Prenatal Gastrointestinal and Hepatobiliary Imaging
Evaluation of the organs in the gastrointestinal tract and within the peritoneal cavity is a standard part of the full prenatal ultrasound scan for anatomy screening.1 In circumstances when ultrasound cannot accurately determine the extent or nature of an abnormality, fetal magnetic resonance imaging (MRI) can be useful. Sequence selection includes standard single-shot fast spin echo (SSFSE) T2-weighted images at 3- to 5-mm slice thickness and steady-state free-precession imaging (SSFP) at overlapping intervals. Echo-time lengths range from 80 to 250 ms; longer lengths are advantageous for delineation of cystic abnormalities. T1-weighted fast gradient echo (GRE) sequences may be useful in the fetal abdomen, specifically for identification of the liver, which is mildly hyperintense, and very bright signal meconium.2 Imaging modalities using ionizing radiation have no role in the evaluation of a fetus with a gastrointestinal (GI) tract abnormality, which can be categorized into one of several general categories: obstructions, ventral wall defects, masses and solid organ abnormalities, echogenic bowel, and peritoneal abnormalities.
Intestinal Obstructions
Etiology: Interruption of the fetal GI tract can occur at any point from the esophagus to the anus, and the etiology for the loss of continuity is not the same at each site. The cause of esophageal atresia (EA) with or without a fistula (communicating between the trachea and the esophagus distal to the atretic level) is not known but is believed to be a result of failure of the tracheal bud to develop normally from the primitive foregut (see Chapter 97).3,4 EA occurs in approximately 1 in 4000 live births and can be associated with a number of additional abnormalities in the fetus. Most commonly these abnormalities include those comprising the VACTERL complex (vertebral, anal, cardiac, tracheoesophageal, renal, and limb anomalies) and those seen in Down syndrome, such as common atrioventricular canal, absent or hypoplastic nasal bone, and nuchal thickening. EA occurs most commonly with a distal fistula (84%).5
Gastric atresia is the rarest atresia and is almost always pyloric with a distended proximal stomach. It likely results from vascular impairment during fetal development and is associated with epidermolysis bullosa.6,7
In contrast to pyloric atresia, duodenal atresia (DA) is relatively common, and along with EA is associated with Down syndrome. The etiology of DA may be exceptional in that it is believed to be due to dysfunction in embryonic recannulation of this segment of the proximal small bowel (see Chapter 103).8
Atresias distal to the duodenum are thought to be the result of ischemia or other focal insult to the bowel and may be multiple. Thus it is prudent not to assume that the most proximal obstruction is the only site. Small bowel atresias are the most common cause of fetal and neonatal bowel obstructions, with colonic segments affected much more rarely (in 10% of all atresias).9,10 Although most atresias are sporadic, some populations are prone to multiple atresia syndromes.11,12 Meconium ileus in persons with cystic fibrosis also can have small bowel atresia, and colonic atresia can be seen in persons with Hirschsprung disease.13,14 Anorectal atresia/malformation deserves special consideration because it often is seen in combination with other malformations, such as VACTERL, caudal regression, cloacal malformation, or the Currarino association.
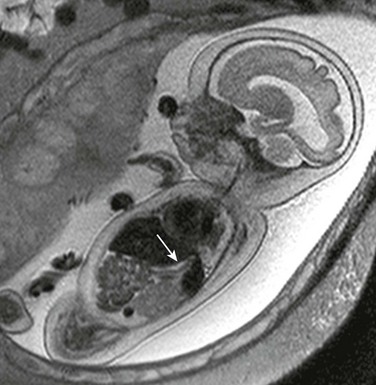
Imaging of the Esophagus: Despite the likelihood of a distal fistula that might allow for flow of fluid into the gut, the most consistent clues to the diagnosis of EA on ultrasound are a persistently small-volume stomach and polyhydramnios. Distension of a proximal esophageal pouch typically is intermittent and therefore unreliably witnessed either on ultrasound or MRI, although in the rare cases where it is vital to make a prenatal diagnosis, serial midline sagittal MRI with cine SSFP sequences may prove useful (Fig. 86-1).15
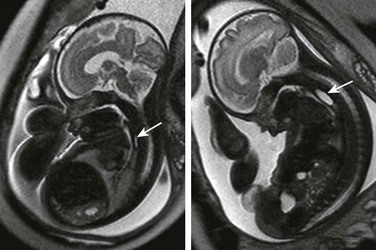
Figure 86-1 Esophageal atresia.
Serial imaging may show intermittent phenomena: A sagittal single-shot fast spin echo image in the neck and chest shows a small amount of fluid in the proximal esophagus that distends to a pouch on a later sequence (arrows). Cine steady-state free-precession sequences are especially useful for serial imaging because of their fast acquisition times.
Imaging of the Stomach: A lack of visualization of the stomach is most likely EA with or without a distal fistula. Other considerations in the differential diagnosis of a small stomach at fetal imaging include congenital microgastria; oligohydramnios/anhydramnios, with a lack of fluid to swallow and distend the stomach; diminished swallowing function (e.g., in arthrogryposis, with little to no fetal movement); or obstruction to normal swallowing, including from increased intrathoracic pressure (Fig. 86-2). A markedly distended stomach, on the other hand, might raise concern for outlet obstruction, as with pyloric atresia. Gastric volvulus rarely has been reported in utero and is more of a risk when the stomach is not in its normal location.16 A right-sided stomach in situs ambiguous or inversus should be evident as long as one is careful to establish the orientation of the fetus to the mother during the examination (Fig. 86-3).
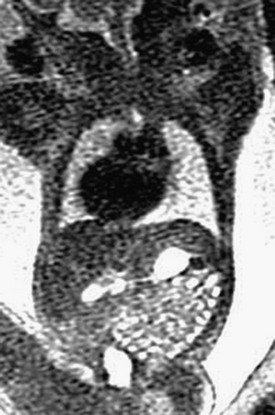
Imaging of the Duodenum: DA appears on imaging as the classic fluid-filled double bubble of dilated stomach and duodenal bulb (Fig. 86-4). Duodenal stenosis sometimes can be distinguished by hyperperistalsis of the dilated proximal duodenum, whereas in DA, no peristalsis may exist at all. DA is associated with malrotation of the bowel in approximately 29% of cases.8 Other causes for proximal obstruction include an adhesive Ladd band in a patient with primary midgut malrotation, or annular pancreas. As with other obstructions to the GI tract above the level of the distal jejunum, a fetus with duodenal obstruction typically has polyhydramnios; the more proximal the atresia, the earlier and the more frequently an abnormally large amniotic fluid volume tends to present.17
Imaging of the Small Bowel: The small bowel is considered to be dilated if it is larger than 7 mm; the colon is usually up to 15 mm in the third trimester, while the normal rectum, which should be the largest segment, can be larger18–20 (Fig. 86-5). In proximal jejunal atresia, more loops will be dilated than just the double bubble seen with DA (“triple bubble” in very proximal jejunal atresia) (Fig. 86-6); distal jejunal atresia and ileal atresia, on the other hand, may be more difficult to distinguish prospectively because multiple dilated, fluid-filled loops are seen in both conditions, and typically a normal amniotic fluid volume is present. Other considerations for a distal bowel obstruction parallel those in newborn imaging and include meconium ileus in a fetus with cystic fibrosis; midgut volvulus; volvulus around a residual of the omphalomesenteric duct; and total colonic aganglionosis (Hirschsprung disease). One clue to the diagnosis of meconium ileus might be the presence of hyperechoic debris filling distended loops near the point of obstruction in the distal small bowel on ultrasound; MRI shows that the internal contents are hyperintense on T1-weighted images but intermediate on T2 imaging, consistent with meconium. One would expect low-signal T2 contents with the more simple intraluminal fluid in the fetus with ileal atresia.21–23 Vigorous hyperperistalsis of the bowel is indicative of obstruction (Fig. 86-7 and Video 86-1). Cases of in utero volvulus, although uncommon, have been prospectively identified when the dilated ischemic segments are thick walled, nonperistaltic, and have internal echogenicity from hemorrhage.24
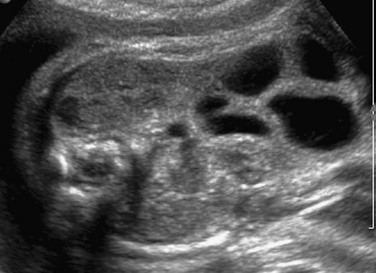
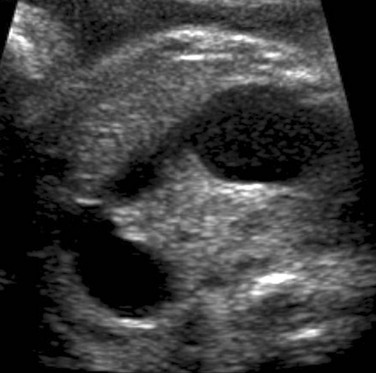
Figure 86-5 Ileal atresia.
A coronal sonogram of the abdomen shows multiple dilated small bowel loops.
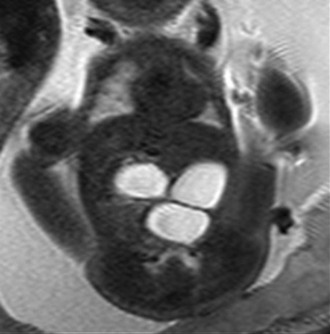
Figure 86-6 Proximal jejunal atresia.
A single-shot fast spin echo coronal image with a dilated stomach, duodenal bulb, and proximal jejunum (“triple bubble”).
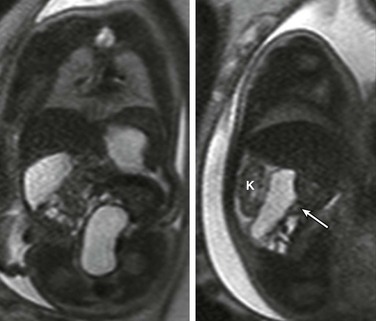
Figure 86-7 Duodenal obstruction due to Ladd’s band in malrotation.
Coronal (left) and sagittal (right) single-shot fast spin echo images show the stomach in the left upper quadrant and a dilated segment of bowel in the right upper quadrant (arrow) ventral to the right kidney (K). The ultrasound clip shown in Video 86-1 is remarkable for hyperperistalsis of this segment.
Imaging of the Colon: Colonic atresias are difficult to diagnose specifically unless the obstructed segment can be identified with certainty in the expected location of the colon, or interhaustral notches can be separated from valvulae conniventes on ultrasound. Meconium typically is seen in the rectum by 20 weeks’ gestation, in the left colon by 24 weeks’ gestation, and in the right colon by 31 weeks’ gestation, but this pattern may be altered by the pathology that is present.25 On FSE T2-weighted sequences, meconium has a very low signal, whereas it shows a high signal on T1 weighting.
In general, when dilated loops are discovered, ultrasound is sufficient to diagnose the level of obstruction as proximal versus distal and to generate a focused differential diagnosis. Fetal MRI is reserved for complex cases or for those in which other abnormalities are suspected and might be confirmed by additional imaging.26 The advantage of MRI is in its larger field of view and reproducible multiplanar capability, showing the anatomy relative to other landmarks.27 This advantage usually is demonstrated well with single-shot T2-weighted sequences in multiple planes, whereas the addition of GRE T1-weighted sequences to show the distribution of high-signal meconium can provide useful additional information (Fig. 86-8). It is crucial to understand that meconium is not exclusively restricted to the colon, can normally be seen in the distal ileum, and may be in dilated loops proximal to distal obstructions (Fig. 86-9).28 Box 86-1 lists causes of nonvisualization of meconium in the colon.
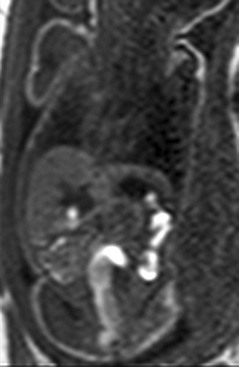
Figure 86-8 Meconium.
A coronal T1-weighted gradient echo image showing T1 high-signal meconium in the colon.
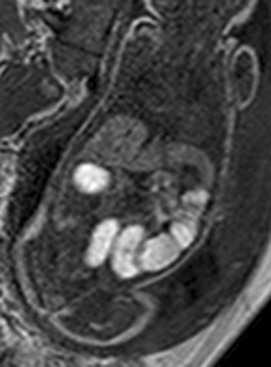
Figure 86-9 Meconium ileus.
A gradient echo T1-weighted image at 32 weeks demonstrates dilated small bowel containing high-signal meconium.
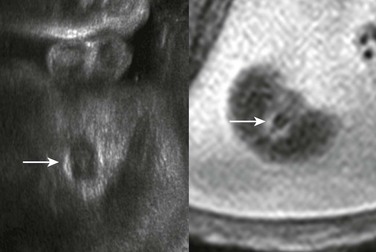
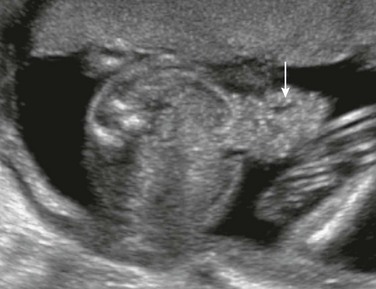
Although one might anticipate that bowel loops would be dilated in cases of anorectal atresia, such dilation is uncommon, and the diagnosis often is made on the basis of a high level of suspicion because of associated features.29 On ultrasound and MRI, the diagnosis may be suspected if the characteristic hypoechoic configuration of the anal musculature (Fig. 86-10) is absent. Also, a cord or ridge of abnormal fibrous tissue at the perineum may be imaged by ultrasound or MRI (Fig. 86-11). If a fistula between the colon and the urinary tract allows mixing of meconium and urine, intraluminal precipitant calcifications may be detected (Fig. 86-12).
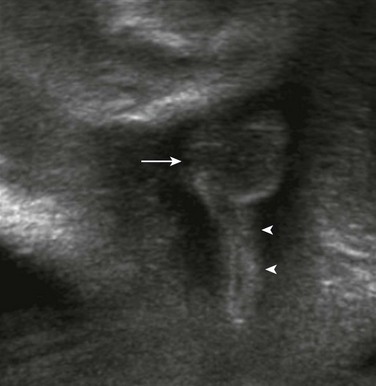
Figure 86-11 Typical configuration of the perineal cord (arrowheads) that may be seen from the genitalia toward the level of anal atresia.
The arrow indicates the scrotum in this male fetus.
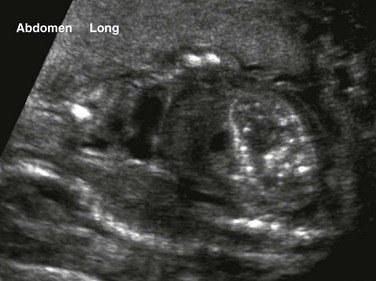
Figure 86-12 Cloacal malformation.
An oblique coronal ultrasound shows multiple small echogenic calcifications within dilated colon in the left abdomen, resulting from mixing of meconium and urine.
Congenital diarrhea syndromes have been reported as showing dilation of the bowel to the rectum but without meconium signal on T1-weighted imaging, reflecting the loss of normal meconium.30 Therefore lack of colonic meconium is not exclusive to mechanical small bowel obstructions. Differential considerations include secretory sodium or chloride diarrheas, megacystis-microcolon-hypoperistalsis syndrome, pseudoobstruction, and total colonic aganglionosis (Hirschsprung disease).25,28,30,31
Treatment and Follow-up: At this time, no criteria have been established for intervention in the fetus based on bowel obstruction unrelated to ventral wall defects, aside from amnioreduction for polyhydramnios. Selected patients may have amniocentesis to evaluate for the possibility of chromosomal abnormalities or cystic fibrosis. Follow-up is performed with ultrasonography, with a plan to have the newborn evaluated at birth by the appropriate pediatric specialists.
Ventral Wall Defects
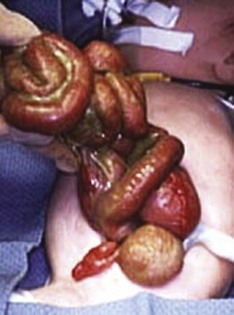
Etiology: Normal herniation of the midgut into the proximal umbilical cord occurs in the eighth gestational week, and after undergoing 270 degrees of rotation, the midgut returns to the abdominal cavity for fixation by the end of the twelfth week. Visualization of either the liver or bowel in the umbilical cord after 12 weeks is abnormal. Ventral wall defects include all abnormalities in which extrusion of internal anatomic structures to the outside of the fetal abdomen and/or pelvis occurs. In general, these abnormalities are thought to occur from a failure of the normal infolding and fusion of the embryonic disc.32 If craniad infolding fails, the resulting defect would be to the abdominal wall and lower chest, resulting in findings in the spectrum of the pentalogy of Cantrell (omphalocele, sternal defect, ventral mediastinal diaphragmatic hernia, pericardial defect, and ectopia cordis) (Fig. 86-13). Lateral fold defects lead to an omphalocele, which is the most common isolated ventral wall defect and is contained by a membrane (parietal peritoneum and amnion) unless ruptured (Fig. 86-14).33 Caudal fold failure results in bladder or cloacal exstrophy, which primarily affect the genitourinary tract. In bladder exstrophy, the ventral wall of the bladder, inferior rectus musculature, and skin are absent, and the residual dorsal wall of the bladder is continuous with the abdominal wall. Cloacal exstrophy is more complex; persistence of the infraumbilical cloacal membrane is thought to prevent normal anterior abdominal wall closure and separation of the urogenital system from the rectum, and as a result, two hemibladders are separated by bowel. Closed spinal defects, renal and genital malformations, and club feet may occur. Both bladder and cloacal exstrophy include diastasis of the pubic symphysis and may have more complex pelvic bone deformities (Fig. 86-15). OEIS complex describes the particular association of omphalocele, bladder exstrophy, imperforate anus, and spinal defect,34 but this complex is believed by many persons to be a synonym for cloacal exstrophy.35
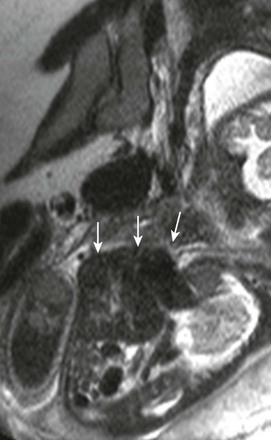
Figure 86-13 Pentalogy of Cantrell.
A sagittal single-shot fast spin echo image through the chest shows herniation of low-signal liver and even lower-signal heart (arrows) through a ventral wall defect.
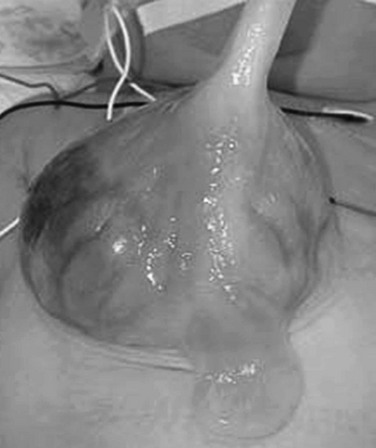
Figure 86-14 Omphalocele.
This photograph of a newborn shows a large anterior wall defect with a surrounding membrane. The umbilical cord is inserting at the apex.
Body stalk anomaly is a severe form of ventral wall defect that results from failure of folding along multiple planes and is not compatible with extrauterine life (Fig. 86-16).36
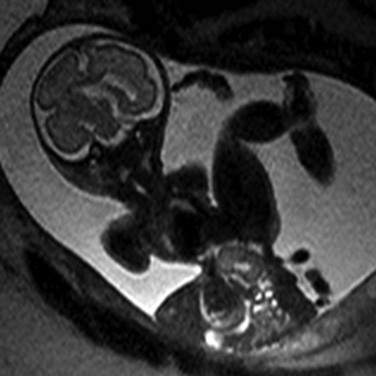
Figure 86-16 Limb-body wall complex.
Extrusion of all abdominal contents in limb-body wall complex. Typically a very short umbilical cord and significant fetal contortion are present.
Gastroschisis is a type of ventral wall defect that may be unrelated to embryonic disc folding. It is possible that a vascular insult results in a through-and-through defect in the layers of the abdominal wall adjacent to the umbilicus, almost universally on the right, through which the bowel herniates without a covering membrane; other hypotheses have been proposed, but the cause is not yet known (Fig. 86-17).37,38 The risk of bearing a fetus with gastroschisis is reportedly increased in young primigravida women and also has been associated with the use of agents that may cause vasoconstriction, such as tobacco and salicylates.39,40
Imaging: Visualization of liver or bowel within the umbilical cord beyond the twelfth week of gestation is evidence of an omphalocele. A giant omphalocele is defined when more than 50% of the liver is extracorporeal or the defect is greater than 5 cm wide.41,42 Wharton’s jelly pseudocysts may be found in association with the defect, located near the cord insertion site on the omphalocele.43 When the defect is small, the differentiation of a small, skin-covered umbilical hernia from omphalocele is difficult. Ultrasound typically can diagnose these defects readily and accurately define the extruded anatomy (Fig. 86-18). When ultrasound fails to reveal the pertinent details, MRI can be useful and relies on standard single-shot FSE T2-weighted imaging; the liver tends to have a higher signal than other abdominal structures on T1-weighted sequences, which is useful for identification of the position of the liver in questionable cases. Characteristically, however, when fetal MRI is requested in cases of omphalocele, it is because of an interest in assessing for additional abnormalities or a need to calculate MR-derived lung volumes. Newborns with giant omphalocele have a tendency to need long-term ventilatory support and may have pulmonary hypoplasia.42,44,45 Echocardiography is indicated, because 50% of fetuses with omphalocele have congenital heart disease. Ascites may be present but tends to decrease through gestation. Note that if a normal bladder is not seen over time or in repeated studies, exstrophy must be considered.
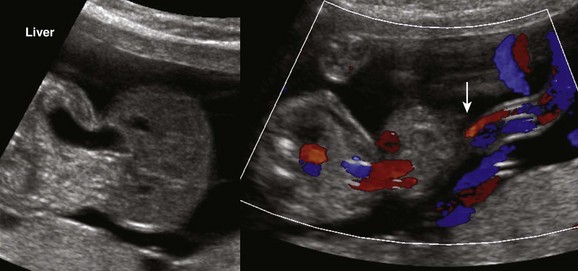
Figure 86-18 Omphalocele containing liver and distal stomach.
The umbilical cord inserts into the omphalocele sac (arrow). The spine is on the left.
Gastroschisis is differentiated from omphalocele by the lack of a covering membrane of the herniated viscera and the normal insertion of the umbilical vessels at the abdominal wall (Fig. 86-19). MRI generally is not necessary for evaluation of this diagnosis because gastroschisis almost always is an isolated defect without additional fetal malformations or lung hypoplasia.32 Closure of the abdominal wall defect in utero with subsequent severing of the extruded bowel, thus leading to extensive atresia/congenital short-gut syndrome, has been reported.46 Additionally, on rare occasions the membrane covering an omphalocele may rupture, thus simulating gastroschisis on imaging because of the free-floating appearance of the bowel. Differentiation is made by examining the relationship of the umbilical vessels to the extruded bowel. If the liver is extruded, it is an omphalocele (Fig. 86-20).
Treatment and Follow-up: Fetuses in whom omphalocele is diagnosed are tested for chromosomal abnormalities because the incidence of aneuploidy is 40% to 60%, especially if only bowel is herniated.47 The outcome for patients with omphalocele is usually dependent on the severity of other anomalies, including cardiac defects.48 Repair of omphaloceles in the newborn period has a high rate of success, especially with surgical techniques emphasizing gradual reduction for larger lesions.33,49,50
Gastroschisis, unlike omphalocele, is not associated with chromosomal abnormalities, although the occurrence of extraintestinal abnormalities is approximately 6%.51 Fetuses with gastroschisis experience complications that occur in the part of the bowel that is directly exposed to amniotic fluid during pregnancy; perforation or atresia occurs in up to 20% of cases.51 Although dilation of the herniated loops is poorly correlated to worsened prognosis, sustained distension of intraabdominal bowel loops is of concern.52 Postnatally, these patients may require resection of portions of their bowel, and short-gut syndrome may develop. These children are at risk for presentation with necrotizing enterocolitis and perforation even months after undergoing repair.53,54
Echogenic Bowel and Peritoneal Abnormalities
Etiology: Echogenic bowel is a nonspecific ultrasound finding that should not be ignored, although in most cases no subsequent fetal or neonatal problem develops.55 In cases in which this finding is a marker for an abnormality, the problem could be as varied in etiology as infection (e.g., cytomegalovirus or parvovirus), intrauterine growth retardation, or aneuploidy (e.g., trisomy 21), or the direct cause of the increased conspicuity of the bowel might remain unclear. In other cases, the etiology may be more obvious; for example, in persons with cystic fibrosis, the meconium is more viscous and may present as hyperechoic contents within the bowel lumen. If bleeding has occurred into either the amniotic fluid or GI tract, swallowed intraluminal fluid can appear bright on ultrasound.
Abnormalities of the peritoneum in the fetus include ascites and calcifications. The etiology of fetal ascites can be classified under five general categories, ranging from serous through chylous, hemorrhagic, bilious, and related to the urinary tract (Box 86-2).56–65
Imaging: When the bowel is as echogenic as the fetal skeleton, it is considered abnormal (Fig. 86-21).66 It has been recommended that transducer frequencies of 5 MHz or less be used for confirmation, because higher frequencies may make the bowel appear unnecessarily bright.67 Although most of these patients are healthy, the finding of abnormally bright bowel in the fetus should prompt a careful ultrasound search for additional abnormalities, with consideration of further imaging by MRI for evaluation of the neural axis if infection is suspected.
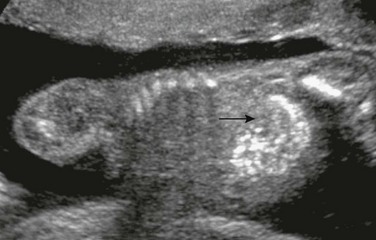
Figure 86-21 Echogenic bowel.
A longitudinal sonogram of a 20-week fetus with echogenic bowel (arrow) that is as echogenic as the adjacent bone. Intrauterine growth retardation was present.
Imaging of fetal ascites should be directed toward determining whether the fluid can be related to a particular organ system. Anatomy should be examined carefully, whether on ultrasound or MRI, for abnormalities of the urinary or reproductive tracts, biliary system, chest, and bowel. The presence of septations or loculation might suggest protein contamination from blood products, bowel pathology, or abdominal lymphangiomatosis. In some case, ascites may be a physiological consequence of a condition (e.g., hypoproteinemia, hydrops, or infection) not directly related to an anatomic abnormality. It is important to look for any evidence of calcifications in the peritoneum, especially along the liver capsule, that would indicate meconium peritonitis and the implied disruption of bowel integrity, as from perforations secondary to obstruction or ischemia. Calcifications in the parenchyma of the liver or spleen would be a clue to the presence of aneuploidy or infection, or rarely may be related to portal emboli.68,69 Calcification inside the bowel lumen is an indication of GI-genitourinary fistula (see Fig. 86-12).70
Treatment and Follow-up: Treatment and follow-up are directed toward the cause of the finding and could include in utero intervention (e.g., intrauterine transfusion) and postnatal surgery, depending on the etiology. Fetuses who have isolated fetal ascites with a normal karyotype and a negative infection screen have a good prognosis.71,72
Masses and Other Organ Abnormalities
Etiology: The etiology of masses in the fetal abdomen may be related to abnormally enlarged or infiltrated viscera or to the presence or development of an abnormal structure.
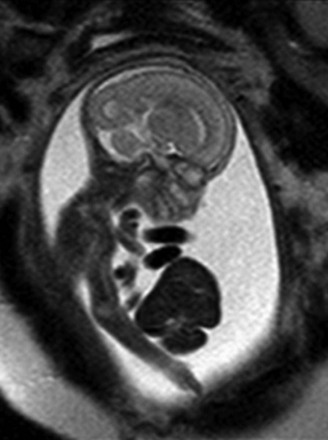
Visceromegaly includes hepatosplenomegaly, which may be the result of viral infection, fetal hydrops, glycogen or lysosomal storage disorders, Beckwith-Wiedemann syndrome, or the presentation in utero of anemia or hematologic malignancy (particularly for trisomy 21), and amniotic fluid testing may be necessary.57,65,73–75 Considerations for isolated hepatomegaly include increased right heart pressure; the liver also might appear enlarged if it lies transversely across the abdomen, as in heterotaxy. Neoplasia also may present with visceromegaly, with the organ being either a repository for metastatic disease, such as neuroblastoma diffusely metastatic to the liver,76,77 or the site of a primary intraperitoneal tumor, most frequently in the fetal liver (Fig. 86-22). Intrauterine torsion also can present with visceromegaly from edema.78–80 A special note should be made of megacystis-microcolon-hypoperistalsis syndrome, which can be considered in patients, more commonly female, with gross bladder enlargement and no colonic meconium on T1-weighted MRI (Fig. 86-23).25,81
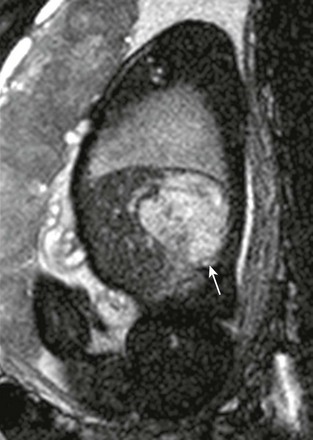
Figure 86-22 Rapidly involuting congenital hemangioma.
A sagittal T2-weighted image of a high-signal mass lesion (arrow) in the posterior right lobe of the liver.
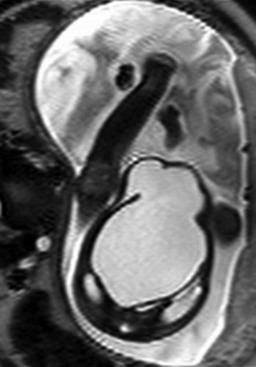
Figure 86-23 Megacystis-microcolon-hypoperistalsis syndrome.
An axial single-shot fast spin echo image in a female fetus with hydronephrosis and marked bladder distension. Normal amniotic fluid volume was present. No meconium could be identified in the bowel on T1-weighted imaging.
The category of abnormal structures presenting as mass lesions includes intraperitoneal cysts or fluid collections, such as meconium peritonitis as a result of perforation of the bowel, or, as recently reported, as a result of reflux of meconium into the peritoneum through the uterus in a fetus with cloacal malformation (Figs. 86-24 and 86-25).82 Box 86-3 lists the origins of cystic masses in the abdomen and pelvis, and Box 86-4 describes solid masses that have been reported. Occasionally a “pseudomass” is described inside of a bowel lumen, such as the stomach; in this particular case, it is thought to represent debris such as blood products and may be seen after a history of intervention (e.g., amniocentesis).83 Note that most commonly, masses in the fetal abdomen are related to the genitourinary tract; these masses are discussed in greater detail in Chapter 113.
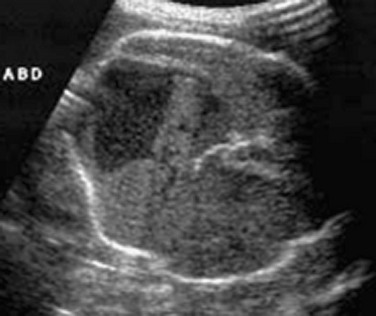
Figure 86-24 Meconium pseudocyst.
A transverse sonogram shows a large loculated heterogeneous collection with a calcified rim within the abdomen. A sigmoid perforation was identified after delivery.
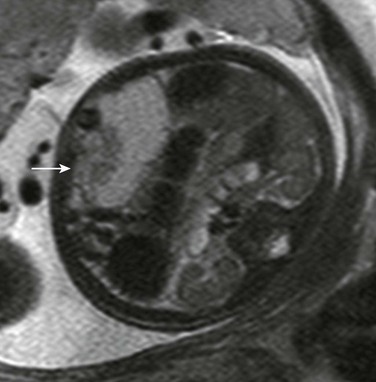
Figure 86-25 Meconium pseudocyst.
Magnetic resonance shows a medium to moderately high signal collection containing debris (arrow) on T2-weighted imaging, consistent with meconium mixing.
Some abnormalities are related to the absence of expected structures. For example, the spleen may be absent, and the gallbladder may be in abnormal position or absent; these findings suggest heterotaxy, which would mandate careful evaluation of the cardiac axis and configuration and other viscerovascular structures. The gallbladder usually can be detected on serial ultrasound84 and is identified consistently on MRI.85 Its absence would suggest biliary atresia and polysplenia if found within the heterotaxy complex. A gallbladder also may be indistinguishable in a fetus with cystic fibrosis.
Calcifications and/or debris in the gallbladder can be a sign of fetal hemolysis, but most are incidental and do not cause complications.86 Small stones typically resolve without intervention. Finally, organs may be displaced from their normal position in persons with splenogonadal fusion, the so-called wandering spleen, and of course with diaphragmatic hernias.
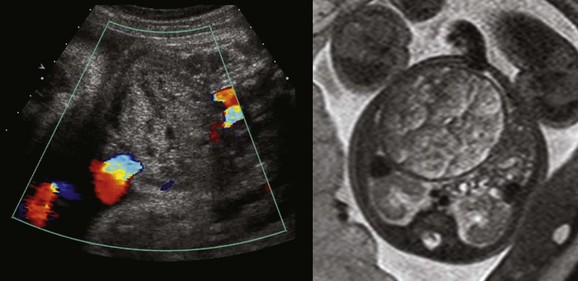
Imaging: Careful delineation of the organ of involvement and of the pertinent anatomy can suggest the correct diagnosis (Fig. 86-26). When calcifications are identified, infection should be considered.87 The use of a high-resolution linear transducer in the appropriate patient can be extremely advantageous in delineating fine detail, such as subtle calcifications, the presence within a large cyst of a daughter cyst indicative of ovarian origin, or the characteristic bowel signature of a duplication cyst. For all abnormalities, in addition to identifying the organ of origin, Doppler evaluation will help to understand whether a primary vascular lesion (increased flow) or torsion/ischemia (decreased flow) should be advanced in the differential diagnosis (Fig. 86-27).
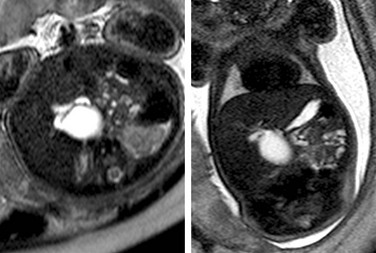
Figure 86-26 Choledochal cyst.
Axial and coronal T2-weighted single-shot fast spin echo images of a cystic mass at the margin of the liver demonstrate a connection to the biliary system.
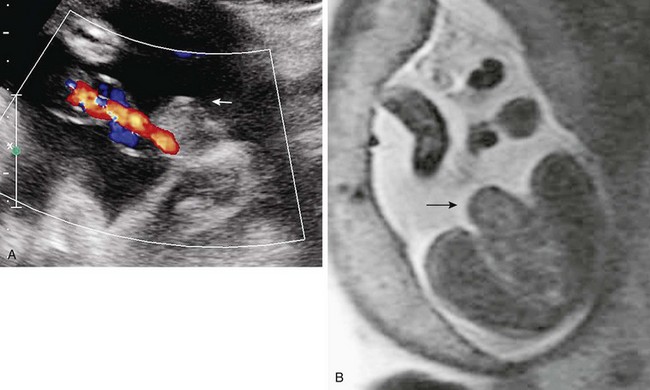
Ultrasound generally delineates well the origin of fetal abdominal masses from solid organs. Masses are investigated by MRI when the organ of origin or the extent of involvement is imprecisely detailed by ultrasonography. In addition to the use of GRE, T1-weighted and FSE T2-weighted sequences, SSFP T2-weighted sequences are useful because of their ability to delineate adjacent structures and high-signal blood flow. This effect can be very helpful in identifying primary vascular lesions (Fig. 86-28) or enlarged vessels supplying tumors. In addition, fetal MRI may be indicated in the evaluation of the liver in conditions of potential iron overload, in which the signal from the liver is abnormally low.88,89
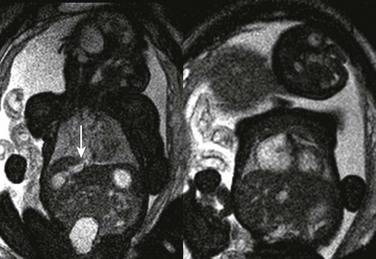
Figure 86-28 Hepatic vascular malformation.
Balanced steady-state free-precession coronal imaging shows an enlarged draining vein (arrow, left) and enlarged right atrium (right) as a result of congenital vascular malformation in the liver.
Umbilical venous varix is a vascular lesion that is variably associated with in utero complications.90 Other vascular anomalies of the venous system, including absence of the ductus venosus or agenesis of the portal veins, have been described and require meticulous delineation of the associated portosystemic shunt pathway by ultrasound Doppler or bright-blood (SSFP) MRI for accurate diagnosis.91,92.
Treatment and Follow-up: Depending on the diagnosis, alterations in planning and timing of delivery may be indicated; amniocentesis and in utero therapies may be considered (e.g., intrauterine transfusion for fetal anemia). In general, therapy is targeted at providing the safest environment in which appropriate treatment can be directed for the fetus or neonate. To date, there are no clear indications for fetal surgery for gastrointestinal anomalies, although imaging evaluation can alter both the timing and mode of delivery. Open fetal surgery has been reserved for conditions determined to be terminal without intervention in a previable fetus, and has been limited to excisions of malformations and tumors of the chest, head and neck, and coccyx (e.g., sacrococcygeal teratoma).93–95
Brugger, PC. MRI of the fetal abdomen. In: Prayer D, ed. Fetal MRI. Berlin: Springer-Verlag, 2011.
Dubois, J, Grignon, A. Abdomen (digestive tract, wall and peritoneum. In: Avni FE, ed. Perinatal imaging: from ultrasound to MR imaging. Berlin: Springer-Verlag, 2002.
Hertberg, BS, Nyberg, DA, Neilsen, IR. Ventral wall defects. In: Nyberg D, McGahan JP, Pretorius DH, et al, eds. Diagnostic imaging of fetal anomalies. Philadelphia: Lippincott, Williams and Wilkins, 2003.
Nyberg, DA, Neilsen, IR. Abdomen and gastrointestinal tract. In: Nyberg D, McGahan JP, Pretorius DH, et al, eds. Diagnostic imaging of fetal anomalies. Philadelphia: Lippincott, Williams and Wilkins, 2003.
Samuel, N, Dicker, D, Feldberg, D, et al. Ultrasound diagnosis and management of fetal intestinal obstruction and volvulus in utero. J Perinat Med. 1984;12(6):333–337.
References
1. American College of Radiology, American College of Obstetricians and Gynecologists, American Institute of Ultrasound in Medicine. Practice guideline for the performance of obstetrical ultrasound (pdf file). http://www.acr.org/~/media/F7BC35BD59264E7CBE648F6D1BB8B8E2.pdf. [Accessed August 7, 2012].
2. Brugger, PC, Stuhr, F, Lindner, C, et al. Methods of fetal MR: beyond T2-weighted imaging. Eur J Radiol. 2006;57(2):172–181.
3. Merei, JM, Farmer, P, Hasthorpe, S, et al. Timing and embryology of esophageal atresia and tracheo-esophageal fistula. Anat Rec. 1997;249(2):240–248.
4. Spilde, TL, Bhatia, AM, Marosky, JK, et al. Fibroblast growth factor signaling in the developing tracheoesophageal fistula. J Pediatr Surg. 2003;38(3):474–477.
5. El-Gohary, Y, Gittes, GK, Tovar, JA. Congenital anomalies of the esophagus. Semin Pediatr Surg. 2010;19(3):186–193.
6. Franken EA, ed. Gastrointestinal Imaging in Pediatrics, 2nd ed, Philadelphia: Harper and Row, 1982.
7. Louw, JH. Congenital intestinal atresia and stenosis in the newborn. Observations on its pathogenesis and treatment. Ann R Coll Surg Engl. 1959;25:209–234.
8. Kimble, RM, Harding, JE, Kolbe, A. Is malrotation significant in the pathogenesis of duodenal atresia? Pediatr Surg Int. 1995;10(5-6):325–328.
9. Dalla Vecchia, LK, Grosfeld, JL, West, KW, et al. Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg. 1998;133(5):490–496. [discussion 496-497].
10. Touloukian, RJ. Diagnosis and treatment of jejunoileal atresia. World J Surg. 1993;17(3):310–317.
11. Gahukamble, DB, Adnan, AR, Al-Gadi, M. Atresias of the gastrointestinal tract in an inbred, previously unstudied population. Pediatr Surg Int. 2002;18(1):40–42.
12. Bilodeau, A, Prasil, P, Cloutier, R, et al. Hereditary multiple intestinal atresia: thirty years later. J Pediatr Surg. 2004;39(5):726–730.
13. Sweeney, B, Surana, R, Puri, P. Jejunoileal atresia and associated malformations: correlation with the timing of in utero insult. J Pediatr Surg. 2001;36(5):774–776.
14. Seo, T, Ando, H, Watanabe, Y, et al. Colonic atresia and Hirschsprung’s disease: importance of histologic examination of the distal bowel. J Pediatr Surg. 2002;37(8):E19.
15. Brugger, PC. Methods of fetal MRI. In: Prayer D, ed. Fetal MRI. Berlin: Springer-Verlag, 2011.
16. Al-Assiri, A, Wiseman, N, Bunge, M. Prenatal diagnosis of intrathoracic stomach (gastric herniation). J Pediatr Surg. 2005;40(2):E15–E17.
17. Kimble, RM, Harding, JE, Kolbe, A. Does gut atresia cause polyhydramnios? Pediatr Surg Int. 1998;13(2-3):115–117.
18. Brugger, PC, Prayer, D. Fetal abdominal magnetic resonance imaging. Eur J Radiol. 2006;57(2):278–293.
19. Malas, MA, Aslankoç, R, Ungör, B, et al. The development of large intestine during the fetal period. Early Hum Dev. 2004;78(1):1–13.
20. Zalel, Y, Perlitz, Y, Gamzu, R, et al. In-utero development of the fetal colon and rectum: sonographic evaluation. Ultrasound Obstet Gynecol. 2003;21(2):161–164.
21. Muller, F, Aubry, MC, Gasser, B, et al. Prenatal diagnosis of cystic fibrosis. II. Meconium ileus in affected fetuses. Prenat Diagn. 1985;5(2):109–117.
22. Zizka, J, Elias, P, Hodik, K, et al. Liver, meconium, haemorrhage: the value of T1-weighted images in fetal MRI. Pediatr Radiol. 2006;36(8):792–801.
23. Carcopino, X, Chaumoitre, K, Shojai, R, et al. Foetal magnetic resonance imaging and echogenic bowel. Prenat Diag. 2007;27(3):272–278.
24. Miyakoshi, K, Ishimoto, H, Tanigaki, S, et al. Prenatal diagnosis of midgut volvulus by sonography and magnetic resonance imaging. Am J Perinatol. 2001;18(8):447–450.
25. Veyrac, C, Couture, A, Saguintaah, M, et al. MRI of fetal GI tract abnormalities. Abdom Imaging. 2004;29(4):411–420.
26. Benachi, A, Sonigo, P, Jouannic, AM, et al. Determination of the anatomical location of an antenatal intestinal occlusion by magnetic resonance imaging. Ultrasound Obstet Gynecol. 2001;18(2):163–165.
27. Biyyam, DR, Dighe, M, Siebert, JR. Antenatal diagnosis of intestinal malrotation on fetal MRI. Pediatr Radiol. 2009;39(8):847–849.
28. Colombani, M, Ferry, M, Garel, C, et al. Fetal gastrointestinal MRI: all that glitters in T1 is not necessarily colon. Pediatr Radiol. 2010;40(7):1215–1221.
29. Harris, RD, Nyberg, DA, Mack, LA, et al. Anorectal atresia: prenatal sonographic diagnosis. AJR Am J Roentgenol. 1987;149(2):395–400.
30. Colombani, M, Ferry, M, Toga, C, et al. Magnetic resonance imaging in the prenatal diagnosis of congenital diarrhea. Ultrasound Obstet Gynecol. 2010;35(5):560–565.
31. Shen, O, Schimmel, MS, Eitan, R, et al. Prenatal diagnosis of intestinal pseudo-obstruction. Ultrasound Obstet Gynecol. 2007;29(2):229–231.
32. Vermeij-Keers, C, Hartwig, NG, van der Werff, JF. Embryonic development of the ventral body wall and its congenital malformations. Semin Pediatr Surg. 1996;5(2):82–89.
33. Langer, JC. Gastroschisis and omphalocele. Semin Pediatr Surg. 1996;5(2):124–128.
34. El-Hattab, AW, Skorupski, JC, Hsieh, MH, et al. OEIS complex associated with chromosome 1p36 deletion: a case report and review. Am J Med Genet A. 2010;152A(2):504–511.
35. Bohring, A. OEIS complex, VATER, and the ongoing difficulties in terminology and delineation. Am J Med Genet. 2002;107(1):72–76.
36. Pumberger, W, Schaller, A, Bernaschek, G. Limb-body wall complex: a compound anomaly pattern in body-wall defects. Pediatr Surg Int. 2001;17(5-6):486–490.
37. Stevenson, RE, Rogers, RC, Chandler, JC, et al. Escape of the yolk sac: a hypothesis to explain the embryogenesis of gastroschisis. Clin Genet. 2009;75(4):326–333.
38. Feldkamp, ML, Carey, JC, Sadler, TW. Development of gastroschisis: review of hypotheses, a novel hypothesis, and implications for research. Am J Med Genet A. 2007;143(7):639–652.
39. Haddow, JE, Palomaki, GE, Holman, MS. Young maternal age and smoking during pregnancy as risk factors for gastroschisis. Teratology. 1993;47(3):225–228.
40. Loane, M, Dolk, H, Morris, JK. Maternal age-specific risk of non-chromosomal anomalies. BJOG. 2009;116(8):1111–1119.
41. Wilson, RD, Johnson, MP. Congenital abdominal wall defects: an update. Fetal Diagn Ther. 2004;19(5):385–398.
42. Lee, SL, Beyer, TD, Kim, SS, et al. Initial nonoperative management and delayed closure for treatment of giant omphaloceles. J Pediatr Surg. 2006;41(11):1846–1849.
43. Sepulveda, W, Gutierrez, J, Sanchez, J, et al. Pseudocyst of the umbilical cord: prenatal sonographic appearance and clinical significance. Obstet Gynecol. 1999;93(3):377–381.
44. Edwards, EA, Broome, S, Green, S, et al. Long-term respiratory support in children with giant omphalocele. Anaesth Intensive Care. 2007;35(1):94–98.
45. Argyle, JC. Pulmonary hypoplasia in infants with giant abdominal wall defects. Pediatr Pathol. 1989;9(1):43–55.
46. Bhatia, AM, Musemeche, CA, Crino, JP. Gastroschisis complicated by midgut atresia and closure of the defect in utero. J Pediatr Surg. 1996;31(9):1288–1289.
47. Nyberg, DA, Fitzsimmons, J, Mack, LA, et al. Chromosomal abnormalities in fetuses with omphalocele. Significance of omphalocele contents. J Ultrasound Med. 1989;8(6):299–308.
48. Mayer, T, Black, R, Matlak, ME, et al. Gastroschisis and omphalocele. An eight-year review. Ann Surg. 1980;192(6):783–787.
49. Pacilli, M, Spitz, L, Kiely, EM, et al. Staged repair of giant omphalocele in the neonatal period. J Pediatr Surg. 2005;40(5):785–788.
50. Schlatter, M, Norris, K, Uitylugt, M, et al. Improved outcomes in the treatment of gastroschisis using a preformed silo and delayed repair approach. J Pediatr Surg. 2003;38(3):459–464.
51. Brantberg, A, Blaas, HG, Salvesen, KE, et al. Surveillance and outcome of fetuses with gastroschisis. Ultrasound Obstet Gynecol. 2004;23(1):4–13.
52. Huh, NG, Hirose, S, Goldstein, RB. Prenatal intraabdominal bowel dilation is associated with postnatal gastrointestinal complications in fetuses with gastroschisis. Am J Obstet Gynecol. 2010;202(4):396. [e1-e6].
53. Benamar, A, Michaud, L, Sfeir, R, et al. Necrotizing enterocolitis in two infants with gastroschisis [in French]. Arch Pediatr. 2008;15(2):149–152.
54. Oldham, KT, Coran, AG, Drongowski, RA, et al. The development of necrotizing enterocolitis following repair of gastroschisis: a surprisingly high incidence. J Pediatr Surg. 1988;23(10):945–949.
55. Kesrouani, AK, Guibourdenche, J, Muller, F, et al. Etiology and outcome of fetal echogenic bowel. Ten years of experience. Fetal Diagn Ther. 2003;18(4):240–246.
56. Chen, CP, Lin, SP, Chang, TY, et al. Prenatal sonographic findings of Klippel-Trenaunay-Weber syndrome. J Clin Ultrasound. 2007;35(7):409–412.
57. Degani, S. Sonographic findings in fetal viral infections: a systematic review. Obstet Gynecol Surv. 2006;61(5):329–336.
58. Piersigilli, F, Auriti, C, Marcellini, M, et al. Isolated fetal ascites due to Budd-Chiari syndrome. Ultrasound Obstet Gynecol. 2008;31(2):222–223.
59. Casaccia, G, Giorlandino, C, Catalano, OA, et al. Prenatal rectal perforation: an unsuspected cause of isolated ascites. J Perinatol. 2006;26(11):717–719.
60. Nitta, A, Suzumura, H, Watabe, Y, et al. Fetal hemophagocytic lymphohistiocytosis in a premature infant. J Pediatr. 2007;151(1):98.
61. Degani, S, Lewinsky, RM. Transient ascites associated with a fetal ovarian cyst. Case report. Fetal Diagn Ther. 1995;10(3):200–203.
62. Truin, G, Guillard, M, Lefeber, DJ, et al. Pericardial and abdominal fluid accumulation in congenital disorder of glycosylation type Ia. Mol Genet Metab. 2008;94(4):481–484.
63. Alkan, M, Ozçelik, Z, Keskin, E, et al. Fetal urinary ascites in a neonate without detectable obstructive uropathy or neurogenic bladder etiology. J Pediatr Urol. 2009;5(2):151–153.
64. Camanni, D, Zaccara, A, Capitanucci, ML, et al. Isolated fetal ascites secondary to persistent urogenital sinus. Obstet Gynecol Int. 2009;2009:219010.
65. Spiegel, R, Raas-Rothschild, A, Reish, O, et al. The clinical spectrum of fetal Niemann-Pick type C. Am J Med Genet A. 2009;149A(3):446–450.
66. Al-Kouatly, HB, Chasen, ST, Streltzoff, J, et al. The clinical significance of fetal echogenic bowel. Am J Obstet Gynecol. 2001;185(5):1035–1038.
67. Vincoff, NS, Callen, PW, Smith-Bindman, R, et al. Effect of ultrasound transducer frequency on the appearance of the fetal bowel. J Ultrasound Med. 1999;18(12):799–803. [quiz 805-806].
68. Simchen, MJ, Toi, A, Bona, M, et al. Fetal hepatic calcifications: prenatal diagnosis and outcome. Am J Obstet Gynecol. 2002;187(6):1617–1622.
69. Achiron, R, Seidman, DS, Afek, K, et al. Prenatal ultrasonographic diagnosis of fetal hepatic hyperechogenicities: clinical significance and implications for management. Ultrasound Obstet Gynecol. 1996;7(4):251–255.
70. Lubusky, M, Prochazka, M, Dhaifalah, M, et al. Fetal enterolithiasis: prenatal sonographic and MRI diagnosis in two cases of urorectal septum malformation (URSM) sequence. Prenat Diagn. 2006;26(4):345–349.
71. El Bishry, G. The outcome of isolated fetal ascites. Eur J Obstet Gynecol Reprod Biol. 2008;137(1):43–46.
72. Zelop, C, Benacerraf, BR. The causes and natural history of fetal ascites. Prenat Diagn. 1994;14(10):941–946.
73. Ghidini, A, Sirtori, M, Romero, R, et al. Hepatosplenomegaly as the only prenatal finding in a fetus with pyruvate kinase deficiency anemia. Am J Perinatol. 1991;8(1):44–46.
74. Smrcek, JM, Baschat, AA, Germer, U, et al. Fetal hydrops and hepatosplenomegaly in the second half of pregnancy: a sign of myeloproliferative disorder in fetuses with trisomy 21. Ultrasound Obstet Gynecol. 2001;17(5):403–409.
75. Lipitz, S, Yagel, S, Shalev, E, et al. Prenatal diagnosis of fetal primary cytomegalovirus infection. Obstet Gynecol. 1997;89(5 Pt 1):763–767.
76. Jennings, RW, LaQuaglia, MP, Leong, K, et al. Fetal neuroblastoma: prenatal diagnosis and natural history. J Pediatr Surg. 1993;28(9):1168–1174.
77. Jaffa, AJ, Many, A, Hartoov, J, et al. Prenatal sonographic diagnosis of metastatic neuroblastoma: report of a case and review of the literature. Prenat Diagn. 1993;13(1):73–77.
78. Woodward, PJ, Sohaey, R, Kennedy, A, et al. From the archives of the AFIP: a comprehensive review of fetal tumors with pathologic correlation. Radiographics. 2005;25(1):215–242.
79. Applegate, KE, Ghei, M, Perez-Atayde, AR. Prenatal detection of a solitary liver adenoma. Pediatr Radiol. 1999;29(2):92–94.
80. Catanzarite, V, Hilfiker, M, Daneshmand, S, et al. Prenatal diagnosis of fetal hepatoblastoma: case report and review of the literature. J Ultrasound Med. 2008;27(7):1095–1098.
81. Garel, C, Dreux, S, Phillippe-Chomette, P, et al. Contribution of fetal magnetic resonance imaging and amniotic fluid digestive enzyme assays to the evaluation of gastrointestinal tract abnormalities. Ultrasound Obstet Gynecol. 2006;28(3):282–291.
82. Hamada, T, Hirose, R, Kosaka, T, et al. Giant cystic meconium peritonitis associated with a cloacal anomaly: case report. J Pediatr Surg. 2008;43(3):E21–E23.
83. McNamara, A, Levine, D. Intraabdominal fetal echogenic masses: a practical guide to diagnosis and management. Radiographics. 2005;25(3):633–645.
84. Shen, O, Rabinowitz, R, Yagel, S, et al. Absent gallbladder on fetal ultrasound: prenatal findings and postnatal outcome. Ultrasound Obstet Gynecol. 2011;37(6):673–677.
85. Brugger, PC, Weber, M, Prayer, D. Magnetic resonance imaging of the fetal gallbladder and bile. Eur Radiol. 2010;20(12):2862–2869.
86. Brown, DL, Teele, RL, Doubilet, PM, et al. Echogenic material in the fetal gallbladder: sonographic and clinical observations. Radiology. 1992;182(1):73–76.
87. Imbar, T, Lev-Sagie, A, Cohen, S, et al. Diagnosis, surveillance, and treatment of the anemic fetus using middle cerebral artery peak systolic velocity measurement. Prenat Diagn. 2006;26(1):45–51.
88. Cassart, M, Avni, FE, Guibaud, L, et al. Fetal liver iron overload: the role of MR imaging. Eur Radiol. 2011;21(2):295–300.
89. Marti-Bonmati, L, Baamonde, A, Poyatos, CR, et al. Prenatal diagnosis of idiopathic neonatal hemochromatosis with MRI. Abdom Imaging. 1994;19(1):55–56.
90. Zalel, Y, Lehavi, O, Heifetz, S, et al. Varix of the fetal intra-abdominal umbilical vein: prenatal sonographic diagnosis and suggested in utero management. Ultrasound Obstet Gynecol. 2000;16(5):476–478.
91. Achiron, R, Gindes, L, Kivilevitch, Z, et al. Prenatal diagnosis of congenital agenesis of the fetal portal venous system. Ultrasound Obstet Gynecol. 2009;34(6):643–652.
92. Perles, Z, Nir, A, Nadjari, M, et al. Absent ductus venosus in the fetus: review of the literature and first report of direct umbilical venous drainage to the coronary sinus. Fetal Diagn Ther. 2003;18(4):247–251.
93. Adzick, NS, Harrison, MR, Flake, AW, et al. Fetal surgery for cystic adenomatoid malformation of the lung. J Pediatr Surg. 1993;28(6):806–812.
94. Hirose, S, Sydorak, RM, Tsao, K, et al. Spectrum of intrapartum management strategies for giant fetal cervical teratoma. J Pediatr Surg. 2003;38(3):446–450.
95. Hirose, S, Farmer, DL. Fetal surgery for sacrococcygeal teratoma. Clin Perinatol. 2003;30(3):493–506.