Chapter 31
Preoperative Management
Matthew J. Eagleton, Jeanwan Kang
Based on a chapter in the seventh edition by Tamara N. Fitzgerald and Alan Dardik
Preoperative assessment and management of vascular patients are of utmost importance because patients with vascular disease have multisystem involvement, including cardiac, cerebrovascular, renal, and peripheral arterial disease, as well as multiple other comorbid conditions. Consequently, they are at high risk for complications after surgery. A focused preoperative evaluation should identify potential treatable risk factors so that complications can be minimized and optimal choices can be made for the timing of elective surgery.
General Preoperative Risk Assessment
Every patient undergoing elective vascular surgery should have a preoperative assessment that includes a thorough history and physical examination, blood analysis, and electrocardiogram (ECG). In addition, if there are patient-specific areas of concern, these should receive a more detailed, directed evaluation. A complete blood count should be obtained to screen for the presence of infection, to ensure an adequate red blood cell volume, and to rule out a serious hematologic abnormality. Serum electrolyte concentrations should be evaluated and corrected when abnormalities exist. Of special importance are serum potassium, calcium, and magnesium levels because if they are abnormal and not corrected, they can lead to deleterious cardiac effects. Furthermore, because renal disease is so prevalent in vascular patients and some vascular interventions may compromise renal function, a baseline creatinine level should be obtained. All patients should also have serum glucose concentration measured, and in diabetic patients, glucose levels should be controlled before, during, and after intervention. Measures of coagulation, such as the prothrombin time and international normalized ratio, should be determined to identify coagulation abnormalities, and in patients taking warfarin or other anticoagulants, an appropriate anticoagulation scheme should be decided on before surgery (Box 31-1).
Because cardiac disease is so prevalent among patients with peripheral vascular disease, ECG should be performed in all patients. ECG not only provides critical information about the presence of a rhythm abnormality or previous infarction but also provides a baseline assessment should an adverse cardiac event occur in the postoperative period. Chest radiography may be helpful in some patients if undiagnosed underlying disease is suspected on the basis of the history and physical examination. In select patients, more advanced testing may be required, such as dobutamine stress echocardiography or pulmonary function tests when cardiac or pulmonary disease is suspected. Finally, overall assessment of health can be quantified by the American Society of Anesthesiology classification (Table 31-1).
Table 31-1
American Society of Anesthesiology (ASA) Classification
| ASA I | Normal, healthy patient with good exercise tolerance |
| ASA II | Controlled medical conditions without significant systemic effects |
| ASA III | Medical conditions with systemic effects; functional compromise |
| ASA IV | Medical condition with significant dysfunction; potential threat to life |
| ASA V | Critical medical condition; little chance of survival with or without surgery |
| ASA VI | Brain death; anesthesia performed for organ donation |
Specific Risk Factor and Organ System Evaluation
Cardiac Evaluation
Heart disease is present in more than 50% of patients with peripheral vascular disease, so it is imperative that all vascular patients be appropriately screened for cardiac disease. Cardiac disease is discussed in more detail in Chapter 39, but a brief overview of the preoperative evaluation is provided here.
Factors that identify patients with heart disease include coronary artery disease, heart failure, previous myocardial infarction (MI), aortic stenosis, and advanced age. Other predictors are diabetes, hypertension, and poor functional capacity. During the preoperative evaluation, the surgeon should acquire a detailed cardiac history, including information on current exercise tolerance, angina, dyspnea, fatigue, syncope, arrhythmias, previous MI, and prior cardiac interventions. A thorough cardiovascular examination should also be performed and an ECG obtained in all patients both to serve as a baseline assessment and to identify dysrhythmias or signs of current or previous myocardial ischemia. On the basis of this information and the clinical presentation of the patient, the decision is then made as to whether a more detailed preoperative cardiac assessment is necessary.
The American College of Cardiology Foundation and the American Heart Association have provided recommendations to guide physicians with regard to pursuing further cardiac workup (Table 31-2).1
Table 31-2
ACCF/AHA Recommendation for Perioperative Cardiac Assessment
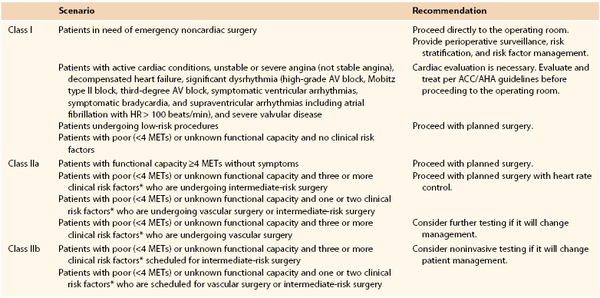
* Clinical risk factors include ischemic heart disease, compensated or prior heart failure, diabetes mellitus, renal insufficiency, and cerebrovascular disease.
Class I recommendations suggest that procedures/treatments should be performed; Class IIa recommendations suggest that it is reasonable to perform the procedure/treatment; Class IIb recommendations imply that the procedure/treatment should be considered; and in Class III the intervention should not be performed because it may not be helpful and may potentially be harmful to the patient.
ACCF/AHA, American College of Cardiology Foundation/American Heart Association; AV, atrioventricular; HR, heart rate; METs, metabolic equivalents.
Major vascular procedures represent the highest risk procedures. Endovascular aortic aneurysm repair and carotid endarterectomy, however, are considered within the intermediate-risk category, distinct from the open vascular surgery procedures. Dipyridamole-thallium imaging or dipyridamole stress echocardiography may be indicated for patients at intermediate or high cardiac risk who are undergoing vascular surgery (as outlined before), but it is probably unnecessary in low-risk individuals. In addition, those who have undergone coronary revascularization within 5 years or have normal findings on coronary angiography or cardiac stress testing within 2 years may also proceed to surgery without further cardiac evaluation provided there have been no significant functional changes since that time.
Preoperative Medical versus Interventional Therapy for Cardiac Disease
Perioperative use of beta blockers has long been the standard of care for most patients with cardiac disease undergoing vascular surgery. Their use, however, has engendered significant controversy during the past several years. The POISE (PeriOperative Ischemic Evaluation) trial confirmed a reduction in primary cardiac events with perioperative beta-blockade therapy.2 Nearly 8000 patients undergoing noncardiac surgery were randomized to a fixed higher dose, extended-release metoprolol started the day of surgery, or no treatment. Whereas patients receiving beta-blockade therapy experienced a reduction in cardiovascular death, MI, and cardiac arrest, this benefit was offset by an increased risk of stroke and total mortality. The results suggested that the administration of high-dose beta blockers in the absence of dose titration may be harmful in beta-blocker naive patients. In the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography trial (DECREASE-IV), patients were randomized to receive beta blockade only, statin only, beta blockade and statin, or neither medication before elective noncardiac surgery.3 Beta-blocker use, initiated well before surgery and titrated for heart rate (50-70 beats/min), proved to be cardioprotective to intermediate-risk patients, without an increase in perioperative stroke or death. The results of this trial, however, have been called into question.3a On the basis of these studies as well as several others, the American College of Cardiology Foundation/American Heart Association adjusted their recommendations for perioperative beta-blocker use in 2009 (Table 31-3).
Table 31-3
ACCF/AHA Guidelines for Perioperative Beta-Blocker Use
| Scenario | Recommendation | |
| Class I | Patients undergoing surgery who are receiving beta blockers for treatment of conditions with ACCF/AHA Class I guidelines for the drugs | Continue perioperative beta blockers. |
| Class IIa | Patients undergoing vascular surgery who are at high cardiac risk owing to CAD Preoperative assessment for vascular surgery identifies high cardiac risk as defined by more than one clinical risk factor* Preoperative assessment identifies CAD or high cardiac risk (identified by the presence of >1 clinical risk factor*) in patients who are undergoing intermediate-risk surgery |
Beta blockers titrated to heart rate and blood pressure are reasonable. |
| Class IIb | Patients undergoing either intermediate-risk procedures or vascular surgery in whom preoperative assessment identifies a single clinical risk factor* in the absence of CAD Patients undergoing vascular surgery with no clinical risk factors who are not currently taking beta blockers |
The usefulness of beta blockers is uncertain. |
| Class III | Patients undergoing surgery who have absolute contraindication to beta blockade Routine administration of high-dose beta blockade in the absence of dose titration in patients not currently taking beta blockers who are undergoing noncardiac surgery |
Use is contraindicated. |
* History of ischemic heart disease, heart failure, cerebrovascular disease, diabetes mellitus, and renal insufficiency.
ACCF/AHA, American College of Cardiology Foundation/American Heart Association; CAD, coronary artery disease.
In addition to beta blockade, the patient’s current cardiovascular regimen, including other antihypertensives and statins, should be maintained. Historically, some recommended that aspirin be discontinued before surgery to avoid hemorrhagic complications; current guidelines, however, recommend continued aspirin perioperatively, given its value in cardiovascular health and low risk of associated bleeding.4 Few data are available to recommend the cessation or continuation of clopidogrel before major vascular surgery, although most consider there to be increased risk of bleeding associated with its continued use. Before considering stopping of antiplatelet agents, however, one must take into consideration whether the patient has had a recent percutaneous coronary intervention. If aspirin and clopidogrel need to be discontinued perioperatively, elective surgery should be postponed 4 to 6 weeks after placement of a bare metal stent and 12 months after a drug-eluting stent.4 If surgery cannot be postponed, aspirin therapy should be continued throughout the perioperative period. In general, statin agents should not be discontinued before surgery but should be continued throughout the hospitalization, in particular in patients with vascular disease. There is evidence demonstrating the effectiveness of statins in preventing cardiovascular events among high-risk patients through their effects on endothelial function, reduction in vascular inflammation, and stabilization of atherosclerotic plaque.1
It is often debated whether coronary artery revascularization should be performed before elective peripheral vascular surgery. In support of a conservative management plan, procedure-related complications in patients undergoing coronary artery revascularization are frequent and often lead to delays in the intended vascular surgery. Furthermore, patients who have recently (<5 years) undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) before vascular surgery do not have an obvious survival advantage over patients at high cardiac risk without previous coronary interventions. In addition, the Coronary Artery Revascularization Prophylaxis (CARP) trial demonstrated that in patients with stable coronary artery disease, coronary artery revascularization (with either CABG or PCI) before elective major vascular surgery does not improve long-term survival or reduce short-term postoperative outcomes such as death, MI, or length of hospital stay.5 The trial did exclude patients with unstable coronary syndromes, more than 50% stenosis of the left main coronary artery, and left ventricular ejection fraction below 20%. In further support of these results, the DECREASE-V pilot study screened 1880 patients scheduled to undergo major vascular surgery to identify a high-risk cohort.6 The final cohort of 101 patients were randomized to best medical therapy alone or best medical therapy and revascularization before vascular surgery. Thirty-day and 1-year all-cause death and nonfatal MI were similar between the two groups. Thus, current recommendations are that CABG or PCI be reserved for patients with unstable cardiac symptoms or advanced coronary disease, for whom a survival benefit with CABG or PCI is clear.1
Preoperative Management of Hypertension
Hypertension must be carefully controlled in the perioperative period because excessive swings in blood pressure and heart rate place a patient with coronary artery disease at risk for cardiac ischemia. In addition, because chronic hypertension resets cerebral circulatory autoregulation, reductions in blood pressure may induce cerebral ischemia. Therefore, an appropriate history should be obtained for each patient, including onset of hypertension, degree and strategy of control, and current medications. Although most hypertension is primary, the surgeon should take care to assess the possibility of hypertension from a secondary source, such as renal artery stenosis, coarctation of the aorta, pheochromocytoma, Conn’s syndrome, Cushing’s syndrome, or other causes that may prove dangerous during a surgical procedure.
Blood pressure should always be measured in a relaxed setting with an appropriately sized cuff to prevent erroneous values. The diagnosis of hypertension is made when two or more readings of diastolic blood pressure are higher than 90 mm Hg or systolic blood pressure is higher than 140 mm Hg. Accelerated hypertension is defined as markedly elevated blood pressure plus grade 3 retinopathy (hemorrhage and exudates), whereas malignant hypertension is defined as markedly elevated blood pressure plus papilledema. Complicated hypertension includes cardiovascular end-organ damage, such as stroke, renal failure, MI, or aortic aneurysm.
Several measures can be taken to reduce the operative risks associated with hypertension. Beta blockade is a good choice for blood pressure control in patients with vascular disease because it is also valuable for cardioprotection. In addition, beta blockade has been shown to limit the magnitude of the rise in blood pressure during intubation and the amount of ischemic changes on the ECG during surgical procedures. The use of α2 agonists, such as clonidine, can lead to reduced rates of perioperative MI and mortality. Patients should be maintained with their current antihypertensives as withholding of these medications before surgery may result in rebound hypertension. The exceptions are angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and diuretics, which should be withheld the day of surgery, particularly in patients with renal insufficiency.
Elective surgery should be delayed if systolic blood pressure is higher than 200 mm Hg or if diastolic blood pressure is higher than 120 mm Hg, and acute control within hours of surgery is inadvisable. Pain can elevate blood pressure; therefore, appropriate analgesia with nonsteroidal anti-inflammatory drugs or opioids should be administered to alleviate pain. Agents with minimal hemodynamic effects should be considered, such as fentanyl rather than morphine. Before induction of anesthesia, patients should also be well hydrated because volume loading can minimize the drastic swings in blood pressure that are characteristic of hypertensive patients. Refer to Chapter 30 for a more detailed discussion of hypertension in vascular patients.
Pulmonary Evaluation
A thorough discussion of pulmonary care is provided in Chapter 40, but a brief review of preoperative pulmonary assessment is covered here. Postoperative pulmonary complications play a significant role in the risk for surgery and anesthesia. As such, preoperative pulmonary evaluation should be completed in all patients and should begin with a history and physical examination, which are the most sensitive evaluation to identify “at-risk” patients. Risk factors for postoperative pulmonary complications include patient-related risk factors (i.e., chronic obstructive pulmonary disease, age older than 60 years, American Society of Anesthesiology class II or higher, functionally dependent, and congestive heart failure). Obesity and mild to moderate asthma do not increase the risk of pulmonary complications.7 The surgeon should pay particular attention to any history of smoking, exercise intolerance, unexplained dyspnea, or coughing. On physical examination, decreased breath sounds, wheezes, crackles, or prolonged expiratory phase should be noted. Procedure-related risks should likewise be assessed. The duration of surgery, the need for emergent surgery, and the surgical site all influence the risk of perioperative pulmonary complications. Aortic aneurysm repair, thoracic surgery, abdominal surgery, upper abdominal surgery, emergency surgery, and vascular surgery all increase the risk of pulmonary complications.7
In some situations, the at-risk patient will require further pulmonary evaluation and risk stratification. Whereas clinicians frequently obtain chest radiographs as part of a routine preoperative evaluation, few show unexpected abnormalities or influence the management of the patient. Despite this, there is some evidence that chest radiographs may be helpful for patients with known cardiopulmonary disease and those older than 50 years who are undergoing upper abdominal, thoracic, and open abdominal aortic aneurysm surgery.7 The value of pulmonary function testing before lung resection surgery and in determining the candidacy for coronary artery revascularization is well documented. Its value outside of these cases, however, remains controversial. In addition, available data do not suggest a prohibitive threshold on these studies below which the risks of surgery are not acceptable. Currently the American College of Physicians recommends preoperative pulmonary function testing only in patients who are thought to have undiagnosed chronic obstructive pulmonary disease. Other factors, such as low serum albumin concentration (<35 g/L), are powerful, independent markers of increased risk for postoperative pulmonary complications.8 Several indices incorporating multiple factors can be used to stratify pulmonary risk.
Pulmonary risk factors have been added to the Goldman Cardiac Risk Index and include the following: obesity (body mass index >27 kg/m2), cigarette smoking within 8 weeks of surgery, productive cough within 5 days of surgery, wheezing within 5 days of surgery, ratio of forced expiratory volume in 1 second to forced vital capacity of less than 70%, and PaCO2 within 45 mm Hg. Patients with a combined score of more than 4 points (of 10) are 17 times more likely to have complications.
Once a patient is identified as at risk for pulmonary complications, risk reduction strategies should be used. These include smoking cessation, inspiratory muscle training, optimization of nutritional status, intraoperative strategies, and postoperative lung expansion techniques when appropriate. Patients who currently smoke have a twofold increased risk for postoperative complications, with the highest risk in patients who have smoked within the last 2 months. It has been questioned, therefore, whether preoperative smoking cessation could reduce the perioperative risk. In a prospective randomized trial in men undergoing hip and knee replacement surgery, patients were randomly assigned to usual care versus a smoking cessation program including weekly meetings and nicotine replacement.9 Overall complications were lower in the intervention group (18% vs 52%), but this was primarily due to fewer wound complications and urinary tract infections. No differences in pulmonary measures were identified, but there was a trend toward shorter hospital stays and fewer cardiac complications in the intervention group. Smoking cessation may paradoxically increase postoperative complications for smokers who stop or reduce smoking shortly (<2 months) before surgery.10 The cessation may transiently increase mucus production as a result of improved mucociliary activity while reducing coughing because of less bronchial irritation. Patients who have quit smoking for more than 6 months have a risk similar to that of those who do not smoke, and the beneficial effects of smoking cessation, including improvement in ciliary function and a decrease in sputum production, occur gradually during a period of several weeks. Strategies to improve the quit rate for smoking include counseling, nicotine replacement therapy, bupropion, and varenicline. Nicotine replacement therapy is available in several formulations, including transdermal patch, gum, nasal spray, inhaler, and lozenge. Bupropion is an atypical antidepressant, and varenicline is a partial agonist of the α4β2 nicotinic acetylcholine receptor. Second-line agents are nortriptyline, a tricyclic antidepressant, and clonidine, an antihypertensive drug. All of the available treatments appear to be similarly effective, with the exception of varenicline, which has been shown to offer significant improvement in abstinence rates over antidepressants.
Patients with a history of bronchospasm and chronic obstructive pulmonary disease should also be optimized before surgery. International guidelines suggest the use of inhaled bronchodilators, β2 agonists, and anticholinergics as the mainstay of symptomatic therapy. Systemic corticosteroids are recommended when the forced expiratory volume in the first second of expiration is less than 80% of predicted. In addition, it has been demonstrated that the instances of bronchospasm during intubation in patients with bronchial hyperreactivity who were not previously receiving bronchodilators is reduced with the use of albuterol and steroids. The safety of perioperative corticosteroid use has been established, and it is not associated with death or serious infections. However, in patients who have received systemic steroids for more than 3 weeks within the past 6 months, stress-dose steroid coverage should be provided perioperatively.
Lung expansion techniques, such as incentive spirometry, chest physical therapy, cough, postural drainage, ambulation, and continuous positive airway pressure, have been employed to limit postoperative pulmonary complications. Available data suggest that for patients undergoing abdominal surgery, any type of lung expansion intervention is better than no prophylaxis at all, although no one modality is clearly superior and there is little benefit to combining modalities.7 In addition, the selective use of nasogastric tube decompression after abdominal surgery likely decreases rates of pneumonia and atelectasis, with earlier return to oral intake and no differences in aspiration rates. Despite the association of hypoalbuminemia with the development of postoperative pulmonary complications, the use of preoperative nutritional supplementation (either parenteral or enteral) has not been demonstrated to reduce postoperative pulmonary complications and should not be instituted solely for this purpose.
Renal Evaluation
In addition to the information presented in this text, a more detailed description of renal issues is provided in Chapter 41. Vascular patients are frequently found to have kidney dysfunction at initial evaluation because of the shared risk factors of diabetes, hypertension, and atherosclerosis, and therefore kidney function must be carefully evaluated preoperatively. The preoperative evaluation should include an assessment of renal failure symptoms such as fatigue, dry skin, muscle cramps, decreased urine output, recurrent urinary tract infections, edema, history of renal disease, and history of dialysis or transplantation. The physical examination should focus on signs of hyperkalemia, volume status, anemia, and bleeding.
In all patients, a baseline creatinine level should be obtained, both to identify problems with renal clearance preoperatively and to establish baseline renal function should renal compromise occur. The glomerular filtration rate should also be calculated because it has been shown to be a more sensitive assessment of renal function and, in certain settings, is a better predictor of postoperative morbidity than serum creatinine concentration alone is. In addition, as noted previously, laboratory tests should assess electrolyte status, the presence of anemia, and coagulation ability, and the chest radiograph should be examined carefully for signs of fluid overload. If the patient is being maintained with dialysis, careful examination of the vascular access site should be performed. If a dialysis catheter is present, it should be inspected for signs of infection, and functionality of the catheter should be verified. If an arteriovenous fistula or graft is present, the fistula or graft should be assessed for thrill and bruit, infection should be excluded, and the examiner should verify that there is adequate blood flow to the hand. The fistula or graft should also be protected before and during surgery, commonly by wrapping the site in loose gauze. The nursing and technical staff should also be advised to avoid drawing blood from the arm used for access.
Hyperkalemia is common in patients with chronic renal insufficiency or end-stage renal disease (ESRD). It is important to correct potassium levels before surgical intervention because hyperkalemia may cause cardiac instability. Treatment options include polystyrene binding resins, insulin in combination with intravenous dextrose, calcium carbonate for cardiac stabilization, intravenous bicarbonate, and dialysis. Polystyrene binding resins can be given orally or as retention enemas and, like dialysis, remove excess stores of potassium, although retention enemas should be used with care to prevent colonic necrosis. Patients maintained with dialysis should undergo dialysis the day before surgery. In addition to correction of electrolytes, acid-base balance should be achieved before surgery because acidosis can decrease the effectiveness of some local anesthetics.
Uremia can cause platelet dysfunction, which can result in increased perioperative bleeding. Bleeding time is the most sensitive indicator of platelet dysfunction and should be assessed to determine whether preoperative treatment is needed. Options to improve the bleeding time include dialysis, desmopressin, cryoprecipitate, conjugated estrogens, and transfusion of red blood cells or platelets (or both). Antiplatelet agents, including aspirin and dipyridamole, should be avoided before surgery in patients with ESRD. In addition, drug-induced minor platelet effects may be exaggerated in ESRD patients, and drugs associated with such problems include diphenhydramine, nonsteroidal anti-inflammatory drugs, chlordiazepoxide, and cimetidine.
Anemia is of particular concern in patients with ESRD because of decreased production of erythropoietin. If necessary, red blood cells can be transfused to correct the hematocrit before surgery. However, unnecessary transfusion should be avoided because antibody formation may result in higher rejection rates if the patient is a candidate for future renal transplantation. In addition, transfusion may result in red blood cell lysis and contribute to hyperkalemia. If surgery is elective, it should be delayed until erythropoietin can be administered and the hematocrit allowed to increase from the patient’s own production of red blood cells. Erythropoietin is also an important option for patients who refuse transfusion for religious reasons.
Contrast-induced nephropathy is an emerging problem as more patients are receiving iodinated contrast media for computed tomography, angiography, and endovascular therapies. Care must be taken in patients with marginal renal function to avoid or to minimize the volume of contrast material and to protect against nephropathy when it must be used. If an iodinated contrast agent is to be given, the patient should be hydrated with a bicarbonate solution and premedicated with N-acetylcysteine, which has been shown to aid in the preservation of renal function. In contrast, the role of dopamine in renal function protection and prevention of contrast nephropathy is controversial. The use of a gadolinium-based agent, such as commonly employed in magnetic resonance imaging, should likewise be avoided. It has been associated with the development of nephrogenic systemic sclerosis, a rare but serious syndrome that involves fibrosis of the skin, joints, eyes, and internal organs.
Diabetes
Surprisingly, the prevalence of undiagnosed diabetes in surgical patients is high despite the association of diabetes with multiple perioperative complications. High glucose levels are associated with increased morbidity and mortality after surgery and in the intensive care unit. Independent of previous cardiac disease, diabetes, or other comorbid conditions, hyperglycemia at the time of carotid endarterectomy has also been associated with an increased risk for perioperative stroke, MI, and death. In addition, poor perioperative glycemic control is associated with an unfavorable outcome after peripheral bypass surgery. Therefore, it is imperative that blood glucose be controlled before, during, and after vascular surgery in diabetic patients. In addition to this review, refer to Chapter 28 for a more complete discussion of diabetes.
Preoperatively, the surgeon should elicit a complete diabetic history, including a history of metabolic control and diabetic complications such as heart disease, nephropathy, neuropathy, retinopathy, and previous episodes of diabetic ketoacidosis or hyperosmolar hyperglycemic nonketotic coma. In addition, evidence of diabetic autonomic neuropathy should be sought because this predisposes patients to perioperative hypotension, and gastroparesis increases a patient’s risk for aspiration. Every effort should also be made to ensure that glucose levels are controlled preoperatively. When possible, glucose control should be attained weeks before surgery, and hemoglobin A1c is a useful diagnostic test for assessing long-term glucose control. However, some patients will require preoperative hospitalization and endocrine consultation to ensure diabetic control before surgery. When necessary, an insulin drip with hourly glucose monitoring can be used to stabilize blood glucose levels.
Although there has been substantial observational evidence linking hyperglycemia in hospitalized patients with or without diabetes to worsening health outcomes, the optimal blood glucose ranges remain ill-defined. Until recently, achievement of nearly normal glycemia was considered beneficial in the intensive care unit setting and during the postoperative period, predominantly on the basis of studies evaluating outcomes after cardiac surgery. Subsequent trials have not replicated the initial findings with tight glycemic control using intensive insulin therapy. The use of intensive insulin therapy has been demonstrated to have no effect on mortality, sepsis, or wound infections.11 Similarly, the need for strict intraoperative glycemic control has not been demonstrated.
There is insufficient evidence to provide strict recommendations for glycemic targets for hospitalized patients. The American Association of Clinical Endocrinologists and the American Diabetes Association provided recommendation in the form of a consensus statement on inpatient glycemic control.12 In the intensive care unit, the glucose goal should be maintained between 140 and 180 mg/dL. For general medical and surgical patients, a premeal glucose target below 140 mg/dL and a random blood glucose concentration below 180 mg/dL should be obtained. Different regimens have been recommended for routine preoperative treatment of diabetic patients undergoing surgical procedures. Patients with type 2 diabetes who are being treated by diet alone are to abstain from oral intake overnight, and hydration may be maintained with intravenous solutions. Hyperglycemia is treated with supplemental short-acting insulin. In patients treated with oral agents, these agents are generally withheld the day of surgery, and hyperglycemia can be corrected with insulin. Metformin should be stopped 24 hours before surgery to prevent the possibility of lactic acidosis if the patient’s renal function becomes compromised. For patients receiving insulin therapy and for the postoperative patient, recommendations are listed in Table 31-4. Specific sliding scale insulin regimens are typically standardized on the basis of hospital practices, but guidelines for their implementation are available13 (Box 31-2).
Table 31-4
Recommendations for Thromboprophylaxis in Various Risk Groups*
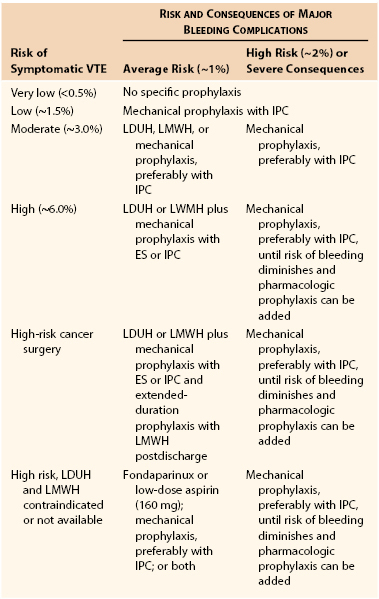
* Reproduced with permission from Gould MK, et al: Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141(Suppl):e227S-e277S, 2012.
VTE, Venous thromboembolism; IPC, intermittent pneumatic compression; LDUH, low-dose unfractionated heparin; LMWH, low-molecular-weight heparin; ES, elastic stockings.
Deep Venous Thrombosis, Thromboembolism, and Antithrombotic Therapy
The risk for deep venous thrombosis (DVT) in surgical patients can be stratified by their risk factors and the type of operation. Before surgery, the surgeon should obtain a history of the patient’s coagulation status and previous history of thrombotic events. A family history or previous episode of DVT may indicate a genetic predisposition to abnormal clotting, such as factor V Leiden or prothrombin gene mutation. A pharmacologic history, including current and previous exposure to drugs that modify coagulation, such as aspirin, antiplatelet therapy, warfarin, unfractionated heparin, low-molecular-weight heparin, and direct thrombin inhibitors, should also be obtained. Particular attention should be paid to adverse reactions, such as aspirin allergy or previous development of heparin-induced thrombocytopenia. In addition, physicians should be aware of the possibility of other procoagulant states, such as antithrombin III deficiency, protein C and S deficiency, antiphospholipid syndrome, disseminated intravascular coagulation, and abnormal platelet function, all of which may confound the underlying disease process responsible for the vascular insult. Pharmacologic prophylaxis for venous thromboembolism includes unfractionated heparin, low-molecular-weight heparin, fondaparinux, warfarin, antiplatelet therapy, and direct thrombin inhibitors. Mechanical devices, such as graduated compression stockings, intermittent pneumatic compression, and venous foot pumps, are also effective modalities for prophylaxis of venous thromboembolism. Recently updated guidelines for DVT prophylaxis have been reported.14 Recommendations specific to vascular surgery are limited because of the lack of large randomized trials evaluating outcomes in patients undergoing specific vascular procedures. Given this, recommendations for vascular surgery are pooled with recommendations for patients undergoing general, urologic, gynecologic, bariatric, and plastic or reconstructive surgeries (see Table 31-4).14
Refer to Chapters 49 and 51 for a detailed review of DVT prophylaxis and management.
The perioperative management of patients who are receiving vitamin K antagonists has received much attention, and recent guidelines pertaining to this have been updated.4 Current recommendations for vitamin K antagonists include cessation of the medication 5 days before surgery with resumption 12 to 24 hours after surgery and when there is adequate hemostasis. In patients with a mechanical heart valve, atrial fibrillation, or venous thromboembolism at high risk for thromboembolism, bridging of the anticoagulation with the use of unfractionated heparin or low-molecular-weight heparin is recommended. In these settings, if the risk for thromboembolism is low, no bridging is necessary. Assessing the risk for thromboembolism during the perioperative interruption of antithrombotic therapy is distinct from assessing the risk of venous thromboembolism. Patients considered at high risk are those with a mitral valve prosthesis, any caged-ball or tilting disk aortic valve prosthesis, recent stroke or transient ischemic attack, atrial fibrillation in the setting of rheumatic valvular heart disease, recent (within 3 months) venous thromboembolism, or severe thrombophilia. Low-risk patients include those with bileaflet aortic valve prosthesis without atrial fibrillation and no other risk factors for stroke and those with venous thromboembolism more than 12 months prior with no other risk factors. Cessation of bridging unfractionated heparin should occur 4 to 6 hours before surgery; low-molecular-weight heparin should be last administered subcutaneously 24 hours before surgery. Low-molecular-weight heparin should not be resumed until 48 to 72 hours after surgery if there is high risk of bleeding.
Nutrition
Up to half of surgical patients have preoperative malnutrition that contributes to an increased risk for postoperative complications, such as impaired wound healing and infection, pulmonary complications, longer hospital stay, higher health cost, and overall increased morbidity and mortality. Vascular surgical patients are an elderly population with poor mobility and diet and multiple comorbidities that contribute to a higher prevalence of malnutrition, which may be exacerbated by specific vascular disease processes such as diabetes, chronic mesenteric ischemia, and renal failure. Lower preoperative nutritional indices are associated with more severe systemic inflammatory responses after major vascular surgery. Serum albumin is the standard laboratory assessment of nutritional status, and hypoalbuminemia (<3.5 g/dL) from malnutrition is an independent predictor of major adverse events and death after major open vascular surgery.15 A similar relationship, however, was not observed for endovascular procedures, suggesting that other preoperative factors (congestive heart failure, preoperative hematocrit level, and creatinine concentration) were more predictive of postoperative complications.
A nutritional assessment should be performed for all vascular surgical patients, including a history evaluating changes in weight and appetite as well as the patient’s functional status (which may impair the ability to obtain or prepare food). Symptoms of nausea, vomiting, dysphagia, constipation, diarrhea, and related gastrointestinal complaints should be assessed, particularly in patients with suspected mesenteric ischemia. In general, weight loss of more than 5% in 1 month or 10% in 6 months can signify an increased risk for postoperative complications.16 The physical examination should focus on body habitus and findings of general weakness, edema, pallor, decubitus ulcers, petechiae, ecchymoses, poor skin turgor, fissured tongue, inflamed gums, ulceration of the oral mucosa, brittle hair, and nail abnormalities.
A number of laboratory values can be used to assess nutritional status, but serum albumin is the most appropriate. Albumin is the most abundant plasma protein and has a long half-life (18 to 21 days); it is a marker of chronic protein status and a better evaluation of nutritional status than anthropometric measurements. Serum prealbumin and transferrin levels have a shorter half-life and are more sensitive to the short-term response to nutritional support than as assessment tools for chronic malnutrition. The prognostic nutritional index, which relates operative risk to nutritional status, is determined on the basis of nutritional parameters including serum albumin, serum transferrin, triceps skinfold measurement, and skin test of delayed hypersensitivity.17 It is seldom used in clinical practice.
Despite the nutritional assessment’s ability to predict poor outcomes, there are no clear guidelines as to who would benefit from preoperative nutritional support, especially in patients undergoing vascular surgery. There is evidence that preoperative nutritional support can lower postoperative complication rates in patients undergoing intra-abdominal surgery, particularly in those with severe malnutrition (albumin <2.1 g/dL). Up to 25 to 35 kcal/kg/day, based on adjusted body weight in obese patients, should be provided, incorporating 1.5 to 2 g/kg/day of protein. A recent Cochrane evaluation of preoperative nutritional support in patients undergoing gastrointestinal surgery demonstrated that the use of parenteral nutrition significantly reduced postoperative complications.16 Similar results with oral supplementation or enteral nutrition were not observed, although most experts agree that enteral nutrition is preferred to parenteral because it is safer and more cost-effective. It is recommended that nutritional support be provided for 10 to 14 days before major surgery.18 Interestingly, immune-enhancing nutritional supplementation can significantly reduce postoperative complications. Immune-enhancing formulas intend to bolster function of the immune system by providing immunonutrients, including arginine, glutamine, and ω-3 polyunsaturated long-chain fatty acids. The generalizability of these modalities to vascular surgical patients, however, is unknown.
Adrenal Evaluation
Patients who have been chronically exposed to steroids may not be able to mount a stress response and will thus be at risk for perioperative adrenal crisis. Therefore, a careful history should be performed to screen for steroid use. In addition, hypothalamic or pituitary dysfunction should be considered in patients with tumor, previous brain irradiation, or sarcoidosis. Furthermore, primary adrenal insufficiency may be present in patients with tuberculosis, acquired immunodeficiency syndrome, and autoimmune endocrine syndromes.
However, there is significant individual variation in the dose and duration of therapy that leads to suppression of the hypothalamic-pituitary axis. In general, steroid therapy of less than 3 weeks’ duration will not lead to significant suppression, and inhaled or cutaneous steroids are not likely to put a patient at risk for perioperative adrenal crisis. In contrast, patients who have been taking at least 20 mg of prednisone per day for more than 3 weeks or patients with cushingoid features should be assumed to have suppression. Recovery from suppression of the hypothalamic-pituitary axis can take up to 12 months, so steroid use within the previous year must be considered potentially indicative of hypothalamic-pituitary axis suppression. Corticotropin stimulation testing should be considered when hypothalamic-pituitary axis function is uncertain because of intermediate steroid doses (5 to 20 mg prednisone) or an unclear medication history. The standard test uses 250 µg of cosyntropin administered intravenously or intramuscularly and measures cortisol at baseline and then at 60 minutes. A plasma cortisol level greater than 20 µg/dL (552 nmol/L) at either time is the diagnostic level for adequate function. As an alternative, the low-dose stimulation test uses 1 µg cosyntropin intravenously and measures cortisol at baseline and 30 minutes. A plasma cortisol level greater than 18 µg/dL (497 nmol/L) is the diagnostic level for adequate function in the low-dose stimulation test.
Excessive steroid use may be associated with impaired wound healing, increased blood glucose concentration, or increased susceptibility to infection, and therefore steroids should be given in anticipation of the minimum requirement. For major surgery, 100 mg of hydrocortisone should be given intravenously before induction of anesthesia and then 100 mg every 8 hours for 24 hours. For moderately stressful procedures, 100 mg of hydrocortisone can be given as a single dose. The steroid dose should be tapered rapidly (50% per day) to the patient’s maintenance dose.
Musculoskeletal Considerations
The musculoskeletal assessment is an often overlooked but important part of the preoperative evaluation. For procedures involving the neck, such as carotid endarterectomy or central catheter placement, the degree of neck mobility is critical for proper surgical access. In addition, obesity or the presence of an extremely large goiter may limit positioning and access to critical structures in the neck.
Adequate mobility is also required for retroperitoneal and extremity positioning. Orthopedic spine problems, previous musculoskeletal injuries, and body habitus may all limit the flexibility of the patient and thus restrict operative access. With the incidence of obesity on the rise, the surgeon must also consider the weight limitations of the standard operating table and whether special arrangements will be needed to accommodate an obese patient. This is of particular concern in the endovascular and imaging suites, in which moving tables may have more stringent weight limitations.
Infection
Patients with infection in the preoperative period should be identified because of their increased risk for surgical infection. Common sources of infection, both preoperatively and postoperatively, include the urinary tract, the pulmonary system, and an ischemic peripheral extremity. If any prosthetic material has been implanted, it can be a nidus for infection as well. A careful history should be performed to elicit signs of infection, such as recent fever, cough, sputum production, urinary symptoms, tenderness or erythema over a graft site, or purulence from an open wound or ischemic extremity. The physical examination should assess abnormal breath sounds, and careful inspection of the extremities and feet should identify any ulceration, tenderness, or erythema. Urinalysis, a chest radiograph, and a white blood cell count should be obtained to evaluate for specific sites of infection if indicated. If infection is identified, elective surgery should be delayed until the patient’s health has been optimized. Choice of antibiotics should be tailored to the specific microbe and sensitivities identified from microbiology laboratory cultures. In the absence of an acute infection, perioperative prophylactic antibiotic administration is recommended in patients undergoing vascular surgery. Antibiotics should be administered within 1 hour before incision and should be discontinued within 24 hours postoperatively unless an underlying infection is being treated. Acceptable prophylactic antibiotics include cefazolin and cefuroxime. Vancomycin is an acceptable alternative in the setting of β-lactam allergy, methicillin-resistant Staphylococcus aureus (MRSA) colonization or infection, chronic wound care or dialysis, acute inpatient hospital extended care or nursing home care in the previous 12 months, or preoperative length of stay of 24 hours or longer.
Antibiotic resistance is a growing problem, in particular with MRSA, which has become a predominant cause of S. aureus infections in both health care and community settings, with up to 65% of health care–associated S. aureus infections due to MRSA. Unlike for traditional S. aureus infection, treatment options for MRSA infection are limited and less effective. MRSA is typically transmitted person to person, and colonization generally precedes infection. Colonization is frequently long-lasting (months to years). Successful prevention of MRSA transmission is possible through Centers for Disease Control and Prevention–recommended core and supplemental strategies. Core strategies target preventing transmission from colonized to uncolonized persons and preventing infection in colonized patients. Core preventive strategies include assessing hand hygiene practices, implementing contact precautions of MRSA-colonized/infected patients, recognizing previously colonized patients who have not met criteria allowing discontinuation of isolation, rapid laboratory reporting of MRSA identification, and providing MRSA education to health care providers. Supplemental strategies including active surveillance testing and decolonization have been recommended by many but met with controversy. Whereas several successful MRSA preventive collaboratives have used active surveillance testing, studies evaluating its utility have demonstrated mixed results. Active surveillance testing involves culture or polymerase chain reaction testing (frequently from the nares) done at the time of patient admission, typically focusing on high-risk patients such as those admitted to the intensive care unit. Once it is identified, however, no data to date exist to support programs of pharmacologic decolonization.
Preoperative Anemia
Low hematocrit levels increase the postoperative risk for death and cardiac events in elderly men undergoing noncardiac surgery. A history of chronic renal disease or anemia, such as sickle cell disease or thalassemia, should alert the physician to the potential for anemia, and a preoperative hematocrit level should always be obtained to provide information on the red blood cell status of the patient. However, because transfusion is not without side effects and blood is costly with limited availability, anemic patients should be carefully evaluated to determine whether transfusion is likely to be of benefit. In addition, the patient’s cardiac status may influence the decision to transfuse inasmuch as anemia worsens outcomes in patients with coronary artery disease who undergo cardiac or noncardiac surgery. Although recommendations for transfusion have been debated, most current guidelines recommend adhering to a restrictive transfusion strategy when possible.17 In adult and pediatric intensive care unit patients, transfusion should be considered at hemoglobin concentrations of 7 g/dL or less. In postoperative surgical patients, transfusion should be considered at a hemoglobin concentration of 8 g/dL or less or for symptoms including chest pain, orthostatic hypotension, and tachycardia unresponsive to fluid resuscitation or congestive heart failure. In hemodynamically stable patients with preexisting cardiovascular disease, transfusion should be considered for a hemoglobin concentration of 8 g/dL or less or for symptoms. Transfusion should also be considered in patients with symptomatic anemia, in those who are actively bleeding, or if anticipated blood loss from surgery will result in the hemoglobin level’s falling below the transfusion thresholds just listed. For elective surgery, autologous transfusion or administration of erythropoietin should be considered to boost the patient’s red blood cell volume from endogenous sources.
Urologic Evaluation
Both hypertension and antihypertensive medications probably contribute to the onset of erectile dysfunction, and vascular risk factors such as diabetes mellitus, hypertension, smoking, hyperlipidemia, and sedentary lifestyle correlate with an increased likelihood of having abnormal penile vascular parameters. Indeed, it has even been suggested that erectile dysfunction be considered a marker for underlying silent cardiac and vascular disease processes.19 Some vascular procedures, such as open abdominal aortic aneurysm repair, can also disturb the pelvic neurovascular plexus and result in impaired erectile function postoperatively. Therefore, it is imperative that patients receive a proper preoperative evaluation for erectile dysfunction and be educated about the risk of vascular disease and vascular surgery as they relate to urologic function. All patients in whom surgery could affect erectile function should provide an appropriate history of sexual function. A formalized evaluation can be accomplished with validated questionnaires, such as the International Index of Erectile Function or the Sexual Health Inventory for Men.20 A quantitative assessment of penile vascular status can also be obtained with the penile-brachial index, which is calculated by comparing penile systolic blood pressure with brachial systolic blood pressure at rest and after exercise. The penile-brachial index should normally be 0.75 or higher, and values lower than 0.6 suggest vascular compromise.
Perioperative Medication Management
Perioperative medication management starts with a complete medication history, including dosage and duration. Table 31-5 lists common medications with their perioperative effects and management guidelines.21–23 Detailed discussions about preoperative management of beta blockers, antiplatelet agents, and diabetic regimen are covered in the previous sections of this chapter.
Table 31-5
Recommended Perioperative Management of Various Chronic Medications
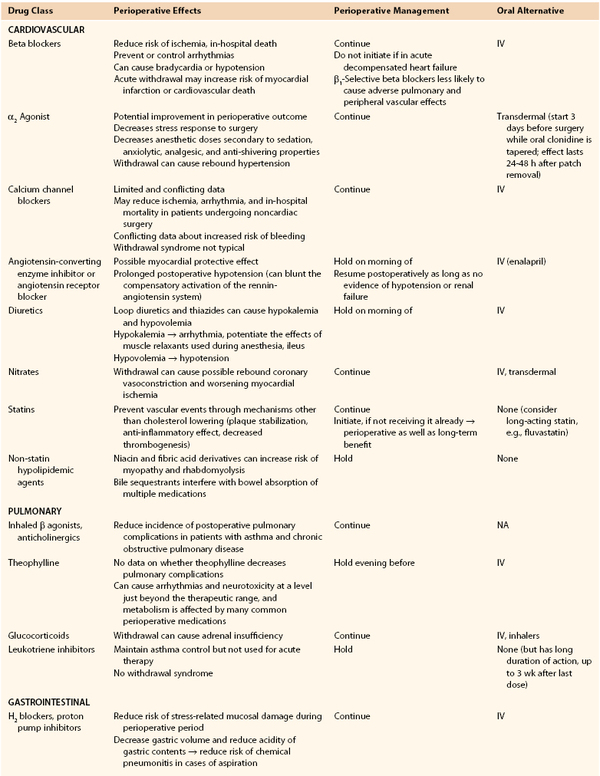


CNS, Central nervous system; HPA, hypothalamic-pituitary axis; IM, intramuscular; IV, intravenous; VTE, venous thromboembolism.
In general, medications with known complications with abrupt withdrawal should be continued or tapered. Medications with increased risk of anesthetic or surgical complications and not essential short term should be held. Medications with protective effects during the perioperative period, such as beta blockers, should be continued or initiated whenever possible. Postoperatively, an alternative route of administration (intravenous, subcutaneous, transdermal) should be considered with appropriate dose conversions if gastrointestinal absorption has been affected by the procedure. Finally, drug-drug interactions with anesthetic agents or perioperative medications such as antibiotics or analgesics need to be considered (see Table 31-5).
Ethical and Legal Concerns
As part of the decision to proceed with surgery, both the physician and the patient must consider ethical and legal matters. In the preoperative evaluation, situations pertaining to patient confidentiality, reporting of abuse, and informed consent may arise. Furthermore, should issues arise in the perioperative or postoperative period that ultimately result in legal action, a medical damages claim must establish three things to prove successful: it must be shown that (1) the provider had a duty to care for the patient, (2) the duty of care was breached in a deviation from the standard of care, and (3) this breach caused loss or damage to the patient. Therefore, the preoperative setting is ideal for both the patient and the physician to come to a mutual understanding about the expectations of the physician-patient relationship and the treatment decided on to minimize the chance that legal action will be initiated.
Patient confidentiality is becoming an increasingly important topic since the passage of new legislation (Health Insurance Portability and Accountability Act [HIPAA]) in the United States to address the privacy and release of health information. Physicians have a duty to maintain confidentiality with their patients, which means that they may not disclose any medical information discovered in connection with the treatment of a patient, thereby protecting the physician-patient relationship. Information contained in a patient’s medical record may be released to third parties only if the patient provides consent. Breaches in confidentiality can lead to mistrust, lawsuits, or disciplinary action. Exceptions can be made if patients threaten bodily harm to themselves or to others. State laws vary on whether a physician is required to report suspected child, elder, or spousal abuse.
Informed consent must be obtained from all patients before surgical intervention and is a process of communication that results in the patient’s agreeing to undergo a medical intervention. To provide consent, the patient must have an understanding of the diagnosis, the purpose of a proposed procedure, the risks and benefits associated with the procedure, the alternative treatments, and the risks and benefits of not undergoing a procedure. This may seem straightforward, but it has been shown that surgeon characteristics influence how risks are presented to vascular patients.28 Care must be taken while providing the risks and benefits of the surgery so that patients do not think that they are being coerced into a specific decision. The informed consent, although not designed as such, is an instrument through which trust between physician and patient can be further enhanced.29 Although informed consent is a process, for the purpose of protection from litigation it is important that this communication be documented. If the patient does not have decision-making capacity, a family member or someone with power of attorney will need to be designated to make decisions on behalf of the patient. A patient need not have the same level of awareness at all times to provide legally adequate informed consent. In these situations, consultation with the psychiatry and ethics teams can be helpful. Regardless, physicians should make themselves familiar with federal and state laws regarding confidentiality, reporting of abuse, and informed consent (Box 31-3).
Selected Key References
Castanheira L, Fresco P, Macedo AF. Guidelines for the management of chronic medication in the perioperative period: systematic review and formal consensus. J Clin Pharm Ther. 2011;36:446.
Review of the management of multiple chronic medications in the perioperative period.
Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, Dunn AS, Kunz R, American College of Chest Physicians. Perioperative management of antithrombotic therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(Suppl):e326S.
Review and recommendations for the management of antithrombotic agents in the perioperative period.
Fleischer LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2009;120:e169.
Review of the perioperative cardiovascular evaluation and care of the noncardiac surgery patient.
Pichardo-Lowden A, Gabbay RA. Management of hyperglycemia during the perioperative period. Curr Diab Rep. 2012;12:108.
Review of the management of hyperglycemia in the preoperative period.
Qaseem A, Snow V, Fitterman N, Hornbake ER, Lawrence VA, Smetana GW, Weiss K, Owens DK, Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144:575.
Review of current guidelines for the evaluation and management of patients with pulmonary disease undergoing nonthoracic surgery.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Fleischer LA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2009;120:e169.
2. Devereaux PJ, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomized controlled trial. Lancet. 2008;371:1839.
3. Dunkelgrun M, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg. 2009;249:921.
3a. Bouri S, et al. Met-analysis of secure randomized controlled trials of Beta-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2013.
4. Douketis JD, et al. Perioperative management of antithrombotic therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(Suppl):e326S.
5. McFalls EO, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795.
6. Poldermans D, et al. A clinical randomized trial to evaluate the safety of noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49:1763.
7. Qaseem A, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144:575.
8. Smetana GW, et al. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581.
9. Moller AM, et al. Effect of preoperative smoking intervention on postoperative complications: a randomized clinical trial. Lancet. 2002;359:114.
10. Bluman LG, et al. Preoperative smoking habits and postoperative pulmonary complications. Chest. 1998;113:883.
11. Pichardo-Lowden A, et al. Management of hyperglycemia during the perioperative period. Curr Diab Rep. 2012;12:108.
12. Moghissi ES, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119.
13. Pichardo-Lowden AR, et al. Management of hyperglycemia in the non-intensive care patient: featuring subcutaneous insulin protocols. Endocr Pract. 2011;17:249.
14. Gould MK, et al. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(Suppl):e227S.
15. Boitano LT, et al. Differential effect of nutritional status on vascular surgery outcomes in a Veterans Affairs versus private hospital setting. Am J Surg. 2012;204:e27.
16. Burden S, et al. Pre-operative nutritional support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev. 2012;11:1.
17. Carson JL, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49.
18. Weiman A, et al. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clin Nutr. 2006;25:224.
19. Kapur V, et al. The relationship between erectile dysfunction and cardiovascular disease. Part I: pathophysiology and mechanisms. Rev Cardiovasc Med. 2007;8:214.
20. Ramanathan R, et al. Predictive correlation between the International Index of Erectile Function (IIEF) and Sexual Health Inventory for Men (SHIM): implications for calculating a derived SHIM for clinical use. J Sex Med. 2007;4:1336.
21. Castanheira L, et al. Guidelines for the management of chronic medication in the perioperative period: systematic review and formal consensus. J Clin Pharm Ther. 2011;36:446.
22. Whinney C. Perioperative medication management: general principles and practical applications. Cleve Clin J Med. 2009;76:S126.
23. Mercado DL, et al. Perioperative medication management. J Med Clin North Am. 2003;87:41.
24. Stathatos N, et al. Perioperative management of patients with hypothyroidism. Endocrinol Metab Clin North Am. 2003;32:503.
25. Huyse FJ, et al. Psychotropic drugs and the perioperative period: a proposal for a guideline in elective surgery. Psychosomatics. 2006;47:8.
26. Movig KL, et al. Relationship of serotonergic antidepressants and need for blood transfusion in orthopedic surgical patients. Arch Intern Med. 2003;163:2354.
27. Oppedal K, et al. Preoperative alcohol cessation prior to elective surgery. Cochrane Database Syst Rev. 2012;7:1.
28. Berman L, et al. Informed consent for abdominal aortic aneurysm repair: assessing variations in surgeon opinion through a national survey. J Vasc Surg. 2008;47:287.
29. Eyal N. Using informed consent to save trust. J Med Ethics. 2012.