine toxicity include euphoria, dizziness, tinnitus, confusion, and a metallic taste in the mouth. Signs of severe lidocaine toxicity include seizures, respiratory failure, loss of consciousness, and circulatory collapse. In asthmatics, lidocaine has been reported to precipitate bronchoconstriction by a nonhistamine-mediated mechanism.107
B Cocaine
Cocaine, a naturally occurring ester anesthetic, is used primarily for anesthesia of the nasal mucosa when the nasal route is planned for AI.108 It has a vasoconstrictor property that makes it particularly useful for this application, because the nose is highly vascularized and bleeding can make FOI impossible. It is available commercially as a 4% solution (each drop containing 3 mg), and can be applied to the nasal mucosa using cotton pledgets or cotton-tipped swabs. The maximum recommended dosage for intranasal application is 1.5 mg/kg. After topical application of cocaine to the nasal mucosa, peak plasma levels are achieved in 30 to 45 minutes, and the drug persists in the plasma for 5 to 6 hours.
C Other Local Anesthetics
Benzocaine is a water-insoluble ester-type local anesthetic agent that is mainly useful for topical application. The onset of action is rapid (<1 minute), and the effective duration is 5 to 10 minutes. Benzocaine is most commonly available as a 20% aerosol spray that is easily applied to the oropharyngeal mucosa. The limiting factor in benzocaine use is the potential development of clinically significant methemoglobinemia. In some patients, this can occur with as little as 1 to 2 seconds of spraying.109 The risk increases by almost 20-fold when benzocaine exposure is repeated within 1 week. Although most patients tolerate benzocaine airway topicalization without any adverse effects, it is not possible to identify in advance those patients who are at risk for significant methemoglobinemia. Some have advocated for the cessation of benzocaine use across the board.110 Symptoms of early methemoglobinemia toxicity can be seen with methemoglobin levels of 5% and include cyanosis, tachycardia, and tachypnea. As levels increase to 20% to 30%, patients may develop chest pain, ischemic changes on ECG, hypotension, altered mental status, syncope, or coma. The most severe cases have led to neurologic hypoxic injury, myocardial infarction, and death. Should symptomatic methemoglobin occur, treatment is with methylene blue 1 to 2 mg/kg IV given over 5 minutes.110,111
Tetracaine is an amide local anesthetic agent with a longer duration of action than lidocaine or cocaine. It is available as 0.5% to 1% solutions for local use. It is metabolized through hydrolysis by plasma cholinesterase. Tetracaine is rapidly absorbed from the respiratory and gastrointestinal tract when used to anesthetize the airway, and aerosol doses as small as 20 mg have been shown to precipitate toxicity.112 Severe toxic reactions after tetracaine overdose include convulsions, respiratory arrest, and circulatory collapse.113 Fatalities have been reported with topical application of tetracaine 100 mg used to anesthetize mucous membrane.114 These issues have limited its use as a primary local anesthetic for airway topicalization.
Cetacaine is a topical application spray containing 14% benzocaine, 2% tetracaine, and 2% butyl aminobenzoate (a local anesthetic similar to benzocaine). Like 20% benzocaine spray, this combination produces rapid airway anesthesia, but with a prolonged duration of action compared to benzocaine alone. The risk of methemoglobinemia is still a consideration, and cases of severe toxicity have been reported.115,116
EMLA cream (eutectic mixture of local anesthetic) contains 2.5% lidocaine and 2.5% prilocaine and is considered a topical anesthetic for use on intact skin. Although the manufacturer does not recommend its use on mucosal surfaces because of faster systemic absorption, it has been employed safely as a topical anesthetic for AI. Larijani and colleagues described 20 adult patients who underwent awake FOI using 4 g of EMLA applied over the upper airway. The measured peak plasma concentrations of lidocaine or prilocaine did not reach toxic levels, and methemoglobin levels did not exceed normal values (1.5%).117
Benzonatate, an oral antitussive chemically similar to ester-type local anesthetics, has also been used for airway topicalization. The dose used is 200 mg; the capsules are pierced with a needle, and the patient is instructed to crush the capsules with the teeth or to hold the capsules in the back of the mouth. Oropharyngeal anesthesia is quickly obtained. In a study by Mongan and Culling, intubating conditions using benzonatate were comparable to those achieved with topical benzocaine and superior laryngeal nerve blocks, with no adverse effects noted.17
D Application Techniques
1 Atomizers
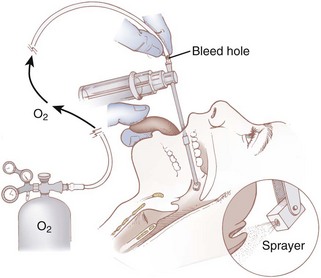
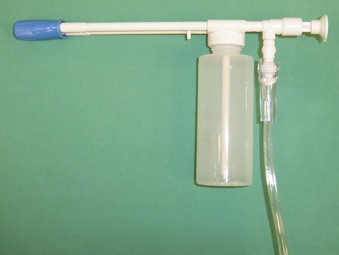
A common method for applying local anesthetic to the airway is with an atomizer. One system for local anesthetic atomization involves the use of a standard DeVilbiss atomizer with the bulb removed. The atomizer reservoir is filled with 2% to 4% lidocaine. O2 tubing is connected from the atomizer to an O2 cylinder with a flow rate of 8 to 10 L/min. A bleed hole is cut in the O2 tubing; this allows for intermittent application of the local anesthetic when a thumb is placed over the hole in the tubing. The atomizer spray is directed toward the soft palate and posterior pharynx to topicalize the mucosa (Fig. 11-1). Any residual anesthetic agent in the oropharynx should be suctioned out to reduce absorption from the gastrointestinal tract. Disposable plastic atomizers are available for this purpose as well (Fig. 11-2). The device is attached to an O2 tank, and the phalange is depressed to deliver the local anesthetic solution to the oropharyngeal mucosa. A disadvantage with these methods is the difficulty of controlling the exact amount of local anesthetic administered.
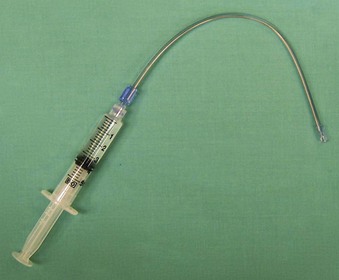
Alternatively, the MADjic Mucosal Atomization Device (Wolfe Tory Medical, Inc., Salt Lake City, UT) is an inexpensive, disposable, latex-free device that, when attached to a Luer fitted syringe containing local anesthetic, can be used to dispense a fine mist to the oropharyngeal or nasal mucosa (Fig. 11-3). The tubing is malleable, allowing for delivery of local anesthetic to deeper pharyngeal structures and to the glottis. Because a syringe is used, a known amount of local anesthetic can be administered.
2 Nebulizers
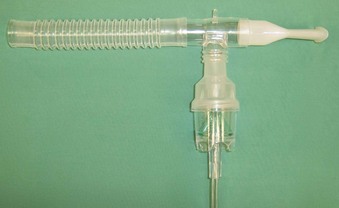
Nebulizers may be used to apply local anesthetic to the airway. The advantages of this technique include ease of application and safety. A standard mouthpiece-type nebulizer (Fig. 11-4) can topicalize the oropharynx and trachea. If nasal cavity anesthesia is needed, a facemask-type nebulizer (Fig. 11-5) can be used; the patient is instructed to breathe in through the nose. This approach is especially advantageous for patients with increased intracranial pressure (ICP), open eye injury, or severe coronary artery disease.118 Foster and Hurewitz compared the administration of lidocaine using two different modalities (nebulizer-atomizer combination and atomizer alone) in patients undergoing bronchoscopy.119 They found that the nebulizer-atomizer combination was more efficacious, resulting in a reduction of the dose required to anesthetize the upper airway. A typical dose of lidocaine used in a standard nebulizer is 4 mL of 4% lidocaine. This results in a total dose of 160 mg of lidocaine, which is well within the safe dosage range. Studies using 6 mg/kg of 10% lidocaine showed that peak plasma concentrations are much lower than would be expected if all the lidocaine had been absorbed—evidence that some of the local anesthetic is lost during exhalation.105
3 “Spray-As-You-Go”
In addition to using a nebulizer or an atomizer to anesthetize the vocal cords and trachea, a technique termed “spray-as-you-go” through the FFB can be performed. The technique is noninvasive and involves injecting local anesthetics through the suction port of the FOB. Two methods have been described. The first requires attaching a triple stopcock (Fig. 11-6) to the proximal portion of the suction port to connect O2 tubing from a regulated O2 tank set to flow at 2 to 4 L/min. Under direct vision through the bronchoscope, targeted areas are sprayed with aliquots of 0.2 to 1.0 mL of 2% to 4% lidocaine. The physician then waits 30 to 60 seconds before advancing to deeper structures and repeating the maneuver. The flow of O2 allows higher delivery of a higher fraction of inspired oxygen (FIO2), keeps the FOB lens clean, disperses mucous secretions away from the lens, and aids in nebulizing the local anesthetic. The second method involves passing a multiorifice epidural catheter (0.5- to 1.0-mm inner diameter [ID]) through the suction port of an adult FOB.120 These techniques are especially useful in patients who are at risk for aspirating gastric contents because the topical anesthetic is applied only seconds before the intubation is accomplished, allowing the patient to maintain airway reflexes as long as possible.
VI Nerve Blocks
Because of the multitude of nerves innervating the airway (Fig. 11-7), there is no single anatomic site where a physician can perform a nerve block and anesthetize the entire airway. Even though topicalization of the mucosa serves to anesthetize the entire airway adequately in most patients, some require supplementation to ablate sensation in the nerve endings that run deep to the mucosal surface, such as the periosteal nerve endings of the nasal turbinates and the stretch receptors at the base of the tongue, which are involved in the gag reflex. Some studies have shown superior patient comfort and hemodynamic stability when a combined regional block technique was used rather than nebulized local anesthetic.121 The following nerve blocks are remarkable for their ease of performance, their minimal risk to the patient, and their speed of onset. Nerve blocks are applied to the nasal cavity and nasopharynx,4,12,102,122–127 the oropharynx,12,102,122,128–134 the larynx,9,12,25,102,113,122,128,135–140 and the trachea and vocal cords.12,25,102,113,122,141
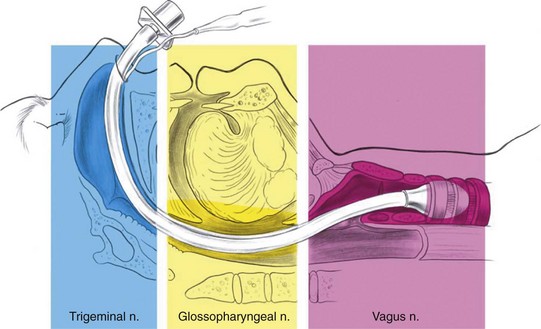
Figure 11-7 Innervation of the upper airway.
(From Brown D, editor: Atlas of regional anesthesia, ed 2, Philadelphia, 1999, Saunders.)
A Nasal Cavity and Nasopharynx
1 Anatomy
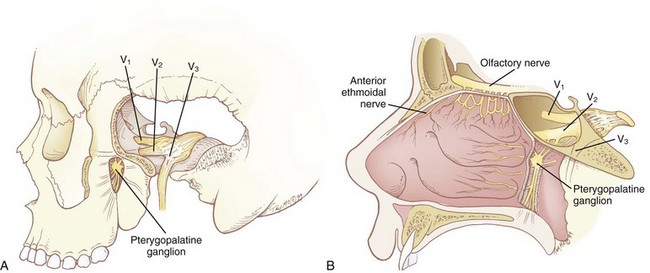
Most of the sensory innervation of the nasal cavity is derived from two sources: the sphenopalatine ganglion and the anterior ethmoidal nerve. The sphenopalatine ganglion (pterygopalatine, nasal, or Meckel’s ganglion) is a parasympathetic ganglion that is located in the pterygopalatine fossa (Fig. 11-8A), posterior to the middle turbinate. Its sensory root is derived from sphenopalatine branches of the maxillary nerve, cranial nerve (CN) V2. As they pass through the sphenopalatine ganglion, these sensory branches form the greater and lesser palatine nerves, which provide sensory innervation to the nasal cavity as well as the roof of the mouth, soft palate, and tonsils. The anterior ethmoidal nerve (see Fig. 11-8B) is one of the sensory branches of the ciliary ganglion, which is located within the orbital cavity and inaccessible to nerve blocks. It provides sensory innervation to the anterior portion of the nasal cavity.
2 Nasal Pledgets or Nasopharyngeal Airways
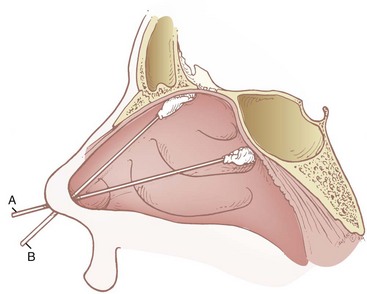
Regardless of which technique is used to anesthetize the nasal cavity, the nares should first be inspected for a deviated septum by using a nasal speculum and asking the patient to breathe deeply through each individual naris while the opposite naris is occluded. Patency can also be determined by using bayonet forceps to place cotton pledgets soaked in either 4% cocaine or 4% lidocaine with epinephrine 1 : 200,000 along the floor of the nasal cavity. The pledgets are advanced posteriorly to the level of the posterior nasopharyngeal wall. The benefits of this technique are that initial topicalization of the nasal cavity is achieved, the angle of ETT insertion can be predicted, and dilation of the nasal cavity is initiated. The pledgets are then followed by placement of nasopharyngeal airway (nasal trumpet) coated in 2% to 4% viscous lidocaine (a 34-F nasal trumpet predicts easy passage of a 7.0-mm ID ETT).4,102
3 Sphenopalatine Nerve Block
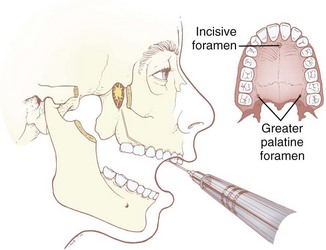
a Oral Approach
With the patient in the supine position, the physician stands facing the patient on the contralateral side of the nerve to be blocked. Using the nondominant index finger, the physician identifies the greater palatine foramen. It is located on either side of the roof of the mouth between the second and third maxillary molars, approximately 1 cm medial to the palatogingival margin, and can usually be palpated as a small depression near the posterior edge of the hard palate. In approximately 15% of the population, the foramen is closed and inaccessible. A 25-G spinal needle, bent 2 to 3 cm proximal to the tip to an angle of 120 degrees, is inserted through the foramen in a superior and slightly posterior direction to a depth of 2 to 3 cm. An aspiration test is performed to ascertain that the sphenopalatine artery has not been cannulated, and 1 to 2 mL of 2% lidocaine with epinephrine 1 : 100,000 is injected. The epinephrine is used as a vasoconstrictor for the sphenopalatine artery, which runs parallel to the nerves, to decrease the incidence of epistaxis. The injection of the local anesthetic should be performed in a slow, continuous fashion (preventing acute increases in pressure within the fossa) to decrease sympathetic stimulation.123–125127
This block anesthetizes the greater and lesser palatine nerves as well as the nasociliary and nasopalatine nerves, which also contribute to the sensory innervation of the nasal cavity. Complications include bleeding, infection, nerve trauma, intravascular injection of local anesthetic, and hypertension. Pain on insertion of the needle can be avoided by application of 2% viscous lidocaine with a cotton-tipped applicator for 1 to 2 minutes before the block is applied. See Figures 11-9 and 11-10.
b Nasal Approach
Two noninvasive nasal approaches to the sphenopalatine ganglion have been described, both of which take advantage of the ganglion’s shallow position beneath the nasal mucosa. The first involves the application of long cotton-tipped applicators soaked in either 4% cocaine or 4% lidocaine with epinephrine 1 : 200,000 over the mucosal surface overlying the ganglion (Fig. 11-11A). The applicator is passed along the upper border of the middle turbinate at an angle of approximately 45 degrees to the hard palate and directed posteriorly until the upper posterior wall of the nasopharynx (sphenoid bone) is reached. The applicator is then left in place for 5 to 10 minutes. The second method involves using the plastic sheath of a 20-G angiocatheter placed along the same path (Fig. 11-12). An anesthetic solution (4 mL of 3% lidocaine/0.25% phenylephrine) is then rapidly injected. About 2 minutes should be allowed for the anesthetic to take effect.4,122,123,126
4 Anterior Ethmoidal Nerve Block
The anterior ethmoidal nerve is blocked by insertion of a long cotton-tipped applicator, soaked in either 4% cocaine or 4% lidocaine with epinephrine 1 : 200,000, parallel to the dorsal surface of the nose until it meets the anterior surface of the cribriform plate (see Fig. 11-11B). The applicator is held in position for 5 to 10 minutes.126
B Oropharynx
2 Glossopharyngeal Nerve Block
a Posterior Approach (Palatopharyngeal Fold)
The classic intraoral approach to the GPN block involves injecting local anesthetic at the base of the posterior tonsillar pillar (palatopharyngeal fold).102,128,129 Because of the proximal location of the nerve targeted, this technique has the potential to block both the sensory fibers (pharyngeal, lingual, and tonsillar branches) and the motor branch innervating the stylopharyngeus muscle.
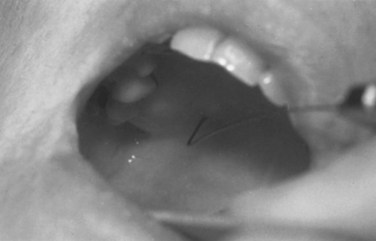
After adequate topicalization of the oropharynx, the patient is placed in a semireclined position with the physician facing the patient on the ipsilateral side of the nerve to be blocked. The patient’s mouth is opened wide. With a tongue depressor held in the nondominant hand, the tongue is displaced caudad and medially, exposing the soft palate, uvula, palatoglossal arch, tonsillar bed, and palatopharyngeal arch (Figs. 11-13 and 11-14). This maneuver stretches both the palatopharyngeal arch and the palatoglossal arch, making them more accessible. A Macintosh size 3 laryngoscope blade may also be useful for retraction, because it provides additional light. The dominant hand holds a 23-G tonsillar needle attached to a syringe. Alternatively, a 22-G, 9-cm spinal needle with the distal 1 cm of the needle bent at a 90-degree angle may be used. The needle is inserted submucosally into the caudad portion of the posterior tonsillar pillar. After an attempt to aspirate blood, 5 mL of 0.5% to 1% lidocaine is slowly injected. If blood is aspirated or the patient complains of headache during injection, the needle should be removed and repositioned. The procedure is repeated on the contralateral side.
Because of the proximity of the GPN at this location to the internal carotid artery, care must taken to avoid intra-arterial injection, which could result in headache or seizure. Hypopharyngeal swelling and mucosal bleeding may occur. Tachycardia may also result from blockade of the afferent nerve fibers of the GPN that arise from the carotid sinus.133
b Anterior Approach (Palatoglossal Fold)
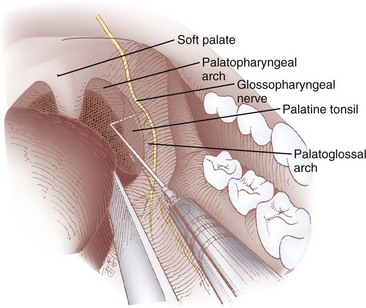
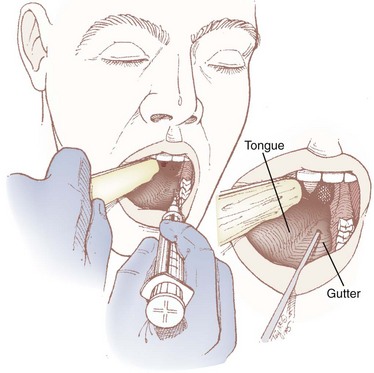
The lingual branch of the GPN provides sensory innervation to the posterior third of the tongue and is the afferent limb of the gag reflex. The anterior approach, which isolates this branch as it passes medial to the base of the anterior tonsillar pillar, is easier and is better tolerated by the patient because it requires less exposure.9,130–132
The patient is placed in the sitting position with the physician facing the patient on the contralateral side of the nerve to be blocked. The patient’s mouth is opened wide with the tongue protruded. With the nondominant hand, the physician uses a tongue blade or a Macintosh size 3 laryngoscope blade to displace the tongue medially, forming a gutter or trough along the floor of the mouth between the tongue and the teeth. The gutter ends in a cul-de-sac formed by the base of the palatoglossal arch (also known as the anterior tonsillar pillar), which is a U– or J-shaped structure that starts at the soft palate and runs along the lateral aspect of the pharynx. A 25-G spinal needle is inserted 0.25 to 0.5 cm deep at the base of the palatoglossal arch, just lateral to the base of the tongue, and an aspiration test is performed (Figs. 11-15 and 11-16). If air is aspirated, the needle has been advanced too deeply (i.e., the tip has advanced all the way through the palatoglossal arch) and should be withdrawn until no air can be aspirated; if blood is aspirated, the needle should be redirected more medially. Two mL of 1% to 2% lidocaine is injected, and the procedure is repeated on the contralateral side.
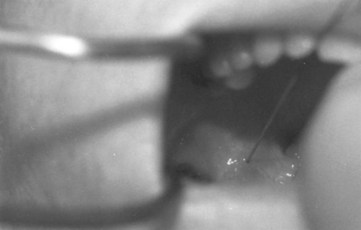
Figure 11-16 Right glossopharyngeal nerve block, anterior approach.
(From Difficult Airway: Teaching Aids. Irvine, University of California, Department of Anesthesia.)
Although this block is targeted at the lingual branch of the GPN, studies using methylene blue dye have shown that, in some cases, retrograde submucosal tracking of local anesthetic occurs, blocking more proximal branches of the GPN (i.e., pharyngeal and tonsillar branches). Serious complications are rare for this approach, although one study reported that 91% of patients undergoing this procedure experienced oropharyngeal discomfort for at least 24 hours after the procedure.134
c External Approach (Peristyloid)
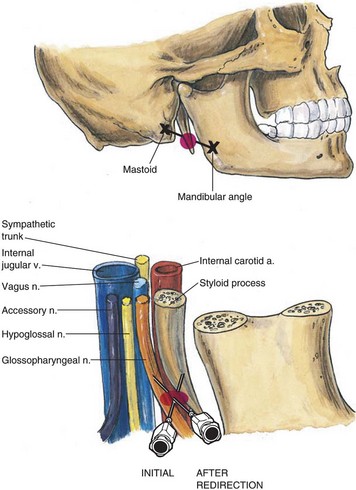
The external approach is most useful when the patient’s mouth opening is insufficient to allow adequate visualization to perform one of the intraoral blocks.102,129 The patient is placed supine with the head in a neutral position. The styloid process is located by identifying the midpoint of the line between the mastoid process and the angle of the jaw. A skin wheal with local anesthetic is made at this location, and a 22-G spinal needle is advanced perpendicularly to the skin until the styloid process is contacted. Depending on the patient’s habitus, this should occur at a depth of 1 to 2 cm. The needle is then redirected posteriorly, and as soon as contact is lost with the styloid process, 5 to 7 mL of 0.5% to 1% lidocaine is injected after a negative aspiration for blood (Fig. 11-17). The procedure is then repeated on the opposite side. Similar precautions should be taken for the peristyloid approach as for the posterior approach because of the proximity of the GPN at this anatomic location to both the internal carotid artery and the internal jugular vein.
C Larynx
1 Anatomy
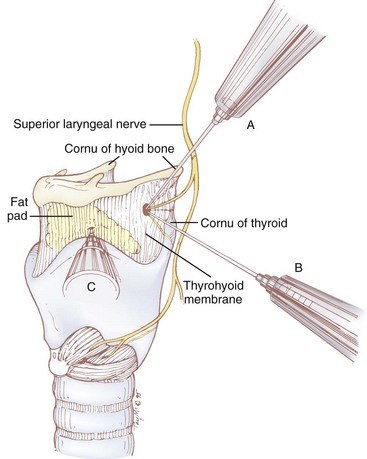
Sensory innervation of the larynx is supplied by the superior laryngeal nerve (SLN), a branch of the vagus nerve. The SLN originates from the ganglion nodosum and descends deep to the carotid artery. It then courses anteriorly, and at the level of the cornu of the hyoid bone it branches into the external and internal branches. The external branch supplies motor innervation to the cricothyroid muscle, which functions to tighten and elongate the vocal cords. The internal branch of the SLN contains sensory fibers that are distributed to the base of the tongue, vallecula, epiglottis, aryepiglottic folds, arytenoids, and glottis down to the level of the vocal cords. It passes medially between the greater cornu of the hyoid bone and the superior cornu of the thyroid cartilage and pierces the thyrohyoid membrane along with the superior laryngeal artery and vein. The nerve then lies in the paraglottic space, a closed space bounded by the thyrohyoid membrane laterally and the laryngeal mucosa medially, where it ramifies (Figs. 11-18 and 11-19).
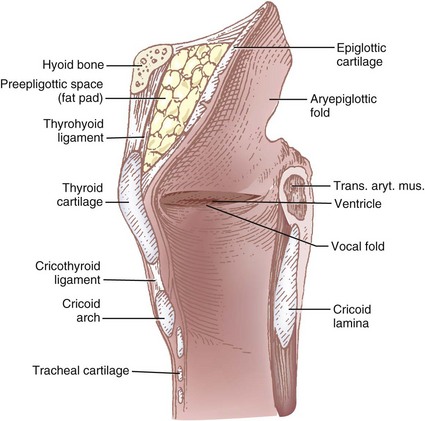
Figure 11-18 Midsagittal view of the larynx showing the pre-epiglottic space containing fat pad and thyrohyoid ligament (see Fig. 11-19).
2 Superior Laryngeal Nerve Block
a External Approach
In the external approach,5,102,113,128,135,136 the patient is placed in the supine position, head slightly extended, with the physician standing on the side to be blocked. The two main anatomic structures that are useful to identify are the hyoid bone and the superior cornu of the thyroid cartilage. The hyoid bone lies above the thyroid cartilage. It is identified by deep palpation; this can be uncomfortable to the patient and is difficult in patients who have a short or thick neck. Because it does not articulate with any other bones, the hyoid is freely movable, which helps in its identification. The greater cornu of the hyoid is the most lateral aspect of the bone that can be palpated. One side can be made more prominent by displacing the contralateral side toward the side being blocked. The superior lateral cornu of the thyroid cartilage can be identified by palpating the superior thyroid notch (“Adam’s apple”) and tracing the upper edge of the thyroid cartilage laterally until the most lateral aspect is identified. Three techniques using different landmarks have been described (see Fig. 11-19). Using 1% to 2% lidocaine, satisfactory sensory blockade is achieved within 5 minutes, with a success rate of 92% to 100%.136
Greater Cornu of the Hyoid
After identifying the greater cornu of the hyoid, the physician uses the nondominant index finger to depress the carotid artery laterally and posteriorly. With the dominant hand, a 25-G needle is “walked off” the cornu of the hyoid bone (see A in Fig. 11-19) in an anterior-inferior direction, aiming toward the middle of the thyrohyoid membrane. A slight resistance is felt as the needle is advanced through the membrane, usually at a depth of 1 to 2 cm (2 to 3 mm deep to the hyoid bone). The needle at this point has entered the pre-epiglottic space. Aspiration through the needle should be attempted. If air is aspirated, the needle has gone too deep and may have entered the pharynx; it should be withdrawn until no air can be aspirated. If blood is aspirated, the needle has cannulated the superior laryngeal artery or vein or the carotid artery; the needle should be directed more anteriorly. After satisfactory needle placement is achieved, 2 to 3 mL of local anesthetic is injected as the needle is withdrawn. The block is repeated on the opposite side. An ultrasound-guided technique for this approach has been described and may be beneficial for patients with abnormal neck anatomy.138
Superior Cornu of the Thyroid
A similar technique to the one previously described uses the superior lateral cornu of the thyroid as the landmark. The benefit of this technique is that, in many patients, this structure is easier and less painful to palpation than the hyoid bone. A 25-G needle is walked off the cornu of the thyroid cartilage (see B in Fig. 11-19) in a superior-anterior direction, aiming toward the lower third of the thyroid membrane. The same precautions as before are taken, and the local anesthetic solution is injected as the needle is withdrawn. The block is repeated on the opposite side.
Thyroid Notch
The easiest landmark to identify in many patients, especially in the morbidly obese, is the thyroid notch (Adam’s apple), the most medial and superficial aspect of the thyroid cartilage. The thyroid notch is palpated, and the upper border of the thyroid cartilage is traced laterally for approximately 2 cm (see C in Fig. 11-19). With a 25-G needle, the thyrohyoid ligament is pierced just above the thyroid cartilage at this location, and the needle is advanced in a posterior and cephalad direction to a depth of 1 to 2 cm from the skin. The tip of the needle is then in the pre-epiglottic space, which normally contains the terminal branches of the internal branch of the SLN imbedded in a fat pad (see Fig. 11-18). After an aspiration test, the entire volume of local anesthetic is injected into the pre-epiglottic space, and the needle is withdrawn. The block is repeated on the opposite side. An added benefit of this approach is the decreased likelihood of blocking the motor branch of the SLN.
b Internal Approach
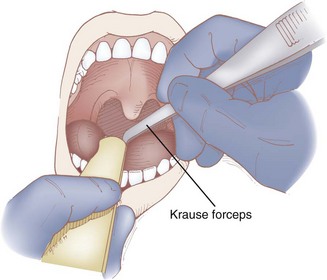
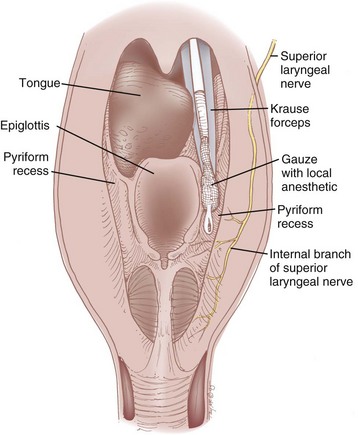
A noninvasive SLN block can be performed by applying local anesthetic to the pyriform recess. At this anatomic location, the internal branch of the SLN lies submucosally, and blockade is possible by diffusion of concentrated local anesthetic.25,113 After adequate topicalization of the oropharynx, the patient is placed in the sitting position with the physician standing on the contralateral side of the nerve to be blocked. The patient’s mouth is opened widely with the tongue protruded. The tongue is grasped with the nondominant hand using a gauze pad and gently pulled anteriorly, or it is depressed with a tongue blade. With the dominant hand, a Krause forceps holding a sponge soaked in 4% lidocaine is advanced over the lateral posterior curvature of the tongue along the downward continuation of the tonsillar fossa (Fig. 11-20). The tip of the forceps is advanced until it cannot be advanced any farther (Fig. 11-21); at that point, the handle of the forceps should be in a horizontal position and the tip should be resting in the pyriform recess. The position of the tip of the forceps may be checked by palpating the neck lateral to the posterior-superior aspect of the thyroid cartilage. The forceps are kept in this position for 5 minutes or longer, and then the process is repeated on the opposite side. This approach requires a considerable length of time and is limited to those patients who can open their mouths sufficiently wide.
3 Cautions, Complications, and Contraindications
Hypotension and bradycardia have also been associated with SLN blockade. A number of possible causes of this reaction have been postulated, including (1) vasovagal reaction related to painful stimulation, (2) digital pressure on the carotid sinus, (3) excessive manipulation of the larynx causing vasovagal reaction, (4) large doses of or accidental intravascular administration of local anesthetic drugs, and (5) direct neural stimulation of the branch of the vagus nerve by the needle.139 It is recommended that anticholinergics be administered before the block is performed.
Contraindications to the external approach include local infection, local tumor growth, and coagulopathy. Although the position is not universally accepted, some have advocated avoidance of SLN anesthesia in patients who are at high risk for aspiration.140
D Trachea and Vocal Cords
1 Anatomy
The alternative is topicalization of the mucosa. In addition to the use of a nebulizer, atomizer, or “spray-as-you-go” technique, topicalization of the trachea may be achieved by translaryngeal (transtracheal) injection. Although this technique does not provide a nerve block in the strictest sense, it is invasive and bears risks similar to those of other airway nerve blocks. In one study comparing transtracheal injection, “spray-as-you-go” topicalization through an FFB, and nebulization, transtracheal injection was most preferred by patients and bronchoscopists.142 Tracheal topicalization is also of particular benefit in cases in which a neurologic examination is required after intubation, because it makes the presence of an ETT more comfortable.
2 Translaryngeal (Transtracheal) Anesthesia
b Technique
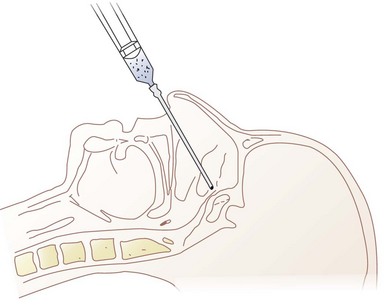
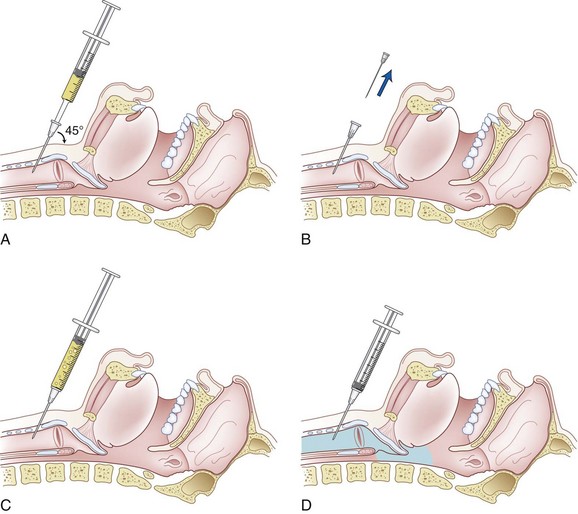
The physician should stand at the side of the patient with the dominant hand closest to the patient. The patient is asked not to talk, swallow, or cough until instructed. The midline of the cricothyroid membrane is identified as the needle insertion site. The index and middle finger of the nondominant hand can be used to mark this spot and stabilize the trachea (Fig. 11-22). Using a tuberculin syringe or a 25-G needle, the physician raises a small skin wheal. A 20-G angiocatheter attached to a 5- to 10-mL syringe containing 3 to 5 mL saline is used. The needle is advanced through the skin perpendicularly or slightly caudally while aspirating. When air is freely aspirated, the sheath of the angiocatheter is advanced slightly, the needle is removed, and a syringe containing 3 to 5 mL of 2% to 4% lidocaine is carefully attached to the catheter sheath that has been left in place. Aspiration of air is reconfirmed, the patient is warned to expect vigorous coughing, and the local anesthetic is injected rapidly during inspiration (Fig. 11-23). The sheath of the angiocatheter may be left in place until the intubation is complete in case more local anesthetic is needed and to decrease the likelihood of subcutaneous emphysema. Coughing helps to nebulize the local anesthetic so that the inferior and superior surfaces of the vocal cords can be anesthetized along with the tracheobronchial tree and inferior larynx. Anesthesia of the epiglottis, vallecula, tongue, and posterior pharyngeal wall are possible but unreliable. The success of translaryngeal anesthesia has been found to be as high as 95% and is attributed to both topicalization of the airway and systemic absorption.
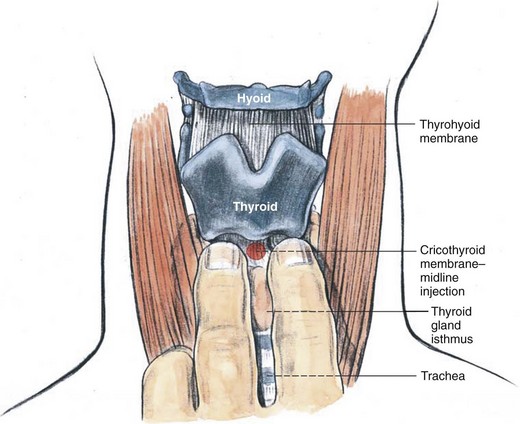
Figure 11-22 Translaryngeal anesthesia, anatomic landmarks.
(From Brown D, editor: Atlas of regional anesthesia, ed 2, Philadelphia, 1999, Saunders.)
This technique may be performed using a standard 20- or 22-G needle. This may, however, increase the risk of airway injury from the sharp metal bevel as the patient coughs. If this technique is used, care should be taken to remove the needle immediately after injection of the local anesthetic. This technique has also been described using a 25-G needle, but this is not recommended, because it introduces the risk of needle breakage as a result of movement of the cricoid cartilage cephalad when the patient coughs.141
c Cautions, Complications, Contraindications
The tip of the needle should never be aimed in a cephalad direction, to avoid laryngeal trauma and to ensure adequate spread of local anesthetic below the vocal cords. Because this procedure eliminates the patient’s ability to protect the airway from aspiration, it should not be performed in patients who are at high risk for aspiration. Coughing elicited by this block may result in increased heart rate, mean arterial blood pressure, ICP, and intraocular pressure. As a result, it is contraindicated in patients with elevated ICP or open globe, and care should be taken in patients with significant cardiac disease. It is also relatively contraindicated in patients with cervical instability, although its routine use in these patients has been described without complications.143 Transtracheal injection should be avoided in patients with local tumor or large goiter.
Potential complications are similar to those described for retrograde intubation (see Chapter 20). They include subcutaneous and intratracheal bleeding, infection, subcutaneous emphysema,144 pneumomediastinum, pneumothorax, vocal cord trauma, and esophageal perforation. These complications are rare, as was illustrated by a review of 17,500 cases of translaryngeal puncture that showed an incidence of complications of less than 0.01%.141
VIII Clinical Pearls
• When faced with a difficult airway (DA), awake intubation (AI) is the gold standard for airway management.
• Preparation begins with a careful history and physical examination and a detailed discussion of the procedure with the patient.
• The goals of premedication are to alleviate anxiety, provide a clear and dry airway, protect against the risk of aspiration, and enable adequate topicalization.
• Sedation may be accomplished with benzodiazepines, opioids, or intravenous hypnotics, either alone or in combination; these agents must be titrated carefully to maintain cooperation and adequate ventilation.
• Adequate topicalization of the airway with local anesthetics is the key to a successful AI.
• If airway topicalization is insufficient, airway nerve blocks may be employed to supplement airway anesthesia.
• There are many choices involved when preparing for AI. Safety should be the primary consideration.
All references can be found online at expertconsult.com.
2 Kopman AF, Wollman SB, Ross K, et al. Awake endotracheal intubation: A review of 267 cases. Anesth Analg. 1975;54:323–327.
5 Thomas JL. Awake intubation: Indications, techniques and a review of 25 patients. Anaesthesia. 1969;24:28–35.
7 Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277. Erratum 10:565, 2004
9 Benumof JL. Management of the difficult adult airway, with special emphasis on awake tracheal intubation. Anesthesiology. 1991;75:1087–1110.
18 Ovassapian A, Krejcie TC, Yelich SJ, et al. Awake fibreoptic intubation in the patient at high risk of aspiration. Br J Anaesth. 1989;62:13–16.
25 Reed AP. Preparation of the patient for awake flexible fiberoptic bronchoscopy. Chest. 1992;101:244–253.
45 Reves JG, Glass PSA, Lubarsky DA, et al. Intravenous nonopioid anesthetics. In: Miller RD, ed. Miller’s anesthesia. ed 6. Philadelphia: Elsevier Churchill Livingstone; 2005:317–378.
57 Fukuda K. Intravenous opioid anesthetics. In: Miller RD, ed. Miller’s anesthesia. ed 6. Philadelphia: Elsevier Churchill Livingstone; 2005:379–438.
102 Simmons ST, Schleich AR. Airway regional anesthesia for awake fiberoptic intubation. Reg Anesth Pain Med. 2002;27:180–192.
126 Hagberg CA. Airway blocks. In: Chelly JE, ed. Peripheral nerve blocks: A color atlas. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2008:177–184.
1 Macewen W. Clinical observations on the introduction of tracheal tubes by the mouth instead of performing tracheotomy or laryngotomy. Br Med J. 1880;2:122–124.
2 Kopman AF, Wollman SB, Ross K, et al. Awake endotracheal intubation: A review of 267 cases. Anesth Analg. 1975;54:323–327.
3 Meschino A, Devitt JH, Koch P, et al. The safety of awake tracheal intubation in cervical spine injury. Can J Anaesth. 1992;39:114–117.
4 Ovassapian A. Fiberoptic airway endoscopy in anesthesia and critical care. New York: Raven Press; 1990.
5 Thomas JL. Awake intubation: Indications, techniques and a review of 25 patients. Anaesthesia. 1969;24:28–35.
6 Practice guidelines for management of the difficult airway: A report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 1993;78:597–602.
7 Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277. Erratum 10:565, 2004
8 Caplan RA, Posner KL, Ward RJ. Adverse respiratory events in anesthesia: A closed claims analysis. Anesthesiology. 1990;72:828–833.
9 Benumof JL. Management of the difficult adult airway, with special emphasis on awake tracheal intubation. Anesthesiology. 1991;75:1087–1110.
10 Reed AP, Han DG. Preparation of the patient for awake fiberoptic intubation. Anesthesiol Clin North Am. 1991;9:69–81.
11 Bailenson G, Turbin J, Berman R. Awake intubation: Indications and technique. Anesth Prog. 1967;14:272–278.
12 Barash PG, Cullen BF, Stoelting RK, et al. Clinical anesthesia, ed 6, Philadelphia: Lippincott Williams & Wilkins, 2009.
13 Miller RD, ed. Miller’s anesthesia, ed 6, Philadelphia: Elsevier Churchill Livingstone, 2005.
14 Evans BJ, Stanley RO, Burrows GD. Measuring medical students’ empathy skills. Br J Med Psychol. 1993;66:121–133.
15 Block MR, Coulehan JL. Teaching the difficult interview in a required course on medical interviewing. J Med Educ. 1987;62:35–40.
16 Farsad P, Galliguez P, Chamberlin R, et al. Teaching interviewing skills to pediatric house officers. Pediatrics. 1978;61:384–388.
17 Mongan PD, Culling RD. Rapid oral anesthesia for awake intubation. J Clin Anesth. 1992;4:101–105.
18 Ovassapian A, Krejcie TC, Yelich SJ, et al. Awake fibreoptic intubation in the patient at high risk of aspiration. Br J Anaesth. 1989;62:13–16.
19 Paul DR, Hoyt JL, Boutros AR. Cardiovascular and respiratory changes in response to change of posture in the very obese. Anesthesiology. 1976;45:73–78.
20 Tsueda K, Debrand M, Zeok SS, et al. Obesity supine death syndrome: Reports of two morbidly obese patients. Anesth Analg. 1979;58:345–347.
21 Zupan J. Fiberoptic bronchoscopy in anesthesia and critical care. In: Benumof JL, ed. Clinical procedures in anesthesia and intensive care. Philadelphia: Lippincott Williams & Wilkins; 2002:253–274.
22 Daos FG, Lopez L, Virtue RW. Local anesthetic toxicity modified by oxygen and by combination of the agents. Anesthesiology. 1962;23:755–761.
23 Benumof JL, Scheller MS. The importance of transtracheal jet ventilation in the management of the difficult airway. Anesthesiology. 1989;71:769–778.
24 Boucek CD, Gunnerson HB, Tullock WC. Percutaneous transtracheal high-frequency jet ventilation as an aid to fiberoptic intubation. Anesthesiology. 1987;67:247–249.
25 Reed AP. Preparation of the patient for awake flexible fiberoptic bronchoscopy. Chest. 1992;101:244–253.
26 Hamard F, Ferrandiere M, Sauvagnac X, et al. Propofol sedation allows intubation of the difficult airway with the Fastrach LMA. Can J Anesth. 2005;52:421–427.
27 Xue F, He N, Liao X, et al. Clinical assessment of awake endotracheal intubation using the lightwand technique alone in patients with difficult airways. Chin Med J. 2009;122:408–415.
28 Doyle DJ. Awake intubation using the GlideScope video laryngoscope: Initial experience in four cases. Can J Anaesth. 2004;51:520–521.
29 Cohn AI, McGraw SR, King WH. Awake intubation of the adult trachea using the Bullard laryngoscope. Can J Anaesth. 1995;42:246–248.
30 Raval C, Patel H, Patel P, et al. Retrograde intubation in a case of ankylosing spondylitis posted for correction of deformity of the spine. Saudi J Anaesth. 2010;4:38–41.
31 Stoelting RK. Anticholinergic drugs. Stoelting RK, ed. Pharmacology and physiology in anesthetic practice, ed 3, Philadelphia: Lippincott Williams & Wilkins, 1999.
32 Gross JB, Hartigan ML, Schaffer DW. A suitable substitute for 4% cocaine before blind nasotracheal intubation: 3% Lidocaine-0.25% phenylephrine nasal spray. Anesth Analg. 1984;63:915–918.
33 Kallar SK, Everett LL. Potential risks and preventive measures for pulmonary aspiration: New concepts in preoperative fasting guidelines. Anesth Analg. 1993;77:171–182.
34 White PF, Recart Friere A. Ambulatory outpatient anesthesia. In: Miller RD, ed. Miller’s anesthesia. ed 6. Philadelphia: Elsevier Churchill Livingstone; 2005:2589–2636.
35 Gibbs CP, Banner TC. Effectiveness of Bicitra as a preoperative antacid. Anesthesiology. 1984;61:97–99.
36 James CF, Modell JH, Gibbs CP, et al. Pulmonary aspiration: Effects of volume and pH in the rat. Anesth Analg. 1984;63:665–668.
37 Feldman M, Burton ME. Histamine-2 receptor antagonists, standard therapy for acid-peptic diseases (1). N Engl J Med. 1990;323:1672–1680.
38 Feldman M, Burton ME. Histamine-2 receptor antagonists, standard therapy for acid-peptic diseases (2). N Engl J Med. 1990;323:1749–1755.
39 Morison DH, Dunn GL, Fargas-Babjak AM, et al. A double-blind comparison of cimetidine and ranitidine as prophylaxis against gastric aspiration syndrome. Anesth Analg. 1982;61:988.
40 Mikawa K, Akamatsu H, Nishina K, et al. The effects of cimetidine, ranitidine, and famotidine on human neutrophil functions. Anesth Analg. 1999;89:218–224.
41 Memis D, Turan A, Karamanlioglu B, et al. The effect of intravenous pantoprazole and ranitidine for improving preoperative gastric fluid properties in adults undergoing elective surgery. Anesth Analg. 2003;97:1360–1363.
42 Lin CJ, Huang CL, Hsu HW, et al. Prophylaxis against acid aspiration in regional anesthesia for elective cesarean section: A comparison between oral single-dose ranitidine, famotidine and omeprazole assessed with fiberoptic gastric aspiration. Acta Anaesthesiol Sin. 1996;34:179–184.
43 Hirota A, Kudo M, Hashimoto H, et al. The efficacy of preanesthetic proton pump inhibitor treatment for patients on long-term H2 antagonist therapy. Anesth Analg. 2005;101:1038–1041.
44 Walsh M, Shorten G. Preparing to perform an awake fiberoptic intubation. Yale J Biol Med. 1998;71:537–549.
45 Reves JG, Glass PSA, Lubarsky DA, et al. Intravenous nonopioid anesthetics. In: Miller RD, ed. Miller’s anesthesia. ed 6. Philadelphia: Elsevier Churchill Livingstone; 2005:317–378.
46 Murphy PJ, Erskine R, Langton JA. The effect of intravenously administered diazepam, midazolam and flumazenil on the sensitivity of upper airway reflexes. Anaesthesia. 1994;49:105–110.
47 Reed AP. Preparation for intubation of the awake patient. Mt Sinai J Med. 1995;62:10–20.
48 Puchner W, Egger P, Löckinger A, et al. Evaluation of remifentanil as single drug for awake fiberoptic intubation. Acta Anaesthesiol Scand. 2002;46:350–354.
49 Lee MC, Absalom AR, Menon DK, et al. Awake insertion of the laryngeal mask airway using topical lidocaine and intravenous remifentanil. Anaesthesia. 2006;61:32–35.
50 Reves JG, Fragen RJ, Vinik HR, et al. Midazolam: Pharmacology and uses. Anesthesiology. 1985;62:310–324.
51 Dixon J, Power SJ, Grundy EM, et al. Sedation for local anaesthesia: Comparison of intravenous midazolam and diazepam. Anaesthesia. 1984;39:372–376.
52 Hendrickx JF, Eger EI, 2nd., Sonner JM, et al. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth Analg. 2008;107:494–506.
53 Lichtenbelt BJ, Olofsen E, Daha A, et al. Propofol reduces the distribution and clearance of midazolam. Anesth Analg. 2010;110:1597–1606.
54 Ben-Shlomo I, Tverskoy M, Fleyshman G, et al. Intramuscular administration of lidocaine or bupivacaine alters the effect of midazolam from sedation to hypnosis in a dose-dependent manner. J Basic Clin Physiol Pharmacol. 2003;14:257–263.
55 White PF, Shafer A, Boyle WA, 3rd., et al. Benzodiazepine antagonism does not provoke a stress response. Anesthesiology. 1989;70:636–639.
56 Amrein R, Hetzel W. Clinical pharmacology of flumazenil. Eur J Anaesthesiol Suppl. 1988;2:65–80.
57 Fukuda K. Intravenous opioid anesthetics. In: Miller RD, ed. Miller’s anesthesia. ed 6. Philadelphia: Elsevier Churchill Livingstone; 2005:379–438.
58 Machata AM, Gonano C, Holzer A, et al. Awake nasotracheal fiberoptic intubation: Patient comfort, intubating conditions, and hemodynamic stability during conscious sedation with remifentanil. Anesth Analg. 2003;97:904–908.
59 Shafer SL, Varvel JR. Pharmacokinetics, pharmacodynamics, and rational opioid selection. Anesthesiology. 1991;74:53–63.
60 Abramson SI, Holmes AA, Hagberg CA. Awake insertion of the Bonfils retromolar intubation fiberscope in five patients with anticipated difficult airways. Anesth Analg. 2008;106:1215–1217.
61 Randell T, Valli H, Lindgren L. Effects of alfentanil on the responses to awake fiberoptic nasotracheal intubation. Acta Anaesthesiol Scand. 1990;34:59–62.
62 Atkins JH, Mirza N. Anesthetic considerations and surgical caveats for awake airway surgery. Anesthesiol Clin. 2010;28:555–575.
63 Mingo OH, Ashpole KJ, Irving CJ, et al. Remifentanil sedation for awake fibreoptic intubation with limited application of local anesthetic in patients for elective head and neck surgery. Anaesthesia. 2008;63:1065–1069.
64 Machata AM, Gonano C, Holxer A, et al. Awake nasotracheal fiberoptic intubation: Patient comfort, intubating conditions, and hemodynamic stability during conscious sedation with remifentanil. Anesth Analg. 2003;97:904–908.
65 Rai MR, Parry TM, Dombrovskis A, et al. Remifentanil target-controlled infusion vs propofol target-controlled infusion for conscious sedation for awake fibreoptic intubation: A double-blinded randomized controlled trial. Br J Anaesth. 2008;100:125–130.
66 Teganeh N, Roshani B, Azizi B, et al. Target-controlled infusion of remifentanil to provide analgesia for awake nasotracheal fiberoptic intubations in cervical trauma patients. J Trauma. 2010;69:1185–1190.
67 Xu YC, Xue FS, Luo MP, et al. Median effective dose of remifentanil for awake laryngoscopy and intubation. Chin Med J (Engl). 2009;122:1507–1512.
68 Cafiero T, Esposito F, Fraioli G, et al. Remifentanil-TCI and propofol-TCI for conscious sedation during fibreoptic intubation in the acromegalic patient. Eur J Anaesth. 2008;25:670–674.
69 Abrams JT, Horrow JC, Bennett JA, et al. Upper airway closure: A primary source of difficult ventilation with sufentanil induction of anesthesia. Anesth Analg. 1996;83:629–632.
70 Scamman FL. Fentanyl-O2-N2O rigidity and pulmonary compliance. Anesth Analg. 1983;62:332–334.
71 Bennett JA, Abrams JT, Van Riper DF, et al. Difficult or impossible mask ventilation after sufentanil-induced anesthesia is caused primarily by vocal cord closure. Anesthesiology. 1997;87:1070–1074.
72 Ackerman WE, Phero JC, Theodore GT. Ineffective ventilation during conscious sedation due to chest wall rigidity after intravenous midazolam and fentanyl. Anesth Prog. 1990;37:46–48.
73 Jaffe TB, Ramsey FM. Alleviation of fentanyl-induced truncal rigidity. Anesthesiology. 1983;58:562–564.
74 Belleville JP, Ward DS, Bloor BC, et al. Effects of intravenous dexmedetomidine in humans, I: Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–1133.
75 Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001;7:221–226.
76 Ebert TJ, Hall JE, Barney JA, et al. The effect of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394.
77 Abdelmalak B, Makary L, Hoban J, et al. Dexmedetomidine as sole sedative for awake intubation in management of the critical airway. J Clin Anesth. 2007;19:370–373.
78 Bergese SD, Khabiri B, Roberts WD, et al. Dexmedetomidine for conscious sedation in difficult awake fiberoptic intubation cases. J Clin Anesth. 2007;19:141–144.
79 Bergese SD, Bender SP, McSweeney TD, et al. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22:35–40.
80 Avitsian R, Lin J, Lotto M, et al. Dexmedetomidine and awake fiberoptic intubation for possible cervical spine myelopathy. J Neurosurg Anesthesiol. 2005;17:97–99.
81 Bergese SD, Candiotti KA, Bokesch PM, et al. A phase IIIb, randomized, double-blind, placebo-controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. Am J Ther. 2010;17:586–595.
82 Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs. midazolam for sedation of critically ill patients. JAMA. 2009;301:489–499.
83 Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs. lorazepam on acute brain dysfunction in mechanically ventilated patients. JAMA. 2007;298:2644–2653.
84 Precedex [package insert]. Lake Forest, IL: Hospira, Inc, 2008.
85 Hall JE, Uhrich TD, Barney JA, et al. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705.
86 Kasuya Y, Govinda R, Rauch S, et al. The correlation between bispectral index and observational sedation in volunteers sedated with dexmedetomidine and propofol. Anesth Analg. 2009;109:1811–1815.
87 Ustün Y, Gündüz M, Erdogan O, et al. Dexmedetomidine vs. midazolam in outpatient third molar surgery. J Oral Maxillofac Surg. 2006;64:1353–1358.
88 Bloor BC, Ward DS, Belleville JP, et al. Effects of intravenous dexmedetomidine in humans, II: Hemodynamic changes. Anesthesiology. 1992;77:1134–1142.
89 Scher CS, Gitlin MC. Dexmedetomidine and low-dose ketamine provide adequate sedation for awake fiberoptic intubation. Can J Anaesth. 2003;50:607–610.
90 Levanen J, Makela ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995;82:1117–1125.
91 Perouansky MA, Hemmings HCJr. Intravenous anesthetic agents. In: Hemmings HC, Jr., Hopkins PM. Foundations of anesthesia: Basic and clinical sciences. ed 2. St. Louis: Mosby; 2006:295–310.
92 Iravani M, Wald SH. Dexmedetomidine and ketamine for fiberoptic intubation in a child with severe mandibular hypoplasia. J Clin Anesth. 2008;20:455–457.
93 Coppen JE, Fox JWC. Endobronchial intubation under neuroleptanalgesia for a patient with severe hemoptysis. Anesth Analg. 1968;47:70–71.
94 Redden RL, Biery KA, Campbell RL. Arterial oxygen desaturation during awake endotracheal intubation. Anesth Prog. 1990;37:201–204.
95 Droperidol [package insert]. Lake Forest, IL: Hospira, Inc, 2004.
96 Adriani J, Zepernick R, Arens J, et al. The comparative potency and effectiveness of topical anesthetics in man. Clin Pharmacol Ther. 1964;5:49–62.
97 Perry LB. Topical anesthesia for bronchoscopy. Chest. 1978;73:691–693.
98 Day RO, Chalmers DRC, Williams KM, et al. Death of a healthy volunteer in a human research project: Implications for Australian clinical research. Med J Aust. 1998;168:449–451.
99 . Case report on death of University of Rochester student issued. Available at http://www.health.state.ny.us/press/releases/1996/wan.htm (accessed January 2012)
100 Ho AMH, Chung DC, To EWH, et al. Total airway obstruction during local anesthesia in a non-sedated patient with a compromised airway. Can J Anesth. 2004;51:838–841.
101 Strichartz GR, Berde CB. Local anesthetics. In: Miller RD, ed. Miller’s anesthesia. ed 6. Philadelphia: Elsevier Churchill Livingstone; 2005:573–604.
102 Simmons ST, Schleich AR. Airway regional anesthesia for awake fiberoptic intubation. Reg Anesth Pain Med. 2002;27:180–192.
103 British Thoracic Society Bronchoscopy Guidelines Committee, a Subcommittee of Standards of Care Committee of British Thoracic Society. British Thoracic Society guidelines on diagnostic flexible laryngoscopy. Thorax. 2001;56(Suppl 1):i1–i21.
104 Langmack EL, Martin RJ, Pak J, et al. Serum lignocaine concentrations in asthmatics undergoing research bronchoscopy. Chest. 2000;117:1055–1060.
105 Parkes SB, Butler CS, Muller R. Plasma lignocaine concentration following nebulization for awake intubation. Anaesth Intensive Care. 1997;25:369–371.
106 Wieczorek PM, Schricker T, Vinet B, et al. Airway topicalization in morbidly obese patients using atomized lidocaine: 2% Compared with 4%. Anaesthesia. 2007;62:984–988.
107 McAlpine LG, Thomson NC. Lidocaine-induced bronchoconstriction in asthmatic patients: Relation to histamine airway responsiveness and effect of preservative. Chest. 1989;96:1012–1015.
108 Donlon JV, Jr., Doyle DJ, Feldman MA. Anesthesia for eye, ear, nose, and throat surgery. In: Miller RD, ed. Miller’s anesthesia. ed 6. Philadelphia: Elsevier Churchill Livingstone; 2005:2527–2556.
109 Novaro GM, Aronow HD, Militello MA, et al. Benzocaine-induced methemoglobinemia: Experience from a high-volume transesophageal echocardiography laboratory. J Am Soc Echocardiogr. 2003;16:170–175.
110 Guay J. Methemoglobinemia related to local anesthetics: A summary of 242 episodes. Anesth Analg. 2009;108:837–845.
111 Kotler RL, Hansen-Flaschen J, Casey MP. Severe methemoglobinemia after flexible fibreoptic bronchoscopy. Thorax. 1989;44:234–235.
112 Weisel W, Tella RA. Reaction to tetracaine (pontocaine) used as topical anesthetic in bronchoscopy: Study of 1000 cases. JAMA. 1951;147:218–222.
113 Roberts JT. Anatomy and patient positioning for fiberoptic laryngoscopy. Anesthesiol Clin North Am. 1991;9:53.
114 Adriani J, Campbell D. Fatalities following topical application of local anesthetic to mucous membranes. JAMA. 1956;162:1527–1530.
115 Douglas WW, Fairbanks VF. Methemoglobinemia induced by a topical anesthetic spray (cetacaine). Chest. 1977;71:587–591.
116 Sandza JG, Jr., Roberts RW, Shaw RC, et al. Symptomatic methemoglobinemia with a commonly used topical anesthetic, cetacaine. Ann Thorac Surg. 1980;30:187–190.
117 Larijani GE, Cypel D, Gratz I, et al. The efficacy and safety of EMLA cream for awake fiberoptic endotracheal intubation. Anesth Analg. 2000;91:1024–1026.
118 Bourke DL, Katz J, Tonneson A. Nebulized anesthesia for awake endotracheal intubation. Anesthesiology. 1985;63:690–692.
119 Foster WM, Hurewitz AN. Aerosolized lidocaine reduces dose of topical anesthetic for bronchoscopy. Am Rev Respir Dis. 1992;146:520–522.
120 Long TR, Wass CT. An alternative to transtracheal infection for fiberoptic intubation in awake patients: A novel noninvasive technique using a standard multiorifice epidural catheter through the bronchoscope suction port. Anesthesiology. 2004;101:1253.
121 Kundra P, Kutralam S, Ravishankar M. Local anesthesia for awake fiberoptic nasotracheal intubation. Acta Anaesthesiol Scand. 2000;44:511–516.
122 Standring S. Gray’s anatomy: The anatomical basis of clinical practice, ed 40. Philadelphia: Churchill Livingstone; 2009.
123 Katz J. Atlas of regional anesthesia, ed 2. New York: Appleton & Lange; 1994.
124 Norton ML, Brown ACD. Atlas of the difficult airway. St. Louis: Mosby; 1991.
125 Raj PP. Handbook of regional anesthesia. New York: Churchill Livingstone; 1985.
126 Hagberg CA. Airway blocks. In: Chelly JE, ed. Peripheral nerve blocks: A color atlas. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2008:177–184.
127 Waldman SD. Atlas of interventional pain management, ed 3. Philadelphia: Saunders; 2009.
128 DeMeester TR, Skinner DB, Evans RH, et al. Local nerve block anesthesia for peroral endoscopy. Ann Thorac Surg. 1977;24:278–283.
129 Brown DL. Atlas of regional anesthesia, ed 4. Philadelphia: Saunders; 2010.
130 Mulroy MF. Regional anesthesia: An illustrated procedural guide, ed 3. Philadelphia: Lippincott Williams & Wilkins; 2002.
131 Henthorn RW, Amayem A, Ganta R. Which method for intraoral glossopharyngeal nerve block is better? Anesth Analg. 1995;81:1113–1114.
132 Saliba DL, McCutchen TA, Laxton MJ, et al. Reliable block of the gag reflex in one minute or less. J Clin Anesth. 2009;21:463.
133 Kodama K, Seo N, Murayama T, et al. Glossopharyngeal nerve block for carotid sinus syndrome. Anesth Analg. 1992;75:1036–1037.
134 Sitzman BT, Rich GF, Rockwell JJ, et al. Local anesthetic administration for awake direct laryngoscopy: Are glossopharyngeal nerve blocks superior? Anesthesiology. 1997;86:34–40.
135 Gotta AW, Sullivan CA. Anaesthesia of the upper airway using topical anaesthetic and superior laryngeal nerve block. Br J Anaesth. 1981;53:1055–1058.
136 Furlan JC. Anatomical study applied to anesthetic block technique of the superior laryngeal nerve. Acta Anaesthesiol Scand. 2002;46:199–202.
137 Stockwell M, Lozanoff S, Lang SA, et al. Superior laryngeal nerve block: An anatomical study. Clin Anat. 1995;8:89–95.
138 Manikandan S, Neema PK, Rathod RC. Ultrasound-guided bilateral superior laryngeal nerve block to aid awake endotracheal intubation in a patient with cervical spine disease for emergency surgery. Anaesth Intensive Care. 2010;38:946–948.
139 Wiles JR, Kelly J, Mostafa SM. Hypotension and bradycardia following superior laryngeal nerve block. Br J Anaesth. 1989;63:125–127.
140 Walts LF, Kassity KJ. Spread of local anesthesia after upper airway block. Arch Otolaryngol. 1965;81:77–79.
141 Gold MI, Buechel DR. Translaryngeal anesthesia: A review. Anesthesiology. 1959;20:181–185.
142 Graham DR, Hat JG, Clague J, et al. Comparison of three different methods used to achieve local anesthesia for fiberoptic bronchoscopy. Chest. 1992;102:704–707.
143 Reasoner DK, Warner DS, Todd MM, et al. A comparison of anesthetic techniques for awake intubation in neurosurgical patients. J Neurosurg Anesthesiol. 1995;7:94–99.
144 Wong DT, McGuire GP. Subcutaneous emphysema following trans-cricothyroid membrane injection of local anesthetic. Can J Anaesth. 2000;47:165–168.