Prenatal Imaging
Scalp
A scalp cyst, although infrequently encountered, is likely to require further assessment by fetal MRI. The most important differential diagnosis is between an ectodermal cyst and a meningocele or encephalocele.1–3 A fetus with an ectodermal cyst should have no underlying skull defect or transiting vessels or membranes, and the underlying brain should be normal. Because of limitations of resolution, small defects and thus the diagnosis of meningocele can be missed when MRI is used (e-Fig. 19-1).4
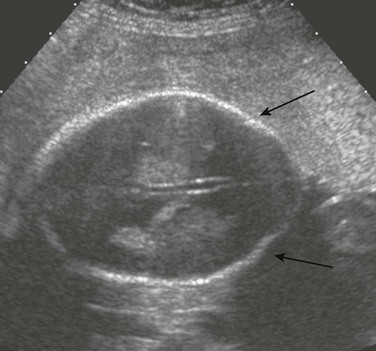
e-Figure 19-1 A scalp cyst.
A, A prenatal ultrasound image shows a parasagittal scalp cyst (arrow). No clear intracranial communication or intracyst contents are present. B, A fetal magnetic resonance image (MRI) shows a cyst (arrow). The subjacent brain appears normal. No skull defect is seen. C, A fetal MRI shows a cyst (arrow) in the sagittal view.
Hemangioma is another scalp abnormality that occurs infrequently but occasionally is reported (Fig. 19-2). The main differential diagnosis is encephalocele.5,6 Further evaluation by fetal MRI can be helpful in demonstrating the absence of a skull defect or an abnormality of the underlying brain.1,7–9
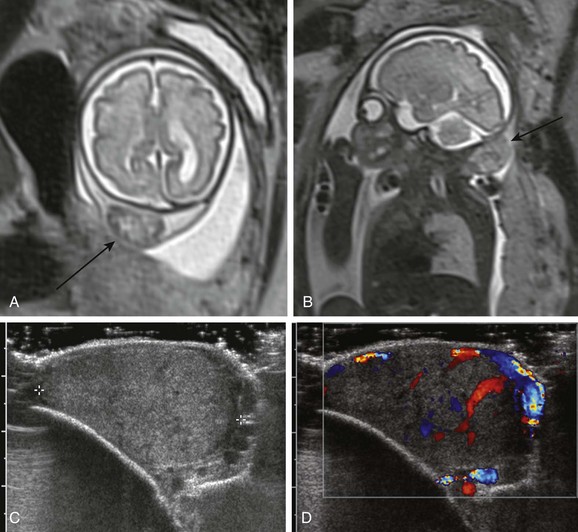
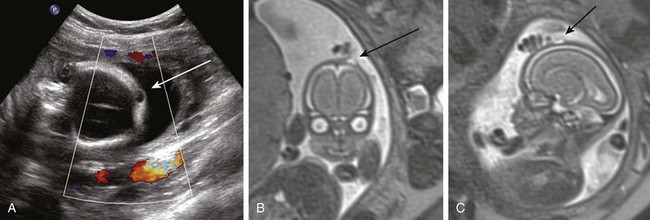
Figure 19-2 A scalp hemangioma.
A, A fetal magnetic resonance image (MRI) shows a heterogeneous mass in the occipital scalp (arrow). The skull appears intact. B, A fetal MRI shows a sagittal view of a mass in the occipital scalp (arrow). C, A postnatal ultrasound image shows a heterogeneous mass in the scalp. The skull appears to be intact. D, A postnatal ultrasound image with Doppler shows vascular flow throughout.
Other lesions that should be considered in the differential diagnosis of scalp abnormalities include lymphatic malformation, edema, a sarcoma, and a teratoma perforating the skull.1,10 Rare abnormalities that have been described on antenatal imaging include gyriform thickening of the scalp in fetuses with cutis verticis gyrata associated with Noonan syndrome11 and hamartomata of the scalp in fetuses with encephalocraniocutaneous lipomatosis.12
Skull
Skull defects are a result of errors in dorsal induction, the process whereby the neural tube forms and closes (neurulation).13,14 A spectrum of anomalies is characterized by absent flat bones of the skull.
Large Bony Defects
“Acalvaria” is the term used when the bony skull vault, meninges, and scalp muscles are absent and a normal brain is covered by skin only. “Acrania” is the term used when the skull vault, scalp muscles, and skin are absent and a dysplastic brain is covered by meninges only.15
Exencephaly
Exencephaly is the combination of acrania with abnormal brain tissue15 and has been demonstrated to be the predecessor of anencephaly.16,17 Exencephaly can be seen in association with amniotic band sequence18–20 and limb body wall complex,21 but can be distinguished from them because they generally form asymmetric skull defects (e-Fig. 19-3).13
e-Figure 19-3 Anencephaly/exencephaly.
A, A fetal magnetic resonance image (MRI) shows anencephaly. The cranium and brain are completely absent (arrow). B, A fetal MRI shows anencephaly in the coronal view. The cranium is absent (arrow) above the orbits. C, A fetal MRI shows exencephaly. The cranium is absent, and persistent dysplastic brain tissue is present (arrow). D, A fetal MRI in the coronal view shows bilateral dysplastic hemispheres (arrows).
Anencephaly
Anencephaly, which is the single most common open neural tube defect,22 is characterized by acrania with complete absence of a normal brain above the brainstem (e-Fig. 19-3, A).13,14 It is the end result of exencephaly with mechanical and chemical attrition of the abnormal brain tissue in utero15; the remaining tissue constitutes a mass of exposed vascular neural tissue. The cartilaginous skull is intact. This condition invariably is fatal. When accompanied by dysraphism of the entire spine, it is known as craniorachischisis.13 Fetal MRI findings have been described in cases associated with omphalocele23 and pentalogy of Cantrell.24
Focal Bony Defects
Focal defects in the skull can allow internal structures to herniate. The terminology changes depending on whether the contents of the hernia include just meninges and cerebrospinal fluid (cephalocele), brain matter (encephalocele), or a ventricle (encephalocystocele). Most defects are in the midline, they generally are occipital (Fig. 19-4),13,14 and they usually contain dysplastic brain tissue and venous sinuses.14 They also can be part of syndromes such as Meckel-Gruber, Dandy-Walker, and Chiari III malformation.13,14 The most common type of encephalocele in Southeast Asia is frontoethmoidal, particularly in children of tea garden workers in Assam, northeastern India.25
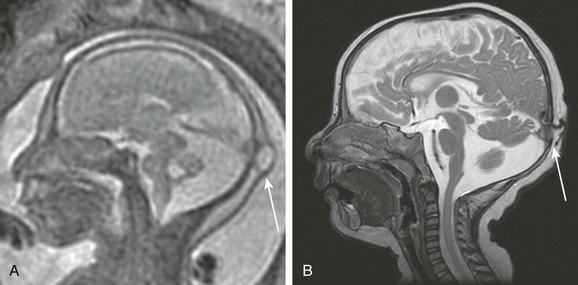
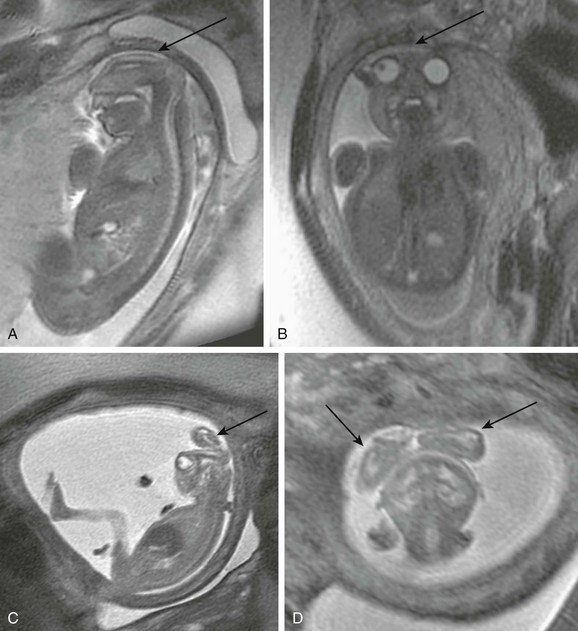
Figure 19-4 A cephalocele.
A, A fetal magnetic resonance image (MRI) shows a small occipital cyst (arrow) with abnormal cerebellar vermis. B, A postnatal MRI shows an atretic cephalocele with a scalp abnormality (arrow). (From Robinson AJ, Blaser S, Toi A, et al. The fetal cerebellar vermis: assessment for abnormal development by ultrasonography and magnetic resonance imaging. Ultrasound Q. 2007;23[3]:211-223.)
Fetal MRI can be useful to differentiate a cephalocele from a scalp cyst and to determine if it contains brain tissue.1,26 Large encephaloceles can appear similar to anencephaly, although the calvarium typically has developed to some extent.13 An atretic cephalocele typically presents as a midline subcutaneous nodule or cyst,27 and underlying venous anomalies often are present that are similar to those seen with enlarged parietal foramina.28–30
Enlarged Parietal Foramina
Enlarged parietal foramina are thought to be the result of defective ossification of the membranous skull. During fetal life they comprise a large central defect that, because of subsequent ossification, including midline ossification (Fig. 19-5), become bilateral symmetric defects.31 In contradistinction, encephaloceles typically are midline or unilateral. Enlarged parietal foramina can be large defects, typically closing almost completely during childhood, leaving only small bilateral foramina.27,32 Although typically benign, they can be associated with hypoplasia or atresia of the straight sinus with a persistent falcine sinus draining to the straight sinus at the level of the anomaly, which has been demonstrated on fetal MRI.27,31 Associated abnormalities of occipital cortical infolding can be seen postnatally.33 Enlarged parietal foramina can be hereditary (autosomal dominant), with genes MSX2 and ALX4 reported to be associated with the condition.34 Enlarged parietal foramina also can be syndromic, as in Potocki-Shaffer syndrome.35
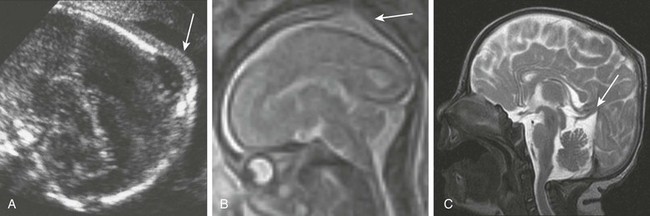
Figure 19-5 An enlarged parietal foramina.
A, A fetal ultrasound image shows a skull defect with intact overlying scalp (arrow). B, A fetal magnetic resonance image (MRI) shows a skull defect that does not contain brain (arrow). C, A postnatal MRI shows a skull defect that now contains brain. An abnormal course of a straight sinus is present (arrow). (From Fink AM, Maixner W. Enlarged parietal foramina: MR imaging features in the fetus and neonate. AJNR Am J Neuroradiol. 2006;27:1379-1381.)
Mineralization Defects
A prenatal ultrasound examination reveals defective skull mineralization in fetuses with several skeletal dysplasias. Plain film radiography of the pregnant patient increasingly is being utilized, and fetal three-dimensional CT is proving to be an emerging technique in the further evaluation of fetal skeletal dysplasias1,14,22,36–42 The main skeletal dysplasias that result in poor skull mineralization are osteogenesis imperfecta type IIa (e-Fig. 19-6), hypophosphatasia congenita, and achondrogenesis.36 A summary of distinguishing features is provided in Table 19-1.
Table 19-1
Skull Abnormalities in Common Lethal Skeletal Dysplasias

Modified from Glanc P, Chitayat D, Unger S. The fetal musculoskeletal system. In: Rumack CM, Wilson SR, Charboneau JW, eds. Diagnostic ultrasound. 3rd ed. St Louis: Elsevier; 2005.
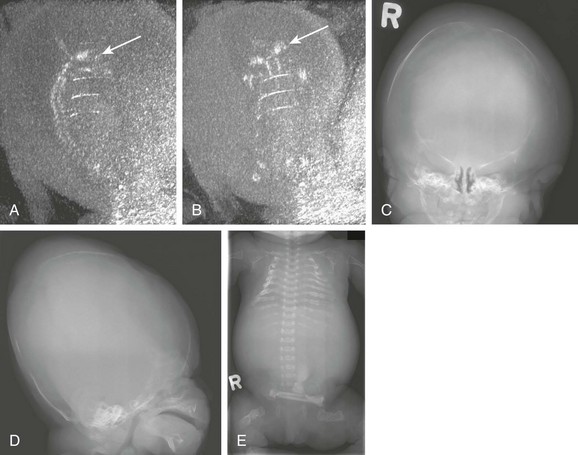
e-Figure 19-6 Osteogenesis imperfecta.
A, In a fetal computed tomography (CT) scan with sagittal reformatting, only the skull base (arrow) and spine are visible. The cranial vault is severely undermineralized. B, A fetal CT scan with coronal reformatting shows that only the skull base is mineralized (arrow). C, A postmortem radiograph shows a severely undermineralized skull vault. D, A postmortem radiograph shows that the skull vault is undermineralized. E, A postmortem radiograph shows short fractured long bones and ribs.
Size and Shape Abnormalities
Microcrania and macrocrania are defined when the head circumference differs more than three standard deviations from the mean in either direction.22
Microcrania
The birth incidence of microcrania is 1 : 1000.37 Microcrania often is the result of an underlying small brain, that is, micrencephaly1 (e-Fig. 19-7). Causes include genetic syndromes,37 chromosomal defects (typically trisomy 1314), hemorrhage, teratogens, infection, radiation,37 and encephalocele.1,14
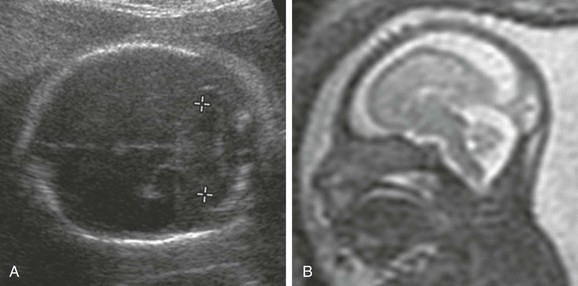
e-Figure 19-7 Micrencephaly.
A, A small cerebellum (calipers). The head circumference and biparietal diameter also were small. B, A fetal magnetic resonance image in the sagittal view shows a small, smooth brain and a backward sloping forehead. (From Robinson AJ, Blaser S, Toi A, et al. The fetal cerebellar vermis: assessment for abnormal development by ultrasonography and magnetic resonance imaging. Ultrasound Q. 2007;23[3]:211-223.)
Macrocrania
Macrocrania typically is seen as a result of fetal hydrocephalus. Other causes include hydrancencephaly,14,38 intracranial teratoma or astrocytoma, and, occasionally, rarer tumors.1,39 Skeletal dysplasias that cause enlargement of the head include achondrogenesis (type 1)36 and thanatophoric dysplasia (e-Fig. 19-8) due to an enlarged brain (megalencephaly), with preferential enlargement of the temporal lobes and coexistent cerebral malformations, in particular, abnormal premature temporal lobe sulcation, which has been demonstrated by both fetal ultrasound and MRI.40,41 Partial callosal dysgenesis also has been reported in fetuses with this condition.42 Table 19-1 summarizes these distinguishing features.
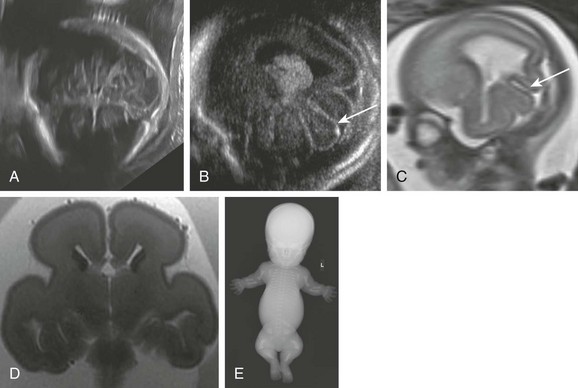
e-Figure 19-8 Thanatophoric dysplasia.
A, A fetal ultrasound scan in the axial view shows a strawberry skull caused by relative enlargement of the temporoparietal compared with the frontal lobe. B, A fetal ultrasound scan shows the parasagittal oblique and thickened abnormal sulci in the temporal lobe (arrow). C, A fetal magnetic resonance image (MRI) shows thickened abnormal temporal lobe sulci (arrow). D, A postmortem T2-weighted MRI scan shows relative enlargement of the temporal lobes compared with the frontal lobes. E, A postmortem radiograph shows typical skeletal features including severe micromelia, a curved femora, and platyspondyly. (A, B, and C From Fink AM, Hingston T, Sampson A, et al. Malformation of the fetal brain in thanatophoric dysplasia: US and MRI findings. Pediatr Radiol. 2010;40[suppl 1]:S134-S137. D from Miller E, Blaser S, Shannon P, et al. Brain and bone abnormalities of thanatophoric dwarfism. AJR Am J Roentgenol. 2009;192[1]:48-51.)
Dysmorphism
Lemon-Shaped Skull and Spina Bifida: A lemon-shaped skull, characterized by a concave or linear contour at the level of the coronal sutures (the “lemon sign”), is a well-known morphologic finding that is highly sensitive for open neural tube defects (see e-Fig. 19-9).43,44 However, it has a poor positive predictive value in low-risk fetuses and is seen in fetuses with other pathologies, including encephalocele and a variety of nonneural tube structural anomalies.22,45–47 This sign may be encountered during fetal MRI evaluation of neural tube defects.48
Cloverleaf Skull (Kleeblattschädel): A cloverleaf appearance occurs as a result of bilateral coronal and lambdoid synostosis and is found in fetuses with type II thanatophoric dysplasia (see Fig. 19-10).1,36,41 Cloverleaf skull also has been used to describe the dysmorphic appearance of the skull in fetuses with syndromic craniosynostosis,49–51 and which in prenatal life is often associated with abnormal karyotype, as described in Chapter 20. Several case reports have described the use of fetal MRI in its antenatal diagnosis.49,52,53
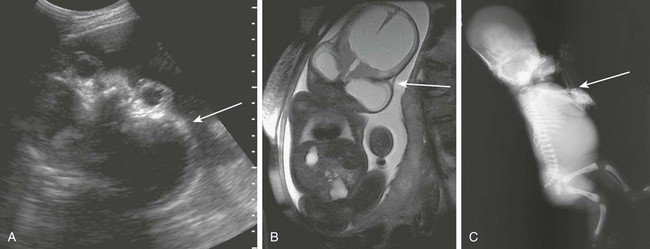
Figure 19-10 Cloverleaf skull.
A, A fetal ultrasound shows bossing of the temporal bones (arrow) and bulging eyes in a case of acrocephalosyndactyly. B, A fetal magnetic resonance image in the coronal view shows bossing of temporal bones (arrow) and frontal bones. Severe ventriculomegaly is noted. C, A postmortem radiograph shows a severe cloverleaf skull with midface hypoplasia. Note elbow ankylosis typical of Pfeiffer syndrome. (A From Robinson AJ, Blaser S, Toi A, et al. Magnetic resonance imaging of the fetal eyes—morphologic and biometric assessment for abnormal development with ultrasonographic and clinicopathologic correlation. Pediatr Radiol. 2008;38:971-981.)
Strawberry Skull: A strawberry-shaped skull, characterized by flattening of the occiput and pointing of the frontal bones, is seen by ultrasound on the submentobregmatic section and is characteristic of fetuses with trisomy 18.54 This appearance also has been described in fetal thanatophoric dwarfism.55
Glanc, P, Chitayat, D, Unger, S. The fetal musculoskeletal system. In: Rumack CM, Wilson SR, Charboneau JW, eds. Diagnostic ultrasound. 3rd ed. St Louis, MO: Mosby Elsevier; 2005:1433–1440.
McGahan, JP. Fetal head and brain. In McGahan JP, Goldberg BB, eds.: Diagnostic ultrasound, 2nd ed, New York: Informa Healthcare, 2008.
Toi, A. The fetal head and brain. In Rumack CM, Wilson SR, Charboneau JW, eds.: Diagnostic ultrasound, 3rd ed, St Louis: Mosby, 2005.
References
1. Stoustrup-Smith, A, Levine, D. MR imaging of the fetal skull, face, and neck. In: Levine D, ed. Atlas of fetal MRI. Boca Raton, FL: Taylor & Francis, 2005.
2. Sepulveda, W, Wong, AE, Sepulveda, S, et al. Fetal scalp cyst or small meningocele: differential diagnosis with three-dimensional ultrasound. Fetal Diagn Ther. 2011;30(1):77–80.
3. Shahabi, S, Busine, A. Prenatal diagnosis of an epidermal scalp cyst simulating an encephalocoele. Prenat Diagn. 1998;18:373–377.
4. Timor-Tritsch, IE, Monteagudo, A, Santos, R. Fine-tuning the diagnosis of fetal scalp cysts: the value of high-frequency sonography. J Ultrasound Med. 2008;27:1363–1368.
5. Bronshtein, M, Bar-Hava, I, Blumenfeld, Z. Early second trimester sonographic appearance of occipital haemangioma simulating encephalocele. Prenat Diagn. 1992;12:695–698.
6. Sherer, DM, Perillo, AM, Abramowicz, JS. Fetal hemangioma overlying the temporal occipital suture, initially diagnosed by ultrasonography as an encephalocele. J Ultrasound Med. 1993;12:691–693.
7. Kashima, H, Unno, N, Hyodo, H, et al. Antenatal sonographic and magnetic resonance images of a giant hemangioma of the fetal skull. Ultrasound Obstet Gynecol. 2005;25:522–523.
8. Boulot, P, Deschamps, F, Montoya, F, et al. Prenatal aspects of giant fetal cranial haemangio-endothelioma. Prenat Diagn. 1996;16:357–359.
9. Miyakoshi, K, Tanaka, M, Matsumoto, T, et al. Occipital scalp hemangioma: prenatal sonographic and magnetic resonance images. J Obstet Gynaecol Res. 2008;34:666–669.
10. Koizumi, K, Abe, E, Kusanagi, Y, et al. Giant immature intracranial teratoma with antenatal cranial perforation. J Obstet Gynaecol Res. 2010;36:1252–1255.
11. Kennedy, A, Perry, D, Battin, M. Antenatal imaging of cutis verticis gyrata. Pediatr Radiol. 2008;38:583–587.
12. Nowaczyk, MJ, Mernagh, JR, Bourgeois, JM, et al. Antenatal and postnatal findings in encephalocraniocutaneous lipomatosis. Am J Med Genet. 2000;91:261–266.
13. Toi, A. The fetal head and brain. In Rumack CM, Wilson SR, Charboneau JW, eds.: Diagnostic ultrasound, 3rd ed, St Louis: Elsevier Mosby, 2005.
14. Robertson, RL, Ball, WS, Barnes, PD. Skull and brain. In: Kirks DR, ed. Practical pediatric imaging. Philadelphia: Lippincott Williams and Wilkins, 1998.
15. Evans, C, Marton, T, Rutter, S, et al. Cranial vault defects: the description of three cases that illustrate a spectrum of anomalies. Pediatr Dev Pathol. 2009;12:96–102.
16. Wilkins-Haug, L, Freedman, W. Progression of exencephaly to anencephaly in the human fetus—an ultrasound perspective. Prenat Diagn. 1991;11:227–233.
17. Timor-Tritsch, IE, Greenebaum, E, Monteagudo, A, et al. Exencephaly-anencephaly sequence: proof by ultrasound imaging and amniotic fluid cytology. Matern Fetal Med. 1996;5:182–185.
18. Rohrbach, M, Chitayat, D, Drake, J, et al. Prenatal diagnosis of fetal exencephaly associated with amniotic band sequence at 17 weeks of gestation by fetal magnetic resonance imaging. Fetal Diagn Ther. 2007;22:112–115.
19. Ferris, NJ, Tien, RD. Amnion rupture sequence with “exencephaly”: MR findings in a surviving infant. AJNR Am J Neuroradiol. 1994;15:1030–1033.
20. Huppert, BJ, Brandt, KR, Ramin, KD, et al. Single-shot fast spin-echo MR imaging of the fetus: a pictorial essay. Radiographics. 1999;19:S215–S227.
21. Chen, CP, Cheng, SJ, Lin, YH, et al. Prenatal imaging of limb-body wall complex by magnetic resonance imaging. Prenat Diagn. 2005;25:521–523.
22. McGahan, JP. Fetal head and brain. In McGahan JP, Goldberg BB, eds.: Diagnostic ultrasound, 2nd ed, New York: Informa Healthcare, 2008.
23. Chen, CP, Liu, YP, Tsai, FJ, et al. Concomitant craniorachischisis and omphalocele in a male fetus: prenatal magnetic resonance imaging findings and literature review. Taiwan J Obstet Gynecol. 2009;48:286–291.
24. Doganay, S, Kantarci, M, Tanriverdi, EC. Pentalogy of Cantrell with craniorachischisis: MRI and ultrasonography findings. Ultraschall Med. 2008;29:278–280.
25. Dutta, HK, Deori, P. Anterior encephaloceles in children of Assamese tea workers. J Neurosurg Pediatr. 2010;5:80–84.
26. Kojima, K, Suzuki, Y, Miyajima, S, et al. Antenatal evaluation of an encephalocele in a dizygotic twin pregnancy using fast magnetic resonance imaging. Fetal Diagn Ther. 2003;18:338–341.
27. Fink, AM, Maixner, W. Enlarged parietal foramina: MR imaging features in the fetus and neonate. AJNR Am J Neuroradiol. 2006;27:1379–1381.
28. Bartels, RHMA, Merx, JL, van Overbeeke, JJ. Falcine sinus and occipital encephalocele: a magnetic resonance venography study. J Neurosurg. 1998;89:738–741.
29. Otsubo, Y, Sato, H, Sato, N, et al. Cephaloceles and abnormal venous drainage. Child Nerv Syst. 1999;15:329–332.
30. Brunelle, F, Baraton, J, Renier, D, et al. Intracranial venous anomalies associated with atretic cephalocoeles. Pediatr Radiol. 2000;30:743–747.
31. Chung, HY, Uster-Friedberg, T, Pentaz, S, et al. Enlarged parietal foramina: findings on prenatal ultrasound and magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;36:521–522.
32. Wilkie, AOM, Mavrogiannis, LA, Enlarged parietal foramina/cranium bifidum (March 30, 2004 [updated March 30, 2010]). Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews [Internet]. University of Washington: Seattle (WA), 1993. http://www.ncbi.nlm.nih.gov/books/NBK1128. [Accessed September 14, 2012].
33. Reddy, AT, Hedlund, GL, Percy, AK. Enlarged parietal foramina: association with cerebral venous and cortical anomalies. Neurology. 2000;14(54):1175–1178.
34. Mavrogiannis, LA, Taylor, IB, Davies, SJ, et al. Enlarged parietal foramina caused by mutations in the homeobox genes ALX4 and MSX2: from genotype to phenotype. Eur J Hum Genet. 2006;14:151–158.
35. Wu, YQ, Badano, JL, McCaskill, C, et al. Haploinsufficiency of ALX4 as a potential cause of parietal foramina in the 11p11.2 contiguous gene-deletion syndrome. Am J Hum Genet. 2000;67:1327–1332.
36. Glanc, P, Chitayat, D, Unger, S. The fetal musculoskeletal system. In Rumack CM, Wilson SR, Charboneau JW, eds.: Diagnostic ultrasound, 3rd ed, St. Louis: Elsevier Mosby, 2005.
37. Cicero, S, Johnson, J, Nicolaides, K. Sonographic markers of fetal chromosomal defects in diagnostic ultrasound. In Rumack CM, Wilson SR, Charboneau JW, eds.: Diagnostic ultrasound, 3rd ed, St Louis: Elsevier Mosby, 2005.
38. Aguirre Vila-Coro, A, Dominguez, R. Intrauterine diagnosis of hydranencephaly by magnetic resonance. Magn Reson Imaging. 1989;7:105–107.
39. Hocwald, O, McFadden, D, Osiovich, H, et al. Congenital gliosarcoma: detailed clinicopathologic documentation of a rare neoplasm. Pediatr Dev Pathol. 2009;12:398–403.
40. Fink, AM, Hingston, T, Sampson, A, et al. Malformation of the fetal brain in thanatophoric dysplasia: US and MRI findings. Pediatr Radiol. 2010;40:S134–S137.
41. Miller, E, Blaser, S, Shannon, P, et al. Brain and bone abnormalities of thanatophoric dwarfism. AJR Am J Roentgenol. 2009;192:48–51.
42. Kalache, KD, Lehmann, K, Chaoui, R, et al. Prenatal diagnosis of partial agenesis of the corpus callosum in a fetus with thanatophoric dysplasia type 2. Prenat Diagn. 2002;22:404–407.
43. Nyberg, DA, Mack, LA, Hirsch, J, et al. Abnormalities of fetal cranial contour in sonographic detection of spina bifida: evaluation of the “lemon” sign. Radiology. 1988;167:387–392.
44. Van den Hof, MC, Nicolaides, KH, Campbell, J, et al. Evaluation of the lemon and banana signs in one hundred thirty fetuses with open spina bifida. Am J Obstet Gynecol. 1990;162:322–327.
45. Gabbe, SG, Mintz, MC, Mennuti, MT, et al. Detection of open spina bifida by the lemon sign: pathologic correlation. J Clin Ultrasound. 1988;16:399–402.
46. Ball, RH, Filly, RA, Goldstein, RB, et al. The lemon sign: not a specific indicator of meningomyelocele. J Ultrasound Med. 1993;12:131–134.
47. Thomas, M. The lemon sign. Radiology. 2003;228:206–207.
48. Saleem, SN, Said, AH, Abdel-Raouf, M, et al. Fetal MRI in the evaluation of fetuses referred for sonographically suspected neural tube defects (NTDs): impact on diagnosis and management decision. Neuroradiology. 2009;51:761–772.
49. Weber, B, Schwabegger, AH, Vodopiutz, J, et al. Prenatal diagnosis of Apert syndrome with cloverleaf skull deformity using ultrasound, fetal magnetic resonance imaging and genetic analysis. Fetal Diagn Ther. 2010;27:51–56.
50. Nazzaro, A, Della Monica, M, Lonardo, F, et al. Prenatal ultrasound diagnosis of a case of Pfeiffer syndrome without cloverleaf skull and review of the literature. Prenat Diagn. 2004;24(11):918–922.
51. David, DJ, Cooter, RD, Edwards, TJ. Crouzon twins with cloverleaf skull malformations. J Craniofac Surg. 1991;2:56–60. [discussion 61].
52. Tonni, G, Panteghini, M, Rossi, A, et al. Craniosynostosis: prenatal diagnosis by means of ultrasound and SSSE-MRI. Family series with report of neurodevelopmental outcome and review of the literature. Arch Gynecol Obstet. 2001;283:909–916.
53. Itoh, S, Nojima, M, Yoshida, K. Usefulness of magnetic resonance imaging for accurate diagnosis of Pfeiffer syndrome type II in utero. Fetal Diagn Ther. 2006;21:168–171.
54. Nicolaides, KH, Salvesen, DR, Snijders, RJ, et al. Strawberry-shaped skull in fetal trisomy 18. Fetal Diagn Ther. 1992;7:132.
55. Seymour, R, Jones, A. Strawberry-shaped skull in fetal thanatophoric dysplasia. Ultrasound Obstet Gynecol. 1994;4:434–436.