CHAPTER 99 PREOPERATIVE AND POSTOPERATIVE NUTRITIONAL SUPPORT: STRATEGIES FOR ENTERAL AND PARENTERAL THERAPIES
Nutritional support is an integral part of trauma and critical care management. Its role has undergone a dramatic evolution over the past two decades as we have developed a deeper understanding of the complex inflammatory and metabolic pathways that accompany surgical stress. The manipulation of this stress response and its inherent catabolic reaction is the focus of emerging nutritional therapies.
PREOPERATIVE NUTRITION
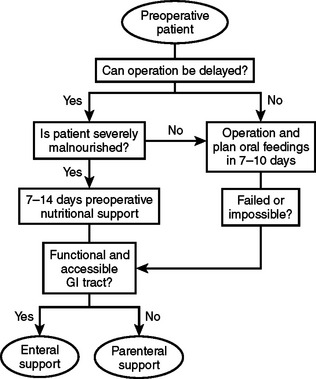
There are two circumstances in which preoperative nutritional support should be considered. One is for patients who will require major operative intervention, but cannot undergo immediate surgery and will have a prolonged fast for more than 5 days. The other circumstance is when operative intervention is delayed to treat patients with significant nutritional deficits that could increase postoperative morbidity (Figure 1).
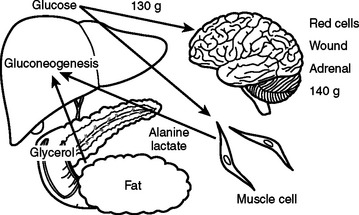
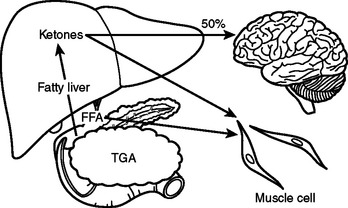
In the preoperative patient, the response to starvation is associated with a redistribution of substrate flow from peripheral tissues to meet metabolic demands. The falling level of insulin promotes the release of fatty acids and amino acids from adipose tissue and skeletal muscle. Although most peripheral tissues can utilize fatty acids as fuel, proteolysis continues to fuel gluconeogenesis in order to support the fuel requirements of the glucose dependent tissues (Figure 2). Over time, there is adaptation to starvation as the brain becomes able to use ketones for 50% of its fuel needs. As fat-derived fuel sources are utilized more, the dependence on protein catabolism decreases from 85% to 35% (Figure 3).
Patients with upper gastrointestinal tract malignancies have the highest incidence of protein-calorie malnutrition, with over 30% of patients demonstrating significant nutritional deficits. Preoperative chemotherapy and radiation, combined with cancer cachexia, obstruction, increased nutrient losses, and abnormal substrate metabolism, increase nutritional risk.1
Prospective studies have shown a decrease in major complications such as anastomotic leak and wound disruption when surgery is delayed and preoperative parenteral nutrition is administered to severely malnourished patients. However, there is an increase in infectious complications without clinical benefit when preoperative parenteral nutrition is administered to patients who are well nourished or onlymildly malnourished. It is important, therefore, to precisely define malnutrition to appropriately select patients for this treatment modality.2
Severe malnutrition can be diagnosed using a clinical nutritional evaluation tool, such as the Subjective Global Assessment.3 In 1982, Baker demonstrated the validity of a clinical assessment relative to one made on the basis of more objective laboratory values. The clinician uses historical information about recent food intake or unintentional weight loss and examines the patient for signs of nutritional depletion. Patients with multiple or severe stigmata of malnutrition or more than 15% weight loss within six months would be considered as seriously depleted. However, in patients with biopsy proven carcinoma, a weight loss of more than 10% in six months would indicate a high-risk group that would benefit from a course of preoperative nutritional support.
After selection of a patient for preoperative nutrition, it is necessary to decide on a formulation and treatment course. Although the optimal duration of therapy has yet to be determined, preoperative therapy from 7–15 days is standard. Total nonprotein calories should be calculated at 150% of basal energy expenditure as measured using indirect calorimetry or derived from the Harris-Benedict equations (Table 1). It is prudent to start patients with severe malnutrition and starvation at a basal energy rate for several days to prevent refeeding syndrome before increasing support to goal rates. After 3 days, support may be increased to 125% of basal requirements and then increased to goal as tolerated.
Data from Van Way CW 3rd: Variability of the Harris-Benedict equation in recently published textbooks. J Parenter Enteral Nutr 16:566–568, 1992.
Preoperative Total Parenteral Nutrition
Total parenteral nutrition (TPN) should be administered to patients who are severely malnourished with nonfunctioning gastrointestinal tracts. Dextrose and lipid formulas are used to provide nonprotein calories, usually in a 70:30 ratio. The caloric values of TPN substrates can be found in Table 2. The amount of dextrose administered should be 4–6 mg/kg/min. However, in patients with chronic obstructive pulmonary disease or diabetes, it is recommended to keep the dextrose administration at 4 mg/kg/min or less. Blood sugars must be monitored and kept tightly controlled between 85 and 120 mg/dl.
| Nutrient | Parenteral (kcal/g) | Enteral (kcal/g) |
|---|---|---|
| Carbohydrate | 3.4 | 4.0 |
| Fat | 9.0 | 9.0 |
| Protein | 3.4 | 4.0 |
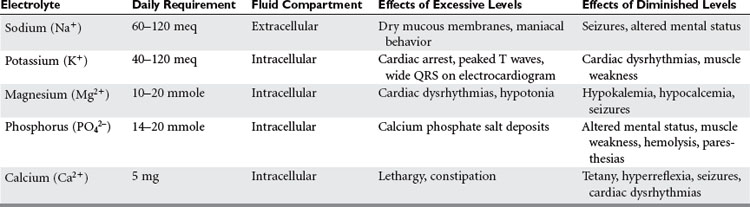
With protein-calorie malnutrition there is loss of the intracellular ions potassium, magnesium, and phosphorus, and a gain in sodium and water. During refeeding, sodium balance may become markedly positive and cause water retention. Potassium, phosphorus, and magnesium levels may drop precipitously upon initiation of nutritional support. It is important to monitor electrolytes and fluid balance to avoid the risk of refeeding syndrome. In addition, potassium and magnesium deficiencies must be corrected if anabolism is to occur (Table 3).
Trace minerals are inorganic compounds, and vitamins are complex organic compounds that regulate metabolic processes (Table 4). The majority act as coenzymes or as essential elemental constituents of enzyme complexes regulating the use of carbohydrates, proteins, and fats. Iron, zinc, copper, chromium, selenium, iodine, and cobalt are known to be necessary for health in man. However, in malnourished and seriously ill patients, requirements for zinc and selenium should be assessed and replenished as necessary.4
| Vitamin or Mineral | Function | Daily Requirement |
|---|---|---|
| Biotin | Coenzyme of carboxylase | 60 mcg |
| Chromium | Insulin utilization | 10–20 mcg |
| Copper | Enzyme systems and ceruloplasmin | 0.1–0.5 mcg |
| Folic acid | Nucleic acid synthesis | 600 mcg |
| Iron | Porphyrin-based compounds, enzymes, mitochondria | 0–2 mg |
| Niacin | Component of nicotinamide adenine dinucleotide and its phosphate (NADP) | 50 mg |
| Pantothenate | Component coenzyme A | 15 mg |
| Pyridoxine | Coenzyme of amino acid metabolism | 5 mg |
| Riboflavin | Coenzymes in redox enzyme system | 5 mg |
| Selenium | Component of glutathione peroxidase | 20–200 mcg |
| Thiamine (B1) | Cocarboxylase enzyme system | 5 mg |
| Vitamin A | Epithelial surfaces, retinal pigments | 2500 IU |
| Vitamin B12 | Nucleic acid synthesis | 12 mcg |
| Vitamin C | Redox reactions, collagen, immune function | 1000 mg |
| Vitamin D | Bone metabolism | – |
| Vitamin E | Membrane phospholipids | 50 IU |
| Vitamin K | Coagulation factors | 1–2 mg |
| Zinc | Enzyme systems | 1–15 mcg |
Preoperative Enteral Nutrition
The initial gastrointestinal barrier function is provided by mucous containing lactoferrin and lysozyme, both of which are effective, nonspecific inhibitors of microbial growth. Normal, undisturbed bacterial flora exert a similar effect. Epithelial tight junctions form the next line of nonspecific defense, with junctional integrity being energy dependent, and at least partially reliant on the presence of intraluminal energy substrates. Specific intestinal immunity is governed by the gut-associated lymphoid tissue (GALT). The inductive sites in the Peyer’s patches provide an interface between antigen-presenting cells and circulating lymphocytes. Animal studies have demonstrated improved immunity in enterally fed groups.5
Patients may have inadequate appetite or gastrointestinal function to maintain optimal nutrition on oral intake alone. Enteral feeding has been used successfully to meet the nutritional needs of patients with a wide range of surgical diseases including cancer, inflammatory bowel disease, and pancreatic disorders. However, its use is contraindicated in cases of bowel obstruction, persistent intolerance, hemodynamic instability, major gastrointestinal bleeding, and inability to access the gastrointestinal tract safely.
Many enteral formulas now contain fiber, which may be soluble or insoluble. Insoluble fiber improves colonic function and bowel transit time, but there is no nutritional benefit or requirement. In contrast, soluble fiber binds to cholesterol and bile salts, and thus lowers serum cholesterol levels. Colonic bacteria digest soluble fiber and produce short-chain fatty acids that are utilized by the colonocyte as a fuel source.
Currently, there are over a hundred enteral products on the market. Most formulas have a caloric density of 1-2 kcal/ml, are lactose-free, and provide the recommended daily allowances of vitamins and minerals in less than 2 liters of formula per day. The majority of patients tolerate standard enteral formulas; however, elemental formulas may be necessary in patients with malabsorption. Recently, excellent results with improved immune function and surgical outcomes have been obtained with the preoperative administration of immunoenhancing formulas.6 Other disease specific formulations have been created for patients with liver disease, renal failure, pulmonary insufficiency, and diabetes. Formulas for patients with liver disease and encephalopathy contain a higher percentage of protein in the form of branched-chain amino acids with almost no aromatic amino acids. Renal failure formulas have very low levels of potassium and phosphorus. However, hepatic and renal formulas have a very low protein content, which must be considered. Patients with advanced pulmonary disease need to receive most of their calories as fat in order to decrease carbon dioxide production. Diabetic formulas also contain additional calories as fat, but also contain soluble fiber to decrease blood sugar levels. It is important to note that all of these specialty formulas are very expensive, and should only be used when a standard formulation with an appropriate nutrient profile has failed.
Preoperative enteral feedings have been demonstrated to decrease postoperative complication rates by 10%–15% of controls, but there is debate over the length of therapy needed to achieve this. The literature supports a course of enteral feedings for 5–20 days prior to surgery. Recently, it has been shown that there may be an advantage to utilizing immune-enhancing formulas, either alone as preoperative supplements, or in combination with postoperative support.6
Postoperative Nutrition
When developing a plan for postoperative nutrition, it is important to anticipate the length of time that the patient may require ventilatory support or have an intestinal ileus. The condition of the patient with attention to premorbid nutrition, and the state of hypermetabolism and catabolism must be assessed to maximize the opportunity for intraoperative enteral access (Figure 4). However, this type of detailed assessment may not be possible in patients scheduled for emergency surgery or those with traumatic injury. In order to simplify these decisions, we developed a model for use in patients who have suffered from traumatic injury (Figure 5). This simple algorithm using the indication for emergency surgery and Injury Severity Score has an accuracy of 88% in selecting trauma patients that are candidates for early nutritional support. In our series, we found that 80% of all trauma patients admitted to the intensive care unit met criteria for nutritional support following traumatic injury.7
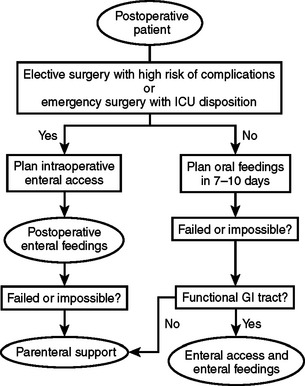
Figure 4 Algorithm for postoperative nutritional support. GI, Gastrointestinal; ICU, intensive care unit.
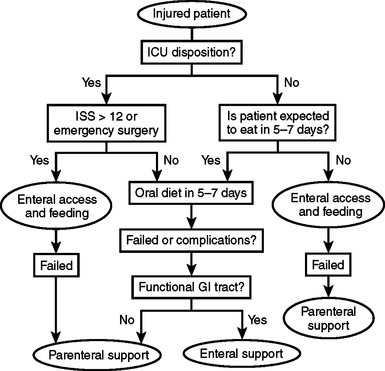
Figure 5 Trauma care nutritional support algorithm. ICU, Intensive care unit; ISS, Injury Severity Score.
(Data from Byers P, Block E, Albornoz J, et al: The need for aggressive nutritional intervention in the injured patient: the development of a predictive model. J Trauma 39:1103, 1995.)
Although protein requirements in stable adults are only 0.6 g/kg/day, the increased catabolism that occurs during critical illness raises the basal needs for balance. Improved nitrogen equilibrium occurs with increased protein synthesis in the majority of postoperative patients when 25–30 kcal/kg of total calories is provided along with 1.5–2.0 g/kg of protein daily. Carbohydrates should be administered with a maximum of 4–6 mg of dextrose per kilogram per minute with serum blood sugars maintained between 85 and 120 mg/dl. Fat should be used to meet less than 30% of total calories.8 If indirect calorimetry is available, it should be utilized to measure caloric needs to avoid overfeeding. Supplements of enteral glutamine should be given in doses of 0.5 g/kg/day, as well as multivitamins and trace minerals, including zinc and selenium.
In addition to monitoring blood sugar, electrolytes, and fluid balance, patients receiving postoperative nutrition should be monitored for efficacy of therapy. Serum protein markers with short half-lives are most effective in measuring improvement in the visceral protein compartment (Table 5). Failure to achieve improvement in these values should prompt assessment of the nutrition administered over the past several days along with a search for untreated infection or inflammation. Nitrogen balance studies may be performed where the amount of protein administered is evaluated against the amount of nitrogen lost in the urine, stool, and wound drainage (Table 6). Critically ill patients should be kept in neutral nitrogen balance while anabolic patients should be kept in a slightly positive balance. After visceral protein markers have normalized, it may become important to evaluate the somatic muscle compartment. If renal function is stable, this can be done by performing a 24-hour urine creatinine measurement and calculating the creatinine height index (Table 7). Improvement in this value will require aggressive physical therapy in addition to nutritional support.
| Protein Marker | Normal Values | Half-Life (Days) |
|---|---|---|
| Albumin | >3.5 g/dl | 20 |
| Transferrin | >200 mg/dl | 8.5 |
| Prealbumin | 20–30 mg/dl | 1.3 |
| Retinol-binding protein | 4–5 mg/dl | 0.4 |
|
N (out) = total urinary Na (g/day) + gastrointestinal losses (2–4 g/day) + cutaneous losses (0–4 g/day)
|
a Total urinary N can either be measured directly or estimated by measuring urine urea N and dividing by 0.8
Table 7 Estimation of Creatinine Height Index
Data from Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S: Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37:478–494, 1983.
Postoperative Parenteral Nutrition
Because most well-nourished patients tolerate inadequate nutrition postoperatively for 7–10 days, there is no justification for the use of routine postoperative parenteral nutrition (see Figure 4). Well-nourished patients with severe stress or preoperatively depleted patients should receive postoperative nutrition when a 7–10-day period of inadequate intake is anticipated. Additional candidates for postoperative parenteral feedings are patients who have been treated with preoperative parenteral nutrition, but are unable to receive postoperative enteral feedings and patients who develop complications that preclude utilization of the gastrointestinal tract. Parenteral nutrition may be life-saving in patients with high-output proximal enterocutaneous fistulae, massive intestinal resection, and end-jejunostomy syndrome.
Central venous access with a designated port for parenteral nutrition must be established. For patients that do not require fluid restriction, dextrose concentrations may be kept between 12%–18% with 5%–6% amino acid solutions. Lipids should be limited to 20% of total calories.8
There are new modalities that may increase the efficacy of postoperative parenteral nutrition. The addition of growth hormone has been shown to improve wound healing in burn patients, but has been harmful in critically ill surgical patients, and cannot be routinely recommended. Intravenous glutamine has also shown promise. In the future, antioxidants may be indicated as part of a nutritional regimen.9 Although scientific studies demonstrating potential benefit are available, more clinical trials proving efficacy are needed.
Postoperative Enteral Nutrition
In patients who undergo laparotomy, enteral access can be best achieved intraoperatively. Postoperative enteral support should begin 12–72 hours following surgery or injury. Hemodynamic stability should always be attained first to avoid intestinal necrosis from ischemia. Continuous tube feedings usually start at 10–20 ml/hr and may be increased by the same amount every 8–24 hours depending on the clinical scenario. Abdominal distension should prompt the immediate decrease in the tube feeding rate by half, and should prompt cessation of feedings if it persists. Gastric feedings should only be advanced if the residual volumes are 200 ml or less. Bolus feedings into the stomach of 200–300 ml every 2–6 hours may also be given and may help to maintain adequate amounts of feeding despite daily care and diagnostic tests. Enteral feedings should be continued until it has been documented that the patient has an adequate dietary intake.
When feeding directly into the jejunum, fully or partially elemental formulas should be utilized. Standard formulas are better tolerated in the stomach and duodenum. Immune-enhancing formulas are now available and have been developed by enriching enteral formulas with specific micronutrients. Newer formulas have added omega-3 fatty acids which decrease inflammation and the resultant tendency toward multisystem organ dysfunction. These formulas also contain glutamine, arginine, and nucleotides. Wound-healing formulas contain higher levels of zinc, Vitamin C, and Vitamin A. Although studies have demonstrated improved outcomes in length of stay and infectious morbidity, there has been no effect on mortality.10
MORBIDITY AND COMPLICATIONS MANAGEMENT
Metabolic Complications
Some complications arise due to administration on exogenous substrates. Malnourished patients may develop electrolyte derangements and congestive heart failure when fed too aggressively. This can be avoided by limiting fluids and sodium, and carefully monitoring phosphorus, potassium, and magnesium, while hypocaloric feedings are initially administered. In addition, feeding with excessive carbohydrates can cause the development of hyperglycemia with an increase in infections, electrolyte derangements, and polyuria with dehydration. Patients with compromised respiratory function may develop hypercarbia and respiratory failure.
SUMMARY AND ALGORITHMS
Preoperative parenteral nutrition should be given to patients who are severely malnourished and need a major operative intervention where healing complications would pose major risk, as long as enteral support is not an option and a course of 7–15 days of support is feasible (see Figure 1). Postoperative parenteral nutrition should be utilized when the postoperative or post-injury period without enteral nutrition is expected to surpass 7–10 days, when the patient has received preoperative nutrition and is not a candidate for postoperative enteral feedings, and when surgical complications develop in the postoperative period that are associated with gastrointestinal dysfunction. Tight serum glucose control is critical in order to use this therapy with minimal morbidity. Enteral feeding is the preferred method of providing nutrients to patients with a functional gastrointestinal tract and is feasible in the majority of patients. Enteral feeding preserves the structure and function of the intestine, and is associated with fewer infectious and metabolic complications (see Figure 4).
1 Studley HO. Percentage of weight loss: a basic indicator of surgical risk in patients with chronic peptic ulcer. JAMA. 1936;106:458-460.
2 Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325:525-532.
3 Baker JP, Detsky AS, Wesson DE, et al. Nutritional assessment: a comparison of clinical judgement and objective measure. N Engl J Med. 1982;306:969-972.
4 Byers PM, Jeejeebhoy KN. Enteral and parenteral nutrition. In: Civetta JM, Taylor RW, Kirby RR, editors. Critical Care. 3rd ed. Philadelphia: Lippincott-Raven; 1997:457-473.
5 Alverdy J, Zaborina O, Wu L. The impact of stress and nutrition on bacterial-host interactions at the intestinal epithelial surface. Curr Opin Clin Nutr Metab Care. 2005;8(2):205-209.
6 Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, DiCarlo V. A randomized controlled trial of preoperative oral supplementation with specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122:1763-1770.
7 Byers PM, Block EJ, Albornoz JC, Pombo H, Martin LC, Kirton OC, Augenstein JS. The need for aggressive nutritional support in the injured patient—the development of a predictive model. J Trauma. 1995;39(6):1103-1109.
8 Hasselmann M, Reimund JM. Lipids in the nutritional support of the critically ill patients. Curr Opin Crit Care. 2004;10(6):449-455.
9 Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med. 2004;31(3):327-337.
10 Moore FA, Moore EE, Kudsk KA, Brown RO, Bower RH, Koruda MJ, Baker CC, Barbul A. Clinical benefits of an immune-enhancing diet for early postinjury enteral feeding. J Trauma. 1994;37:607-615.