Chapter 93B Preoperative portal vein embolization
Technique
Overview
Portal vein embolization (PVE) is performed preoperatively to reduce the risk of extensive surgery in patients with small anticipated remnant livers (Makuuchi et al, 1990; de Baere et al, 1993; Abdalla et al, 2002; Madoff et al, 2005a; see Chapter 30a). PVE redirects portal blood flow to the intended future liver remnant (FLR) in an attempt to initiate hypertrophy of the nonembolized segments, and it has been shown to improve the functional reserve of the FLR before surgery. In appropriately selected patients, PVE has also been shown to reduce perioperative morbidity, and it allows for safe, potentially curative hepatectomy for patients previously considered ineligible for resection based on anticipated small remnant livers (Azoulay et al, 2000; Madoff et al, 2005a; Abulkhir et al, 2008). For this reason, PVE is now performed at many comprehensive hepatobiliary centers worldwide prior to major hepatectomy. This section details the techniques currently used to perform PVE, discusses their advantages and disadvantages, and reviews the potential complications that can occur from this procedure.
Technical Aspects of Portal Vein Embolization
Access Routes to the Portal Venous System
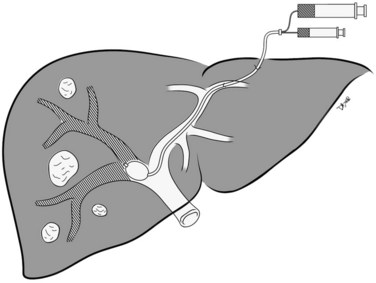
PVE can be performed by any of three standard approaches: 1) the intraoperative transileocolic venous approach, 2) the transhepatic contralateral approach (i.e., percutaneous portal vein is accessed via the FLR), and 3) the transhepatic ipsilateral approach (i.e., percutaneous portal access is via the liver to be resected; Madoff et al, 2005a). It is important to note that the approach to the portal vein is chosen at the discretion of the operator, and the decision may be based on multiple factors, including the extent of the embolization and surgery, the operator’s preference for a specific embolic agent, tumor burden within the liver, and the operator’s level of experience with one technique over another (Avritscher et al, 2008). An alternative approach, portal vein ligation, is typically performed intraoperatively during the first stage of a two-stage resection for hepatic colorectal metastases, and it has been reported to be as effective as percutaneous embolization methods (Aussilhou et al, 2008). Regardless of the technique used, the operator must be aware of variations in portal venous anatomy that may impact the conduct of the procedure (see Chapter 1B).
Transileocolic Venous Approach
The transileocolic venous approach was the first approach described for performing preoperative PVE, and it is still used in many Asian centers. This technique is performed at laparotomy by direct cannulation of the ileocolic vein and introduction of a catheter into the portal system for embolization (Abdalla et al, 2002). Conventional teaching has been that this approach is performed when additional treatment is needed during the same surgical exploration, when a percutaneous approach is not considered feasible, or when an interventional radiology suite is not available (Azoulay et al, 1995; Denys et al, 2002).
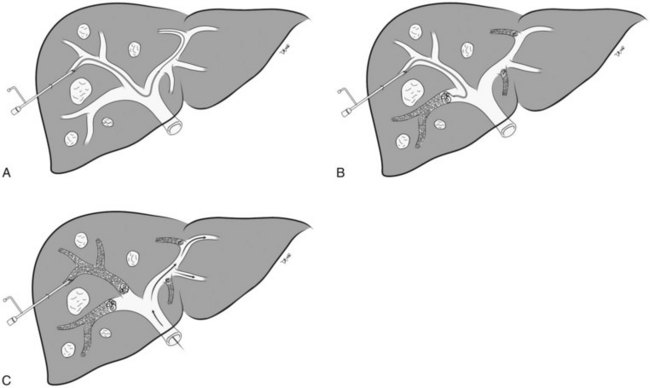
Percutaneous Transhepatic Ipsilateral Approach
The percutaneous transhepatic ipsilateral approach was first described by Nagino and coworkers (1996), and it is now advocated by many other investigators (Madoff et al, 2003, 2005b; Gibo et al, 2007; Tsuda et al, 2006). With this approach, access is obtained through the portal branches within the tumor-bearing liver (Fig. 93B.1). A distinct advantage of the ipsilateral approach is that the FLR is not instrumented. It also allows for straightforward catheterization of segment IV branches, should embolization of segment IV be required. When planning the puncture, the anterior segment of the right portal vein is preferred, because its use is associated with a lower complication rate (Kodama et al, 2002); however, catheterizing the right portal branches can be challenging, owing to the sharp angulation of the right portal veins, which often necessitates using reverse-curve catheters or balloon occlusion catheters with multiple lumina (Nagino et al, 1996, 2000a).
In Nagino’s approach (see Fig. 93B.1), the right anterior portal vein is punctured using sonographic guidance, and a 6-Fr sheath is introduced into the right portal vein system (Nagino et al, 2000a). To make this procedure feasible, the authors designed two types of 5.5-Fr triple-lumen balloon catheters. The first catheter, “type 1,” was designed with one lumen connected to the balloon and two lumina connected to the tip. The second catheter, “type 2,” had two separate lumina opening proximal to the balloon, and the balloons were used to prevent any backflow of embolic material. Both catheters had two separate lumina so that fibrin glue and iodized oil could be injected simultaneously. To facilitate resection, the authors advocated that a proximal right portal vein segment at least 1 cm in length remain patent. Depending on the portal vein anatomy, and the need to spare the proximal right portal vein, type 1 or type 2 catheters were used for embolization. The type 1 catheter was used for embolization of branches distal to the catheter tip, whereas the type 2 catheter was used for embolization of branches proximal to the catheter tip, as mandated by each patient’s portal vein anatomy.
In a recent study, Gibo and coworkers (2007) reported on a modified technique utilizing a four-lumen balloon catheter in eight patients. The authors recommended using this modified catheter because of its larger occlusion balloon and lumina, which allow for safer and easier embolization using fibrin glue. Unfortunately, neither the original nor the modified catheters are available in the United States.
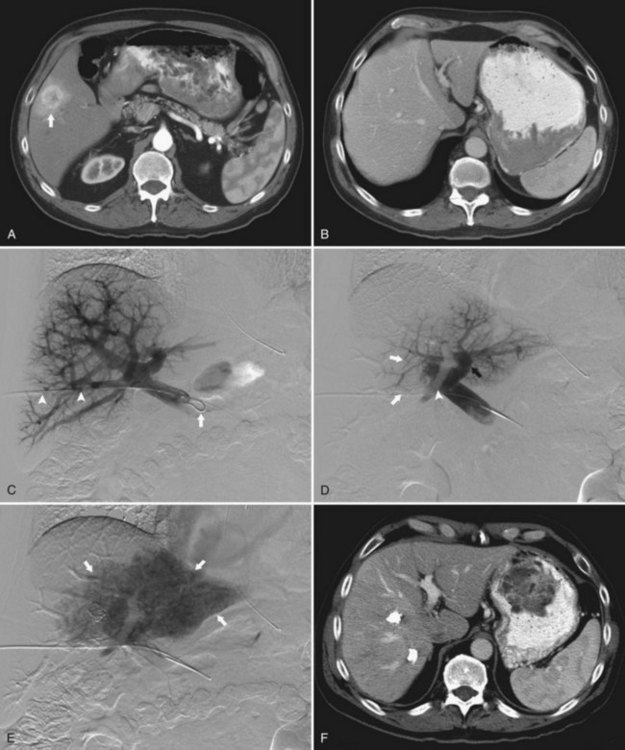
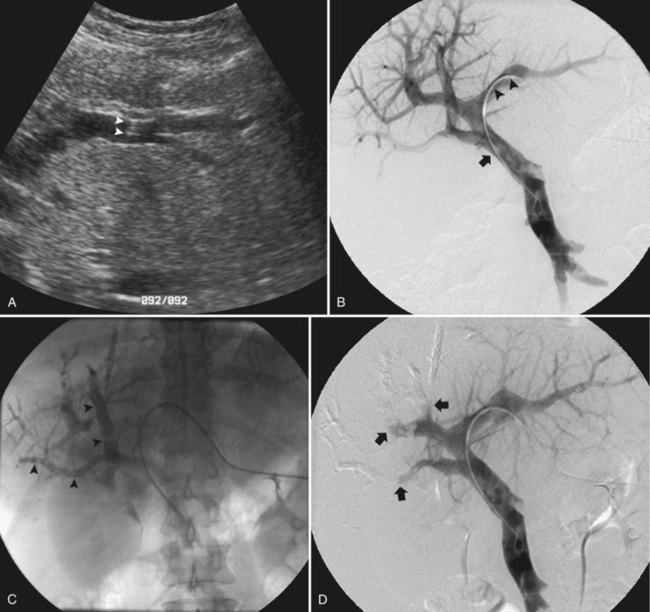
In the early 2000s, Madoff and coworkers (2003, 2005b) described a technique using angiographic catheters commercially available worldwide (Figs. 93B.2 and 93B.3). Under sonographic guidance, a 22-gauge Chiba needle (Neff Percutaneous Access Set; Cook Medical, Bloomington, IN) is used to puncture a distal branch of the right portal system. Subsequently, a 5- or 6-Fr vascular sheath is advanced over a guidewire into the right portal vein branch to aid with subsequent catheter exchanges. Flush portography is performed with a 5-Fr angiographic flush catheter placed within the main portal vein. Anteroposterior and craniocaudal projections are obtained as needed to delineate the portal anatomy.
When embolization of the right portal vein is extended to segment IV (Fig. 93B.4), segment IV embolization should be performed first (Madoff et al, 2005b). This sequence is advocated because of the potential difficulty of exchanging catheters through a previously embolized right portal system and the possible dislodgement of embolic material from the right liver during the subsequent treatment of segment IV, which is usually embolized with a 3-Fr microcatheter placed coaxially through a selective 5-Fr angiographic catheter.
Early on, polyvinyl alcohol (PVA) particles (Contour SE Microspheres; Boston Scientific, Natick, MA) were the embolic agents of choice (Madoff et al, 2003). PVA was administered in a stepwise fashion: smaller particles (355 to 500 µm) were used first to occlude the distal branches, and larger particles (up to 1000 µm) were used subsequently to occlude branches more proximally. Larger particles were not used until the forward portal blood flow was considerably reduced. Additional embolization with the larger particles was then performed, until near complete stasis was achieved. Later, with the advent of spherical embolic agents, tris-acryl gelatin microspheres (EmboGold Microspheres; Biosphere Medical, Rockland, MA) became the embolic agent of choice (Madoff et al, 2005b). EmboGold microspheres ranging from 100 to 700 µm in diameter are administered in a stepwise fashion, similar to the method used for the PVA particles. After particulate embolization is complete, platinum microcoils (Boston Scientific) are placed within the proximal segment IV branches to further reduce portal blood inflow that could lead to recanalization.
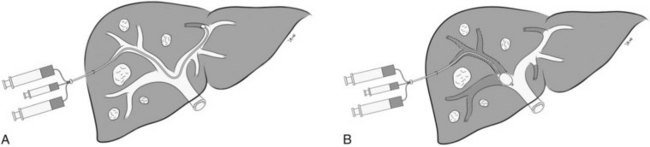
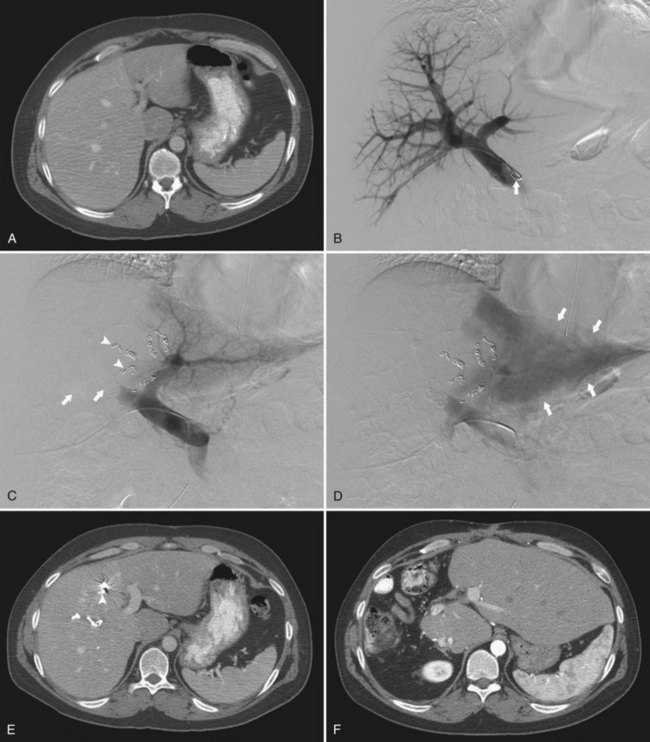
Percutaneous Transhepatic Contralateral Approach
The percutaneous transhepatic contralateral approach was first described by Kinoshita and coworkers (1986) to slow the progression of tumor thrombus within the portal system. Modifications of this technique were later developed for the purpose of causing FLR hypertrophy (de Baere et al, 1993, 1996; Figs. 93B.5 and 93B.6). Similar to the ipsilateral approach, this approach also requires ultrasound-guided percutaneous puncture, using an 18- or 22-gauge needle, of a peripheral portal branch, preferably segment III accessed via the subxiphoid route. Because access is gained through the FLR, great care is taken to limit the number of punctures, and as with the ipsilateral approach, the most peripheral branch possible is targeted to avoid damage to the central structures.
The major advantage of the contralateral approach over the ipsilateral approach is that catheterization of the right portal branches is technically easier and does not have to contend with awkward angles. In addition, embolization is performed with the catheter pointed toward the direction of flow. Some authors report that the contralateral approach allows better visualization of the embolized portal branches during the final portogram (Di Stefano et al, 2005); however, different operators have found that in using the modified ipsilateral approach, the image quality of the final postembolization portography is similar. In addition, with the use of reverse-curve catheters, ipsilateral PVE in the right liver can also be performed safely in the direction of portal venous flow (Madoff et al, 2002).
The main drawback of the contralateral approach is that it requires instrumentation of the left hepatic parenchyma during catheterization of the portal venous branches supplying the FLR, potentially causing injury to the FLR parenchyma and/or to the left portal vein (Madoff et al, 2005a). If there are complications after PVE, these may involve the FLR and make the planned surgical resection more difficult or, at times, impossible.
Additional Approaches
Portal Vein Embolization with Transjugular Access
RPVE by means of the transjugular route has also been described (Perarnau et al, 2003). This technique was tried because of the familiarity gained during the preceding decade with transjugular intrahepatic portosystemic shunts (TIPS). Under sonographic guidance, the right internal jugular vein was accessed, and then with fluoroscopy, a right or left portal branch was punctured from a right, middle, or left hepatic vein. A catheter was placed near the portal bifurcation and used to perform RPVE with a mixture of NBCA and iodized oil. All 15 procedures were technically successful without any serious complications. FLR hypertrophy was deemed sufficient, and right hepatectomy was performed in 12 patients (80%). Although this approach appears safe and effective, the series was small, and further studies are needed before this approach becomes widespread. RPVE in patients with cirrhosis may be an attractive alternative; however, the technical feasibility of RPVE extended to segment IV has yet to be explored.
Portal Vein Embolization Followed by Bland Transarterial Embolization
Other approaches have been used for PVE. The combination of PVE and transarterial embolization (TAE) for complete portal venous and hepatic arterial occlusion has been reported in patients with biliary tract cancer and colorectal metastases who had inadequate hypertrophy after PVE alone (Nagino et al, 2000b; Gruttadauria et al, 2006). Nagino and coworkers (2000b) described a patient who required an extended left hepatectomy, but the FLR volume (i.e., the right posterior liver) did not increase 51 days after PVE. After arterial embolization, the FLR volume increased from 485 cm3 before PVE to 685 cm3 after PVE, an addition of 215 cm3. Another patient required a right hepatectomy, but no significant volume change was seen after PVE (pre-PVE, 643 cm3; post-PVE, 649 cm3). After arterial embolization, the left liver volume enlarged to 789 cm3, an increase of 140 cm3. Both patients underwent uneventful resection after the staged procedures.
Sequential Arterial Embolization and Portal Vein Embolization
In 2004, Aoki and colleagues described their experience with the use of sequential transcatheter arterial chemoembolization followed within two weeks by PVE in 17 patients with hepatocellular carcinoma (HCC). Their justification for this approach was that, first of all, the livers of most patients with HCC are compromised by underlying liver disease such that the liver’s regenerative capability after hepatic resection is weakened, making it hard to predict whether adequate FLR hypertrophy can be achieved after PVE. Second, arterioportal shunts often found in cirrhotic livers and HCC may limit the effects of PVE. Third, since most HCCs are hypervascular, supplied largely by arterial blood flow, termination of portal flow induces compensatory augmentation in arterial blood flow (i.e., “arterialization of the liver”) in the embolized segments that may lead to rapid tumor progression after PVE.
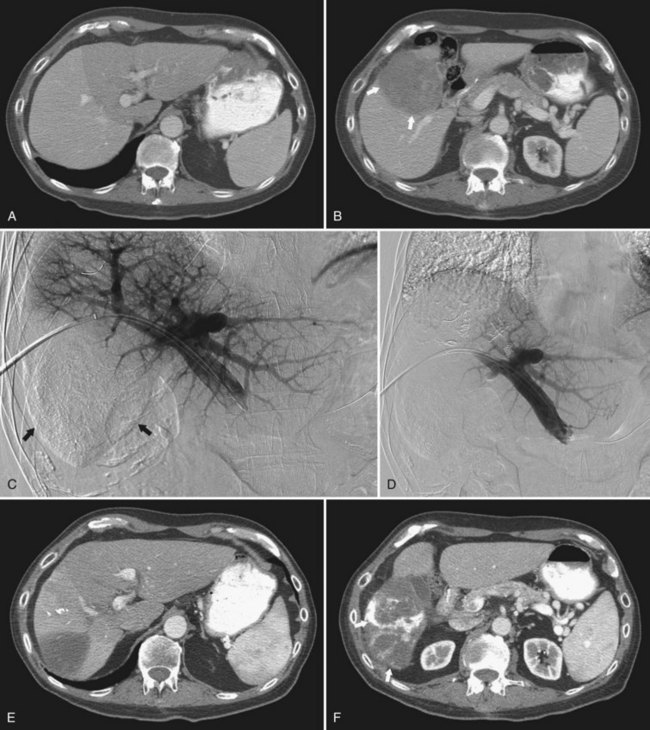
More recently, a French group reported on the use of sequential chemoembolization and PVE in 36 patients with HCC and chronic liver disease prior to right hepatectomy (Ogata et al, 2006; Fig. 93B.7). In their study, 18 patients underwent chemoembolization, followed 3 to 4 weeks later by PVE, and the remaining 18 patients underwent PVE alone. Although PVE was well tolerated in all patients, the mean increase in percentage of FLR volume was significantly higher for patients in the combined chemoembolization and PVE group than in those who underwent PVE alone (P = .022). The incidence of complete tumor necrosis (83% [15/18] vs. 6% [1/18]; P < .001) and 5-year disease-free survival rate (37% vs. 19%; P = .041) were also significantly higher in patients who underwent chemoembolization and PVE. Given the risks of hepatic infarction, the authors recommended that the two procedures should be separated by at least 3 weeks to reduce procedure-related morbidity; however, similar to Nagino’s approach of performing PVE followed by TAE, the downside of the combined approach is that two separate procedures and increased waiting times are required.
Extent of Embolization
The optimal extent of PVE is presently a subject of considerable debate (van Gulik et al, 2008). Currently, most who prepare patients for extended right hepatectomy with PVE occlude only branches of the right portal vein and leave the segment IV portal veins patent (de Baere et al, 1993; Capussotti et al, 2005). Although FLR hypertrophy does occur, full diversion of portal flow to segments II and III ensures the maximal stimulus for FLR hypertrophy (Nagino et al; 1995, 2000; Kishi et al, 2008; Mueller et al, 2008). Further, incomplete embolization of the liver to be resected will also lead to hypertrophy of segment IV, which is undesirable for an extended right hepatectomy because of increased morbidity associated with a larger area of intraoperative parenchymal transection across this hypertrophic segment (Nagino et al, 1995).
Nagino and coworkers (2000) first showed that a greater left lateral bisegment hypertrophy occurs after right PVE extended to segment IV (50% increase in FLR volume) than after only right PVE (31% increase, P < .0005). Recent studies have supported Nagino’s conclusions, and without any increase in PVE-associated complications (Kishi et al, 2008; Mueller et al, 2008). Because segment IV embolization has been shown to be advantageous, the ipsilateral approach for right PVE extended to segment IV has been further refined, which has led to improved FLR hypertrophy and better operative outcomes. Lastly, left PVE is rarely needed because of consistently large volumes of the right posterior liver (segments VI and VII; Nagino et al, 1995; Abdalla et al, 2004; Leelaudomlipi et al, 2002).
Another potential benefit of extending right PVE to include segment IV, from an oncologic perspective, is that the entire tumor-bearing liver is systematically embolized (i.e., right PVE for right hepatectomy, right PVE extended to segment IV for extended right hepatectomy) to reduce the risk of tumor growth that may result from increased portal blood flow and hepatotrophic factors (Madoff et al, 2005a; Ribero et al, 2007; de Graaf et al, 2009). A recent study from a large academic cancer center evaluated 112 patients in whom the entire tumor-bearing liver was systematically embolized and found no increase in the median tumor size during the waiting period (Ribero et al, 2007); however, tumor growth within the nonembolized liver has been discussed upon analysis of a very limited number of patients with primary and secondary liver tumors after right PVE alone, although; comparison to pre-PVE tumor growth rate was made; the true effect of PVE on tumor growth could therefore not be proven (Elias et al, 1999; Kokudo et al, 2001). Furthermore, liver hypertrophy occurs quickly in patients with normal liver, and thus resection can be undertaken in most patients with multiple colorectal metastases within 3 to 4 weeks of PVE.
Embolic Agents
Early in the experience with PVE, gelatin sponge was widely used as an embolic agent; however, recanalization of the embolized portal veins was frequently observed within 2 weeks of the procedure (Makuuchi et al, 1990; Kinoshita et al, 1986; de Baere et al, 1996), and when compared with other embolic agents, it seemed less effective at 4 weeks in terms of causing hypertrophy. Also, fibrin glue combined with ethiodized oil is a commonly used mixture for PVE. This mixture also leads to early recanalization, as it results in less than 75% portal occlusion at 2 weeks and less than 25% portal occlusion at 4 weeks (Makuuchi et al, 1990; de Baere et al, 1996).
In terms of effectiveness, de Baere and coworkers (1996) reported that PVE with NBCA led to a 90% increase in FLR volume after 30 days, and Denys and coworkers (2005) found it useful in inducing hypertrophy in patients with underlying hepatic fibrosis or cirrhosis; however, there are some drawbacks of using NBCA for PVE. For example, the NBCA injections have to be precise because of the increased risk of nontarget embolization; thus it requires a highly experienced operator. Furthermore, NBCA can be difficult to use in patients with reduced hepatopetal flow, as is commonly seen in patients with chronic hepatic disease. These altered flow dynamics have been associated with increased risk of procedural complications (Di Stefano et al, 2005). NBCA also induces an inflammatory process that may make hepatectomy more difficult (de Baere et al, 1996; Avritscher et al, 2008). Lastly, although this agent may be straightforward when the anatomy is favorable (e.g., the anterior and posterior sector portal veins originate from a right portal vein), it may not be the best alternative in situations where variant anatomy is present, or when multiple segment IV veins are to be embolized (i.e., multiple microcatheters are needed, leading to considerable expense and increased risk of nontarget embolization to the FLR).
Absolute ethanol is another efficient embolic agent for PVE. Ogasawara and coworkers (1996) showed near doubling of the FLR volume within 4 weeks for patients with chronic hepatic disease and HCC who underwent PVE with this agent. Unfortunately, of all the embolic agents used for PVE, the most marked changes in liver function tests and the poorest patient tolerance is seen with absolute ethanol.
Recently, the use of particulate agents for PVE was also proposed. In the first clinical report in a single patient, no recanalization of the right portal vein was observed 5 weeks after PVE with PVA particles alone (Brown et al, 2001). Later, Madoff and coworkers (2002, 2003, 2005b) showed that a combination of particles (e.g., polyvinyl alcohol particles [PVA], tris-acryl gelatin microspheres) and coils is safe and effective for PVE. Particles are safe, they cause little periportal reaction, and they generate durable portal vein occlusion, especially when used in combination with coils (Madoff et al, 2002, 2003). In 2003, results from their first 26 patients who had PVE with nonspherical PVA particles, ranging in size from 300 to 1000 µm, and coils were reported; the mean FLR/total estimated liver volume (TELV) increased 7.8% (pre-PVE FLR/TELV, 17.6%; post-PVE FLR/TELV, 25.4%), and the mean absolute FLR increase was 47% (Madoff et al, 2003).
The subsequent advance of spherical particulate embolics has led to even further refinements in technique for PVE by using a stepwise infusion of very small (100 to 300 µm) tris-acryl gelatin microspheres followed by larger spheres (up to 700 µm; Madoff et al, 2005b). This type of distal embolization is thought to limit development of collateral circulation that may potentially reduce hypertrophy because of the improved targeting of distal portal vein branches (nonspherical particles tend to clump and therefore do not always reach the targeted size vessel). Metallic coils are then used proximally to block venous inflow and further reduce the possibility of recanalization. This newer approach was used for right PVE extended to segment IV and led to an absolute increase in FLR volume of 69.0%, an FLR/TELV increase of 9.7%, and a subsequent resection rate of 86% that was a significant improvement over their previously reported method.
Complications of Portal Vein Embolization
As with all transhepatic procedures, complications include subcapsular hematoma, hemoperitoneum, hemobilia, arterioportal shunts, arteriovenous fistula, pseudoaneurysm, portal vein thrombosis, transient liver failure, pneumothorax, and sepsis (Kodama et al, 2002; Di Stefano et al, 2005). Kodama and colleagues (2002) compared complication rates between the contralateral and ipsilateral approaches in 47 patients who underwent PVE and found that in 11 patients who underwent contralateral PVE, 2 (18.1%) experienced complications, and in 36 patients who underwent ipsilateral PVE, 5 (13.9%) experienced complications. This difference was not statistically significant, and the rate of technical complications associated with percutaneous PVE using either approach was 14.9%. The following complications were those found in this patient cohort: two subcapsular hematomas, two pneumothoraces, one inadvertent arterial puncture, one pseudoaneurysm (in a patient who also had a subcapsular hematoma), one hemobilia, and one portal vein thrombosis. Complications more specific to percutaneous PVE included portal vein thrombosis and portal hypertension resulting in esophageal variceal hemorrhage. The authors stressed that given the potential for injury to the FLR when using the contralateral approach, the ipsilateral approach should be tried first.
Di Stefano and coworkers (2005) later reported on 188 patients who underwent PVE using the contralateral approach. They reported that only one patient experienced a major complication (complete portal vein thrombosis) directly related to the contralateral approach that precluded the planned surgical resection. Two other patients experienced inadvertent migration of embolic material into the FLR that required intervention; one needed a portoportal graft during hepatic resection because of portal vein thrombosis. On CT imaging, another 10 patients were found to have embolic material in nontargeted portal venous branches.
In 2007, Ribero and coworkers (2007) reported on 112 patients who underwent PVE with the ipsilateral approach. In this study, only one patient had nontarget embolization to the FLR; however, the overall complication rate was 8.9%, which was not substantially different than the rate reported by Di Stefano and coworkers (2005). If it is taken into account that they considered clinically occult incidental CT findings in their complication rate, the studies reported remarkably similar numbers. Further, the study by Ribero and others (2007) found no difference in the complication rate, whether right PVE was extended to segment IV or not.
Abdalla EK, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-680.
Abdalla EK, et al. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404-410.
Abulkhir A, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57.
Aoki T, et al. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766-774.
Aussilhou B, et al. Right portal vein ligation is as effective as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg. 2008;12:297-303.
Avritscher R, et al. Percutaneous transhepatic portal vein embolization: rationale, technique and outcomes. Semin Interv Radiol. 2008;25:132-145.
Azoulay D, et al. Right portal vein embolization in preparation for major hepatic resection. J Am Coll Surg. 1995;181:266-269.
Azoulay D, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480-486.
Brown K, et al. Portal vein embolization with use of polyvinyl alcohol. J Vasc Interv Radiol. 2001;12:882-886.
Capussotti L, et al. Extension of right portal vein embolization to segment IV portal branches. Arch Surg. 2005;40:1100-1103.
de Baere T, et al. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology. 1993;188:73-77.
de Baere T, et al. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24:1386-1391.
de Graaf W, et al. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009;16:423-430.
Denys A, et al. Indications for and limitations of portal vein embolization prior to major hepatic resection for hepatobiliary malignancy. Surg Oncol Clin N Am. 2002;11:955-968.
Denys A, et al. Portal vein embolization with N-butyl cyanoacrylate before partial hepatectomy in patients with hepatocellular carcinoma and underlying cirrhosis or advanced fibrosis. J Vasc Interv Radiol. 2005;16:1667-1674.
Di Stefano DR, et al. Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 2005;234:625-630.
Elias D, et al. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784-788.
Gibo M, et al. Percutaneous ipsilateral portal vein embolization using a modified four-lumen balloon catheter with fibrin glue: initial clinical experience. Radiat Med. 2007;25:164-172.
Gruttadauria S, et al. Sequential preoperative ipsilateral portal and arterial embolization in patients with colorectal liver metastases. World J Surg. 2006;30:576-578.
Kinoshita H, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803-808.
Kishi Y, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 2008;144:744-751.
Kodama Y, et al. Complications of percutaneous transhepatic portal vein embolization. J Vasc Interv Radiol. 2002;13:1233-1237.
Kokudo N, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267-272.
Leelaudomlipi S, et al. Volumetric analysis of liver segments in 155 living donors. Liver Transpl. 2002;8:612-614.
Madoff DC, et al. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics. 2002;22:1063-1076.
Madoff DC, et al. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness—study in 26 patients. Radiology. 2003;227:251-260.
Madoff DC, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215-225.
Madoff DC, et al. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779-790.
Makuuchi M, et al. Preoperative portal vein embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527.
Mueller L, et al. Major hepatectomy for colorectal metastases: is preoperative portal occlusion an oncological risk factor? Ann Surg Oncol. 2008;15:1908-1917.
Nagino M, et al. Right or left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery. 1995;117:677-681.
Nagino M, et al. Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology. 1996;200:559-563.
Nagino M, et al. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery. 2000;127:155-160.
Nagino M, et al. Portal and arterial embolization before extensive liver resection in patients with markedly poor functional reserve. J Vasc Interv Radiol. 2000;11:1063-1068.
Ogasawara K, et al. Selective portal vein embolization with absolute ethanol induces hepatic hypertrophy and makes more extensive hepatectomy possible. Hepatology. 1996;23:338-345.
Ogata S, et al. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091-1098.
Perarnau JM, et al. Transjugular preoperative portal embolization (TJPE): a pilot study. Hepatogastroenterology. 2003;50:610-613.
Ribero D, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386-1394.
Tsuda M, et al. Ipsilateral percutaneous transhepatic portal vein embolization with gelatin sponge particles and coils in preparation for extended right hepatectomy for hilar cholangiocarcinoma. J Vasc Interv Radiol. 2006;17:989-994.
van Gulik TM, et al. Controversies in the use of portal vein embolization. Dig Surg. 2008;25:436-444.