Chapter 20 Premature Ovarian Failure
INTRODUCTION
Premature ovarian failure is defined as hypergonadotropic hypogonadism before age 40. Premature ovarian failure is commonly but not uniformly associated with depletion of ovarian follicles, as is seen in menopause. It results in cessation of regular menses. This condition affects approximately 1% of all women, with 90% of cases occurring between ages 30 and 40. Premature ovarian failure will be found in 10% to 28% of women presenting with primary amenorrhea and 4% to 18% of women presenting with secondary amenorrhea.1
OVARIAN EMBRYOLOGY
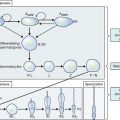
The primordial germ cells are known to originate from the endoderm of the yolk sac. These cells can be identified histologically as early as the end of the third week of gestation and migrate to the genital ridge.2 By 8 weeks of intrauterine life, persistent mitosis increases the total number of oogonia to 600,000.3 From this point on, the oogonial endowment is subject to three simultaneous ongoing processes: mitosis, meiosis, and oogonial atresia. At approximately 20 weeks’ gestation, the ovaries possess the maximal complement of up to 6 million to 8 million primary oocytes, approximately two thirds of which have entered and arrested in the prophase of the first meiotic division.1
From midgestation onward, relentless and irreversible attrition progressively diminishes the germ cell endowment of the gonad.4 Some of the oogonia depart from the mitotic cycle to enter the prophase of the first meiotic division between weeks 8 and 13 of fetal life. This change marks the conversion of these cells to primary oocytes well before actual follicle formation.
A role for steroids has been suggested in the control of ovarian primordial follicle assembly and early follicular development.5 It is generally presumed that usually no oogonia are present at birth. Unconfirmed data from mice has challenged this concept, however.6 These investigators have challenged the dogma that germline stem cells are not present and follicular renewal does not occur in the postnatal mammalian ovary.
Only 1 million germ cells are present at birth.7 This decreases further to approximately 300,000 by the onset of puberty. Of these follicles, only 400 to 500 (i.e., less than 1% of the total) ovulate in the course of a reproductive lifespan.8
NORMAL OVARIAN AGING
Ovarian aging, ultimately leading to ovarian failure and menopause, is a continuum. An early sign is a poor response to ovarian stimulation, followed by menstrual irregularities and eventually ending in cessation of ovarian follicle function. The time interval between the loss of menstrual regularity and the menopause is approximately 6 years, regardless of age at menopause.9 Consistent with this is the finding that the age of last delivery for Canadian women in the nineteenth century showed the same variation as the age of menopause but occurred 10 years earlier.10
Loss of Fertility and Ovarian Aging
Natural fertility is known to decline with maternal age. Normal women experience their peak fertility in their early 20s, and an accelerated decline is observed after age 35, as recorded in natural populations around the world.11 The risk of clinical miscarriage and fetal aneuploidy both increase with maternal age, with a steep rise in the late 30s.12,13 The cause of age-related deterioration of oocyte quality is generally accepted to be meiotic nondisjunction due to accumulation of damage in DNA and the microtubules of the meiotic spindle.10
Elevation of early follicular phase follicle-stimulating hormone (FSH) is a hallmark of diminishing ovarian follicular reserve, heralding the menopause transition.14 Elevated FSH levels noted in the premenopause are believed to cause a more rapid recruitment of the cohort of preantral follicles.15 Thus, a period of accelerated oocyte depletion begins until near-complete exhaustion of the follicles.16
Poor response to ovarian stimulation most likely represents women in a stage between onset of accelerated decline and total loss of fertility, whereas nonresponse corresponds to a total loss of fertility.17 The basis of the link between a poor response and an early menopause lies in the physiology of follicular development and depletion in the ovary. It has been suggested that the size of the antral follicle cohort is a reflection of the actual resting follicle pool.18–21 “Poor responders” also become menopausal earlier.22,23
Menopause
The clinical sequelae of physiologic ovarian failure is menopause, which is defined as the permanent cessation of menses. The median age of menopause in the United States is 51 years, with menopause occurring before age 40 for 1% of women and beyond age 60 for another 1%.24 Despite a progressive prolongation in the mean age of the population and a trend toward accelerated menarche, the age of menopause has remained relatively unaffected over the last century.25 Evidence suggests that the time of the natural menopause is under strong genetic control, although environmental factors can play a significant role.26
PATHOPHYSIOLOGY OF PREMATURE OVARIAN FAILURE
In most cases, the etiology of premature ovarian failure will not be clear, and the majority of cases occur sporadically. However, there is a genetic component in some woman, and the risk of premature ovarian failure in a woman with a first-degree relative with premature ovarian failure is between 4% and 30%.27,28 Multiple known etiologies of premature ovarian failure are presented here (Table 20-1).
Genetic Causes
Single-Gene Defects
Single-gene defects may give rise to ovarian failure. These include mutations of FSH and LH receptors, inhibin, and galactosemia, among others.29,30
Gonadotropin Receptor Polymorphism
A mutation of the FSH receptor has been described in a subset of patients with premature ovarian failure.31 This is associated with loss in receptor function and appears confined to specific families in the Finnish population. A defect in the LH receptor gene associated with ovarian resistance has also been described.32
Inhibin Polymorphism
Mutations in the inhibin α and β genes have been associated with relatively severe symptoms of premature ovarian failure. Amenorrhea usually develops by the second decade of life in 7% of patients exhibiting this mutation, compared to 0.7% of controls.33
Galactosemia
In females affected with this autosomal recessive disorder, the incidence of premature ovarian failure is at least 80%.34 A relevant murine model suggests that this might be due to a decrease in the germ cell number during fetal oogenesis.35 Mutations of the galactose-1-phosphate uridyltransferase gene can also result in premature ovarian failure because of ovarian accumulation of galactose metabolites at toxic levels.36 Other possible mechanisms contributing to premature ovarian failure in these patients include defective isoforms of FSH, and follicular dysfunction that may be related to interference with nucleotide sugar metabolism and the synthesis of galactose-containing glycoproteins and glycolipids consequent to the enzymatic defect.1,37
Other Genetic Abnormalities
Several autosomal loci have been implicated in premature ovarian failure.38 For the chromosome 3 locus, a forkhead transcription factor gene (FOXL2) has been identified, whereby lesions result in decreased follicles. Deficiencies of 17α-hydroxylase and 17,20-desmolase are associated with primary amenorrhea, sexual infantilism, and hypertension.1 The impaired steroidogenesis results in loss of negative feedback and elevation in the endogenous FSH. This in turn has been implicated in recruiting larger cohorts of follicles, resulting in an accelerated exhaustion of the oocyte complement.14–16
HLA-DR3-linked predisposition to premature ovarian failure with autoimmune polyglandular endocrinopathies has been demonstrated.39 Autoimmune polyglandular syndromes are a series of disorders characterized by autoimmunity against two or more endocrine organs. BPEI syndrome (blepharophimosis, ptosis, and epicanthus inversis), an autosomal dominant disorder mapped to chromosome 3q, is associated with development of premature ovarian failure.40,41 Myotonia dystrophica, secondary to a mutation of a gene located on chromosome 19, may be associated with premature ovarian failure.41 Moreover, the genes FRAXA and POF1B have been implicated in premature ovarian failure.42 Autosomal disorders such as mutations of the phosphomannomutase 2 (PMM2) gene have been identified in patients with premature ovarian failure.43 Perrault’s syndrome, with deafness and familial autosomal recessive premature ovarian failure, has also been described.44
Sex Chromosome Abnormalities
Specific sex chromosome anomalies may be identified in some patients presenting with premature ovarian failure.45 Among them, 45,X and 47,XXY are most prevalent, followed by variable mosaicism.46 The X chromosome contains genes critical to ovarian function.
The critical region spans Xq13-26.1 Proximal deletions (Xq13-21) can be associated with primary amenorrhea while the more distal ones are associated with premature ovarian failure. Genes termed POF1 and POF2 have been localized to Xq21.3-27 and Xq13.3-q21.1, respectively.1 The age at menopause is significantly younger with the latter.
Deletions in these genes are typically not associated with short stature. Within the short arm of the X chromosome, some regions have been associated with risk for premature ovarian failure. The chance of having an abnormal karyotype increases with earlier age of onset of the ovarian failure.45 A chromosomal analysis is recommended for patients younger than age 30 because of increased risk of a gonadal tumor associated with the presence of a Y chromosome.47–49
Swyer syndrome, with 46,XY chromosome compliment and a uterus, may result from a defective Y chromosome and usually presents with primary amenorrhea. The frequency of Y chromosome material detected by polymerase chain reaction is high in Turner’s syndrome (12.2%), but the occurrence of a gonadal tumor among these Y-positive patients is low (7% to 10%).50 It has been estimated that 75% of premature ovarian failure patients presenting with primary amenorrhea have a 45,X or mosaic chromosome patterns.51
Fragile X syndrome premutation carriers, typically with mental retardation and developmental delay, intention tremor, ataxia, or dementia, are at an increased risk for premature ovarian failure, with an incidence of 16% to 21%.52 A higher risk for premature ovarian failure (28%) has been proposed for carriers inheriting the premutation from the father, compared to a 4% risk when inherited from the mother.53 Expansion of a triplet repeat within exon 1 of the FMR1 X-linked gene causes the fragile X syndrome. Expansions of between 50 and 200 repeats are premutations. Evidence suggests that female carriers of premutations in the FMR1 gene are at increased risk of premature ovarian failure. Although it is difficult to be obtain precise information, the risk has been reported to be between 22% and 26%.54
Autoimmune Etiologies
Autoimmune premature ovarian failure can be isolated or part of the polyglandular autoimmune syndromes (see Table 20-1).55,56 These syndromes are associated with ovarian failure in more than 60% of patients. Polyglandular autoimmune syndrome type1 is rare, occurs before adulthood, and is inherited in an autosomal recessive manner; its components include hypoparathyroidism, mucocutaneous candidiasis, hypoadrenalism, and primary hypogonadism. The adrenal autoimmunity is directed against the side chain cleavage and the 17-hydroxylase enzymes. Polyglandular autoimmune syndrome type 2 is more common, usually occurs in adults, has a female preponderance, and has a polygenic inheritance related to HLA-DR3 and HLA-DR4; its components include adrenal insufficiency, autoimmune thyroid disease, type 1 diabetes mellitus, and gonadal failure. The adrenal defect involves antibodies to the enzyme 21-hydoxylase.
Histologic evidence of lymphocytic oophoritis has been demonstrated in 11% of patients with premature ovarian failure; 78% of these patients were positive for steroid cell antibodies, suggesting an immune-mediated insult to the ovaries.57 The lympocytic infiltration seems to spare the primordial follicles. A loss of the regulatory/suppressive CD4+ cells may be the underlying mechanism for premature ovarian failure in patients with thymic aplasia, resulting in an exaggerated autoimmune damage to various organs, including the ovary.58 Circulating immunoglobulins that inhibit the binding of FSH to its receptor have been described.59
In a prospective clinical trial of 119 women with karyotypically normal spontaneous premature ovarian failure, testing for hypothyroidism (27%) and diabetes (3%) was judged to be worthwhile. It was suggested that tests for other possible associated diseases be based on associated clinical presentation.60 Steroid cell autoantibodies seen in Addison’s disease may cross-react with the theca interna/granulosa layers of the ovarian follicles, and their presence is a marker for the association of Addison’s disease and premature ovarian failure. Steroidogenesis enzymes can be targets of autoantibodies. Three percent of women with premature ovarian failure develop adrenal insufficiency (a 300-fold increase compared with the general population). Symptoms could include anorexia, weight loss, vague abdominal pain, weakness, fatigue, salt craving, and skin hyperpigmentation.
The lack of consensus on ovary-specific antibodies as markers for ovarian autoimmunity has clinical and research consequences. Variations in detection of ovarian autoantibodies are likely to be due to study design elements such as antibody test format and antigen preparation, in addition to the multiplicity of intraovarian targets potentially involved in ovarian autoimmunity, including ovarian cellular elements and oocyte-related antigens.61 Many studies only assess one target antigen, leaving individuals with ovarian autoimmunity unidentified.
Infectious Agents
A rare type of infection related to premature ovarian failure is mumps oophoritis. It appears that this aberration in menstrual function and fertility may be related to the time during which the infection occurs as well as to the severity of the infection. In addition, it is apparent that mumps oophoritis may be a more frequent cause of premature ovarian failure than commonly suspected.62,63
Iatrogenic Etiologies
Chemotherapy
Many chemotherapeutic agents used for the treatment of malignancies are toxic to the ovaries and cause ovarian failure. Chemotherapy is associated with exaggerated attrition of ovarian follicles through alteration of DNA, direct destruction of dividing granulosa cells, and damage to the oocytes.1,64–73 These effects may be preventable, as discussed in the Management of Premature Ovarian Failure section in this chapter.
Radiation
The effect of radiation depends on age and the X-ray dose.74,75 Duration of time over which exposure occurs may also be important. Steroid levels begin to fall and gonadotropins rise within 2 weeks after radiation of the ovaries. Young women exposed to radiation are less likely to have immediate and permanent ovarian failure, possibly because of the higher number of oocytes present at younger ages.
The risk of premature ovarian failure can be reduced in women undergoing pelvic irradiation by laparoscopically transposing the ovaries out of the pelvis before radiation.76 The risk does not appear to be reduced by treatment with hormone modulators before irradiation.77
The sensitivity of the cells to the adverse influences is related to the nature of the agent, the dose, and the patient’s age at the time of exposure; the younger the patient, the lesser the likelihood of complete cessation of gonad function as an immediate sequel to therapy.1,78 The duration of exposure to toxic agents may also be relevant. Resumption of menses and pregnancy have been reported after radiotherapy and chemotherapy.79
Environmental Toxins
Smoking, among other environmental factors, has been shown to accelerate the onset of menopause by approximately 2 years.80
Idiopathic Etiologies
Many women with premature ovarian failure may have none of the above etiologies. Population-based studies have suggested an association between various epidemiologic parameters and risk of premature ovarian failure. A low socioeconomic status, a higher education level and nulliparity,81 a higher body mass index and anthropometric measurements have all been proposed risk factors for developing premature ovarian failure in different populations.82
Premature Ovarian Failure and Fertility
It is important to appreciate that in premature ovarian failure, despite the occurrence of amenorrhea and elevation of the FSH levels, residual oocytes may still exist, albeit in significantly diminished numbers.1 Therefore the term premature menopause is not correct. Residual follicles, when present, usually exhibit episodic function, as opposed to the virtually inert oocyte–granulosa units seen in age-appropriate menopause.83 As many as 20% of women with premature ovarian failure will exhibit sporadic ovulatory cycles.84 Indeed, pregnancies have been reported in up to 8% of patients.85
DIAGNOSIS OF PREMATURE OVARIAN FAILURE
Premature ovarian failure should be suspected in any woman younger than age 40 who presents with either amenorrhea or signs of hypoestrogenemia and menstrual irregularity. These women may go through a normal puberty and a variable period of cyclic menses followed by oligomenorrhea and amenorrhea (Table 20-2). Therefore, premature ovarian failure should always be included in the differential diagnosis of anovulation.
Table 20-2 Potential Clinical Symptoms and Signs of Premature Ovarian Failure
| Younger than age 40 |
History and Physical Examination
Deafness can be suggestive of Perrault’s syndrome.44 There also appears to be an association of premature ovarian failure with dry eye syndrome.86 Family history of premature ovarian failure and familial mental retardation should be obtained. The risk of FMR1 mutation in patients with premature ovarian failure and no family history is approximately 6%. In patients with a family history of premature ovarian failure the risk is higher. History of mumps infection should be obtained. Exposure to radiation, chemotherapy, tobacco products, and other environmental toxins may result in accelerated ovarian follicular loss and should be noted. All prior ovarian surgeries should be documented. Fatigue, weight loss, anorexia, nausea, postural dizziness, and salt craving may suggest adrenal insufficiency.
Most patients have normal results on physical examination except for urogenital atrophy (Table 20-3). However, features of Turner’s syndrome, such as short stature, low posterior hairline, high arched palate, shield chest with widely spaced nipples, and short fourth and fifth metacarpals, should be sought. Evidence of possible associated autoimmune diseases such as ptosis and goiter should be pursued. Evaluation for neurosensory deafness and a detailed eye examination should be considered. Hyperpigmentation, postural hypotension, and vitiligo may be signs of associated adrenal disease.
Table 20-3 Possible Physical Findings in Premature Ovarian Failure
| Short stature |
| Low posterior hairline |
| High arched palate |
| Shield chest |
| Widely spaced nipples |
| Short 4th and 5th metacarpal |
| Absence of vaginal rugae |
| Ptosis |
| Goiter |
| Skin hyperpigmentation |
| Postural hypotension |
| Vitiligo |
Laboratory Evaluation
Once premature ovarian failure is diagnosed, the patient should be evaluated for associated autoimmune disorders. Testing for antiovarian antibodies is considered to be unreliable at this time. Far more critical is testing for antiadrenal antibodies. If they are positive further testing of adrenal function is necessary (Table 20-4). The most sensitive test is the corticotropin stimulation test.
Table 20-4 Suggested Initial Screening Tests for Associated Medical Disorders
| Antiadrenal antibodies (adrenal disease) |
In amenorrheic women who desire fertility, FSH, LH, and estradiol should be measured weekly for a month to detect any remaining ovarian follicular activity. The lack of significant rise of estradiol or decrease of FSH suggests irreversible ovarian failure, especially in the absence of any demonstrable antral follicles on ultrasound.82
Alternatively, indirect information about the remaining ovarian reserve of follicles in women with some menstrual function may be obtained by measuring day 3 FSH and estradiol,23,87–89 performing a clomiphene challenge test,90 and obtaining an ultrasonographic antral follicle count.91
Ovarian Biopsies
Ovarian biopsies are not necessary for clinical care. When done as part of a research protocol in women with premature ovarian failure, ovarian biopsies show either no follicles or a small number of follicles, sometimes with lymphocytic perifollicular infiltration consistent with an autoimmune process. In patients with the resistant ovary syndrome, many small follicles are found with little progress in folliculogenesis.82
Imaging
Pelvic ultrasonography for antral follicle count has been used as an indirect indicator of ovarian follicle reserve.91 Antral follicles will appear as round or oval echo-free structures up to 5 to 10 mm in diameter. Pelvic ultrasonography will sometimes reveal remaining follicles in patients 6 years after a diagnosis.90
Bone density should be performed as a baseline and as frequently as needed thereafter. However, it is critical to understand that the results need to be interpreted in light of the patient’s age (see Chapters 24 and 25). Treatment protocols may need to be modified to ensure that osteoporosis does not become a problem.
MANAGEMENT OF PREMATURE OVARIAN FAILURE
Prevention
Probably the most important lifestyle event for primary prevention is to stop smoking. Very little can be done to prevent premature ovarian failure caused by cytogenetic or infectious etiologies. Laparoscopic transposition of ovaries out of the pelvis before radiation reduces the risk of premature ovarian failure.92,76 This risk does not appear reduced by prior treatment with hormone modulators.77
Predictable ovarian failure related to premature ovarian aging, such as with familial premature ovarian failure, may allow several preventive intervention options, most of which remain experimental at this time. For the 10% of women in the general population who are expected to become menopausal by age 45, the critical point of 25,000 resting follicles would probably be reached 13 years earlier. By age 32, these women would begin a rapid decline of fertility and possibly have total loss of fertility by age 36. In the years following the diagnosis at age 32 or younger, these women will have a reproductive potential similar to a 37-year-old woman and could experience increased incidence of dizygotic twinning,93–95 increased incidence of aneuploidy88,96 and miscarriage,89 subfertility,90,97 and a relatively poor response to ovarian stimulation.
Under this scenario of fixed time intervals between premenopausal reproductive milestones, menstrual cycles may continue to be regular for 6 to 8 more years following the diagnosis of reduced ovarian follicular reserve.9,10,16,98–101 The diagnosis may be suspected by finding reduced antral follicle counts,18–21,87 elevated FSH on the third day of menses and after a clomiphene challenge test, and lower inhibin B levels.23,88–90 There seems to be a large overlap between basal FSH levels of older and younger women, however.102 Variants of FSH receptor genotype were associated with different basal FSH levels and variable responses to ovarian stimulation with gonadotropins. A mildly elevated basal FSH level does not necessarily mean early ovarian aging.98,103–105 Serum antimüllerian hormone has been suggested as another marker.106,107 It is hoped that “DNA fingerprints” that identify women with a genetic predisposition to early ovarian aging may become available.10 For women at risk, it is wise to advise them to complete their families as soon as possible. For socioeconomic and career priority reasons, some of these women may elect to postpone childbearing, however.
The details of surgical and nonsurgical options to preserve oocytes from being prematurely lost because of chemotherapy or premature ovarian aging are presented elsewhere in this book (see Chapter 32). The surgical approach involves excising some ovarian tissue followed by variable laboratory manipulation of the tissue. The birth of a healthy child from a frozen-thawed ovarian autograft in a 32-year-old woman with Hodgkin’s disease was reported recently.107 Crucial factors in the rapid depletion of the primordial follicles observed in the transplant can be attributed to the still rudimentary technical steps of tissue harvesting, preparation, and localization of the graft. The risk of reintroducing cancer cells through the grafted ovarian tissue calls for an extremely careful selection of patients for this still experimental treatment.108
The nonsurgical approach involves the use of hormonal manipulation to halt follicle depletion related to age or chemotherapy.72,73,109–112 Nuclear cloning techniques could also generate oocytes from stem cells.113 The recently described oogonia stem cells in postnatal ovaries, if confirmed, could also open new horizons in this arena.114 The nonsurgical approach seems to be preferred by a larger number of young American women.114
Counseling
Patients determined to have premature ovarian failure should receive psychological, endocrinologic, and genetic counseling regarding the implications of their disease (Table 20-5).
Table 20-5 Premature Ovarian Failure Patients Referred for Genetic Counseling
| Women with a family history of premature ovarian failure |
| Women with premature ovarian failure and a chromosomal abnormality |
| Women with premature ovarian failure and family history of mental retardation |
Fertility Prognosis
Pregnancies have been reported in women with premature ovarian failure and high gonadotropin levels.115 It has been suggested that approximately one half of young women who have 46,XX spontaneous premature ovarian failure have ovarian follicles remaining in the ovary.116 These follicles function intermittently and unpredictably, and pregnancies can occur without intervention. At present, there are no proven therapies that will improve follicular function for these women. Premature ovarian failure patients still have a 5% to 10% chance to conceive after diagnosis.117
A randomized trial of hormone replacement in this setting showed that folliculogenesis occurred often but was less frequently followed by ovulation and even less frequently by pregnancy (up to 14%). Controlled trials have failed to demonstrate any success with any treatment in excess of the placebo.118,119 Estrogen therapy did not improve the rate of folliculogenesis, ovulation, or pregnancy.118 The clinician should inform premature ovarian failure patients that there is a small likelihood of spontaneous pregnancy. Women desirous of achieving pregnancy are still best served by donor oocytes120; an increased susceptibility to poor ovarian response exists when utilizing a related donor. It has been suggested that corticosteroid treatment may result in normalization of serum gonadotropins, increasing serum estradiol, ultrasonographic evidence of follicular growth, and conception, especially in women with premature ovarian failure associated with autoimmune disease.121 However, this treatment is considered experimental.
Hormone Replacement
The diagnosis of premature ovarian failure results in multiple profound ramifications, including psychological devastation,122 multisystem effects of estrogen deprivation such as osteoporosis,123,124 cardiovascular morbidity,123,125 depression, and cognitive difficulties.126 Bone mineral density scores of approximately 1 standard deviation below the mean for age-comparable women have been demonstrated in patients with premature ovarian failure, despite taking estrogen replacement,1 with a 2.6-fold increase in risk for hip fractures.
A higher mortality rate in association with diminishing age of menses cessation has been reported.127 Snowdown and colleagues127 found an odds ratio (OR) for mortality of 1.95 (CI, 1.24–3.07) for women developing premature ovarian failure. The excess mortality was associated with coronary artery disease and stroke. This persists despite use of estrogen replacement, with the OR for mortality being 3.33 (CI, 1.14–9.72) for patients ever using estrogen replacement therapy. Women in specific pathophysiological subgroups of premature ovarian failure (i.e., autoimmune, X chromosome-related) may be more susceptible to vascular disease on the basis of the underlying defect that caused the premature ovarian failure. Other women with premature ovarian failure (i.e., chemotherapy- or radiation-induced) may be more prone to long-term diseases and mortality by virtue of their primary disease that necessitated chemoradiation therapy.
CONCLUSION
1 Anasti JN. Premature ovarian failure: An update. Fertil Steril. 1998;70:1-15.
2 Baker T. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Biol. 1963;158:417-433.
3 Ohno S, Klinger H, Atkin N. Human oogenesis. Cytogenetics. 1962;1:42.
4 Peters H. Intrauterine gonadal development. Fertil Steril. 1976;27:493-500.
5 Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: Endocrine model of follicle assembly. Endocrinology. 2003;144:8329-8337.
6 Johnson J, Canning J, Kaneko T, et al. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145-150.
7 Himelstein-Braw R, Byskov A, Peters H, Faber M. Follicular atresia in the infant human ovary. J Reprod Fertil. 1976;46:55-59.
8 Franchi L, Mandl A, Zuckermann S. The development of the ovary and the process of oogenesis. In: Zuckerman S, editor. The Ovary. London: Academic Press; 1962:1-88.
9 den Tonkelaar I, te Velde ER, Looman CW. Menstrual cycle length preceding menopause in relation to age at menopause. Maturitas. 1998;29:115-123.
10 te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod. 2002;8:141-154.
11 O’Connor KA, Holman DJ, Wood JW. Declining fecundity and ovarian ageing in natural fertility populations. Maturitas. 1998;30:127-136.
12 Hecht CA, Hook EB. Rates of Down’s syndrome at livebirth by one-year maternal age intervals in studies with apparent close to complete ascertainment in populations of European origin: A proposed revised rate schedule for use in genetic and prenatal screening. Am J Med Genet. 1996;62:376-385.
13 Andersen N, Wohlfahrt J, Christens P, et al. Maternal age and fetal loss: Population based register linkage study. BMJ. 2000;24:1708-1712.
14 Welt CK, McNichill DJ, Taylor AE. Female reproductive aging is marked by a decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84:105-111.
15 Soules MR, Battaglia DE, Klein NA. Inhibin and reproductive ageing in women. Maturitas. 1998;30:193-204.
16 Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: Evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231-1237.
17 Nikolaou D, Trew G. Contribution of assisted reproduction technology to the understanding of early ovarian ageing. In: Studd J, editor. The Management of the Menopause. 3rd ed. London: Parthenon Publishing; 2003:185-198.
18 Gougeon A. Ovarian follicular growth in humans: Ovarian ageing and population of growing follicles. Maturitas. 1998;30:137-142.
19 Reuss ML, Kline J, Santos R, et al. Age and the ovarian follicle pool assessed with transvaginal ultrasonography. Am J Obstet Gynaecol. 1996;74:224-227.
20 Pellicer A, Ardiles G, Neuspiller F, et al. Evaluation of the ovarian reserve in young low responders with normal basal levels of follicle-stimulating hormone using three-dimensional ultrasonography. Fertil Steril. 1998;70:671-675.
21 Scheffer GJ, Broekmans FJM, Dorland M, et al. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845-851.
22 De Boer EJ, Den Tonkelaar I, te Velde ER, et al. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril. 2002;77:978-985.
23 Lawson R, El-Toukhy T, Kassab A, et al. Poor response to ovulation induction is a stronger predictor of early menopause than elevated basal FSH: A life table analysis. Hum Reprod. 2003;18:527-533.
24 McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103-115.
25 van Noord PAH, Dubas JS, Dorland M, et al. Age at natural menopause in a population based screening cohort: The role of menarche, fecundity and lifestyle factors. Fertil Steril. 1997;68:95-102.
26 De Bruin JP, Bovenhuis H, Van Noord PAH, et al. The role of genetic factors in age at natural menopause. Hum Reprod. 2001;16:2014-2018.
27 Conway GS. Premature ovarian failure. Br Med Bull. 2000;3:643-649.
28 Cramer DW, Huijuan XMPH, Harlow BL. Family history as a predictor of early menopause. Fertil Steril. 1995;64:740-745.
29 Aittomaki K. The genetics of XX gonadal dysgenesis. Am J Hum Genet. 1994;58:844-851.
30 Toledo S, Brunner H, Kraaij R, et al. An inactivating mutation of the luteinizing hormone receptor causes amenorrhea in a 46,XX female. J Clin Endocrinol Metab. 1996;81:3850-3854.
31 Aittomaki K, Lucena JLD, Pakarinen Psistonen P, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotrophic ovarian failure. Cell. 1995;82:959-968.
32 Latronico AC, Anasti J, Arnhold IJ, et al. Brief report: Testicular and ovarian resistance to luteinizing hormone caused by inactivating mutations of the luteinizing hormone-receptor gene. NEJM. 1996;334:507-512.
33 Shelling AN, Burton KA, Chand AL, et al. Inhibin: A candidate gene for premature ovarian failure. Hum Reprod. 2000;15:2644-2649.
34 Waggoner DD, Buist NRM, Donnell GN. Long term prognosis in galactosemia: Results of a survey of 350 cases. J Inherit Metab Dis. 1990;13:802-818.
35 Chen YT, Mattison DR, Feigenbaum I, et al. Reduction in oocyte number following prenatal exposure to a diet high in galactose. Science. 1981;214:1145-1147.
36 Guerrero N, Singh R, Manatunga A, et al. Risk factors for premature ovarian failure in females with galactosemia. J Pediatr. 2000;137:833-841.
37 Xu YK, Ng WG, Kaufman FR, et al. Galactose metabolism in human ovarian tissue. Pediatr Res. 1989;25:251-255.
38 Schlessinger D, Herrera L, Crisponi L, et al. Genes and translocations involved in POF. Am J Med Genet. 2002;111:328-333.
39 Walfish PG, Gottesman IS, Shewchuk AB, et al. Association of premature ovarian failure with HLA antigens. Tissue Antigens. 1983;21:168-169.
40 Fraser IS, Shearman RP, Smith A, Russell P. An association among blepharophimosis, resistant ovary syndrome and true premature menopause. Fertil Steril. 1998;50:747-751.
41 Harper PS, Dyken PR. Early onset dystrophia myotonica: Evidence supporting a maternal environmental factor. Lancet. 1972;2:53-55.
42 Bione S, Toniolo D. X chromosome genes and premature ovarian failure. Semin Reprod Med. 2000;18:11-17.
43 Laml T, Preyer O, Umek W, et al. Genetic disorders in premature ovarian failure. Hum Reprod Update. 2002;8:583-591.
44 Fiumara A, Sorge G, Toscano A, et al. Perrault syndrome: Evidence for progressive nervous system involvement. Am J Med Genet. 2004;128:346-349.
45 Dewald G, Spurbeck J. Sex chromosome anomalies associated with premature gonadal failure. Semin Reprod Endocrinol. 1983;1:79.
46 Devi A, Metzger D, Luciano A, Benn PA. 45,X/46,XX mosaicism in patients with idiopathic premature ovarian failure. Fertil Steril. 1998;70:89-93.
47 Giltay J, Ausems M, van Seumeren I, et al. Short stature as the only presenting feature in a patient with isodicentric (Y)(q11.23) and gonadoblastoma: A clinical and molecular cytogenic study. Eur J Pediatr. 2001;160:154-158.
48 Manuel M, Katayama K, Jones JH. The age of occurrence of gonadal tumors in intersex patients with a Y chromosome. Am J Obstet Gynecol. 1976;124:293-300.
49 Troche V, Hernandez E. Neoplasia arising in dysgenetic gonads. Obstet Gynecol Surv. 1986;41:74-79.
50 Gravhold C, Fedder J, Naeraa R, et al. Occurrence of gonadoblastoma in females with Turner syndrome and Y chromosome material: A population study. J Clin Endocrinol Metab. 2000;85:3199-3202.
51 Speroff L, Fritz M. Amenorrhea. In: Speroff L, Fritz M, editors. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2005:401-464.
52 Sherman SL. Premature ovarian failure in fragile X syndrome. Am J Med Genet. 2000;97:189-194.
53 Hundscheid RDL, Sisterman H, Kiemeney LALM, et al. Imprinting effect in premature ovarian failure confined to paternally inherited fragile X permutation. Am J Hum Genet. 2000;66:413-418.
54 Murray A. Premature ovarian failure and the FMR1 gene. Semin Reprod Med. 2000;18:19-66.
55 Myhre A, Halonen M, Eskelin P, et al. Autoimmune polyendocrine syndrome type 1 (APS I) in Norway. Clin Endocrinol (Oxf). 2001;54:211-217.
56 Nelson L. Autoimmune ovarian failure: Comparing the mouse model and the human disease. J Soc Gynecol Invest. 2001;8(Suppl):S55-S57.
57 Hoeck A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997;18:107-134.
58 Nishizuka Y, Sakakura T. Thymus and reproduction: Sex-linked dysgenesis of the gonad after neonatal thymectomy in mice. Science. 1969;166:753-755.
59 Chiauzzi VA, Bussmann L, Calvo JC, et al. Circulating immunoglobulins that inhibit the binding of follicle-stimulating hormone to its receptor: A putative diagnostic role in resistant ovary syndrome? Clin Endocrinol (Oxf). 2004;61:16-54.
60 Kim TJ, Anasti JN, Flack MR, et al. Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol. 1997;89:777-779.
61 Luborsky J. Ovarian autoimmune disease and ovarian autoantibodies. J Womens Health Gend Based Med. 2002;11:785-799.
62 Morrison JC, Givens JR, Wiser WL, Fish SA. Mumps oophoritis: A cause of premature menopause. Fertil Steril. 1975;26:755-759.
63 Taparelli F, Squadrini F, De Rienzo B, et al. Isolation of mumps virus from vaginal secretions in association with oophoritis. J Infect. 1988;17:355-358.
64 Ataya KM, Pydyn E, Sacco A. Effect of “activated” cyclophosphamide on mouse oocyte in vitro fertilization and cleavage. Reprod Toxicol. 1988;2:105-109.
65 Ramahi-Ataya A, Ataya KM, Subramanian M, Struck R. The effect of “activated” cyclophosphamide on rat granulosa cells in vitro. Reprod Toxicol. 1988;2:99-103.
66 Ataya KM, Valeriote FA, Ramahi-Ataya A. Effect of cyclophosphamide on the immature rat ovary. Cancer Res. 1989;49:1660-1664.
67 Ataya KM, Pydyn E, Ramahi-Ataya A. Effect of “activated” cyclophosphamide on human and rat granulosa cell function in vitro. Reprod Toxicol. 1990;4:121-125.
68 Ataya KM, Pydyn E, Young J, Struck R. The uptake and metabolism of cyclophosphamide by the ovary. Selective Cancer Therapeutics. 1990;6:83-92.
69 Teaff N, Moore R, Subramanian M, Ataya KM. Effect of vinblastine on granulosa cells in vitro. Reprod Toxicol. 1990;4:209-214.
70 Pydyn E, Ataya KM. Effect of cyclophosphamide on mouse oocyte in vitro fertilization and cleavage: Recovery. Reprod Toxicol. 1991;5:73-78.
71 Ataya KM, Weintraub A, McKanna J, et al. A luteinizing hormone releasing agonist for the prevention of chemotherapy induced ovarian follicular loss in rats. Cancer Res. 1985;45:3651-3656.
72 Ataya KM, Ramahi-Ataya A. Reproductive performance of female rats treated with cyclophosphamide and/or LHRH agonist. Reprod Toxicol. 1993;7:229-235.
73 Ataya KM, Rao LV, Lawrence E, Kimmel R. LHRH agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995;52:365-372.
74 Gradishar W, Schilsky R. Ovarian function following radiation and chemotherapy. Semin Oncol. 1989;16:425-436.
75 Wallace W, Shalet SM, Crowne E, et al. Ovarian failure following abdominal irradiation in childhood natural history and prognosis. Clin Oncol. 1989;1:75-79.
76 Morice P, Thiam-Ba R, Castaigne D, et al. Fertility results after ovarian transposition for pelvic malignancies treated by external irradiation or brachytherapy. Hum Reprod. 1998;13:660-663.
77 Ataya KM, Pydyn E, Ramahi-Ataya A, Orton CG. Is radiation-induced ovarian failure in rhesus monkeys preventable by LHRH agonists? Preliminary observations. J Clin Endocr Metab. 1995;80:790-795.
78 Ataya KM, Moghissi M. Chemotherapy-induced premature ovarian failure: Mechanisms and prevention. Steroids. 1989;54:607-626.
79 Byrne J, Mulvihill J, Myers M, et al. Effects of treatment on fertility in long-term survivors of childhood cancer. N Eng J Med. 1987;317:1315-1321.
80 Brambilla DJ, McKinley SM. A prospective study of factors affecting age at menopause. J Clin Epidemiol. 1989;42:1031-1039.
81 Testa G, Chiaffarino F, Vegetti W, et al. Case control study on risk factors for premature ovarian failure. Obstet Gynecol Invest. 2001;5:40-43.
82 Harlow BE, Cramer DW, Annis KM. Association of medically treated depression and age at menopause. Am J Epidemiol. 1995;141:1170-1176.
83 Costoff A, Mahesh VB. Primordial follicles with normal oocytes in the ovaries of postmenopausal women. J Am Geriatr Soc. 1975;23:193-196.
84 Nelson LM, Anasti JN, Kimzey LM, et al. Development of leutinized graffian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab. 1994;79:1470-1475.
85 Rebar RW, Connolly HV. Clinical features of young women with hypergonadotrophic amenorrhoea. Fertil Steril. 1990;53:804-810.
86 Smith JA, Vitale S, Reed GF, et al. Dry eye signs and symptoms in women with premature ovarian failure. Arch Ophthalmol. 2004;122:251-256.
87 van Montfrans JM, van Hooff MH, Martens F, Lambalk CB. Basal FSH estradiol and inhibin B concentrations in women with a previous Down’s syndrome affected pregnancy. Hum Reprod. 2002;17:44-47.
88 Trout S, Seifer D. Do women with unexplained recurrent pregnancy loss have higher day 3 serum FSH and estradiol values? Fertil Steril. 2000;74:335-337.
89 Scott RT, Leonardi MR, Hofman GE, et al. A prospective evaluation of clomiphene citrate challenge test screening of the general infertility population. Obstet Gynaecol. 1993;82:539-544.
90 Kalantarundou SN, Nelson LM. Premature ovarian failure is not premature menopause. Ann NY Acad Sci. 2000;900:393-402.
91 Bancsi LJM, Broekmans FJM, Eijkemans MJC, et al. Predictors of poor ovarian response in in-vitro fertilization: A prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328-336.
92 Madsen B, Giudice L, Donaldson S. Radiation-induced premature menopause: A misconception. Int J Radiat Oncol Biol Phys. 1995;32:1461-1464.
93 Turner G, Robinson H, Wake S, Martin N. Dizygous twinning and premature menopause in fragile X syndrome. Lancet. 1994;344:1500.
94 Martin NG, Heath AC, Turner G. Do mothers of dizygotic twins have earlier menopause? Am J Med Genet. 1997;69:114-116.
95 van Montfrans JM, Dorland M, Oosterhuis G, et al. Increased concentrations of follicle-stimulating hormone in mothers with Down’s syndrome. Lancet. 1999;353:1853-1854.
96 Leach RE, Moghissi KS, Randolph JF, et al. Intensive hormone monitoring in women with unexplained infertility: Evidence for subtle abnormalities suggestive of diminished ovarian reserve. Fertil Steril. 1997;68:413.
97 te Velde ER, Scheffer GJ, Dorland M, et al. Developmental and endocrine aspects of normal ovarian ageing. Mol Cell Endocrinol. 1998;145:67-73.
98 te Velde ER, Dorland M, Broekmans FJ. Age at menopause as a marker of reproductive ageing. Maturitas. 1998;30:119-125.
99 van Zonneveld P, Scheffer GJ, Broekmans FJM, te Velde ER. Hormones and reproductive ageing. Maturitas. 2001;38:83-94.
100 van Zonneveld P, Scheffer GJ, Broekmans FJ, et al. Do cycle disturbances explain the age-related decline of female fertility? Cycle characteristics of women aged over 40 years compared with a reference population of young women. Hum Reprod. 2003;18:495-501.
101 Schipper I, de Jong FH, Fauser BCJM. Lack of correlation between maximum early follicular phase serum follicle-stimulating hormone levels and menstrual cycle characteristics in women under the age of 35 years. Hum Reprod. 1998;13:1442-1448.
102 te Velde ER, Scheffer GJ, Dorland M, et al. Age dependent changes in serum FSH levels. Fauser BCJM, editor. Studies in Profertility: FSH Action and Intraovarian Regulation. vol. 6. New York: Parthenon; 1997:145-155.
103 Lambalk CB. Value of elevated basal follicle-stimulating hormone levels and the differential diagnosis during the diagnostic subfertility work-up. Fertil Steril. 2003;79:489-490.
104 Baird DT. A model for follicular selection and ovulation: Lessons from superovulation. J Steroid Biochem. 1987;27:15-23.
105 de Vet A, Laven JSE, de Jong FH, et al. Antimüllerian hormone serum levels: A putative marker for ovarian ageing. Fertil Steril. 2002;77:357-362.
106 Fanchin R, Schonauer LM, Righini C, et al. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323-327.
107 Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
108 Baird DT, Webb R, Campbell BK, et al. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196°C. Endocrinology. 1999;140:462-471.
109 Ataya K, Gitiforooz H: Progesterone and LHRH agonists delay the rate of ovarian follicle loss in rhesus monkeys. Presented to the American Society of Human Genetics, 50th Annual Meeting in Philadelphia, October 2000.
110 Ataya KM: Postponing menopause in rhesus monkeys using LHRH agonist + progesterone by altering the dynamics of follicle growth and atresia. Presented at the Society of Gynecological Investigation, Conference in Toronto, March 2001.
111 Ataya KM: LHRH agonist does not retard physiologic ovarian follicle loss in rhesus monkeys. Presented at the Society of Gynecological Investigation, Conference in Toronto, March 2001.
112 Blumenfeld Z. Ovarian cryopreservation versus ovarian suppression by GnRH analogues: Primum non nocere. Hum Reprod. 2004;19:1924-1925.
113 Hubner K, Fuhrmann G, Christenson LK, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251-1256.
114 Ataya K, McMullen S: Attitude of American young women towards delaying reproductive aging. Presented to the North American Menopause Society, 11th Annual Meeting in Orlando, Florida, September 2000.
115 Ataya KM, Mudawwar F, Allam C, Karam K. Hyper-gonadotropism with pregnancy. Am J Obstet Gynecol. 1983;146:341-343.
116 Nelson LM, Bakalov VK. Mechanisms of follicular dysfunction in 46,XX spontaneous premature ovarian failure. Endocrinol Metab Clin North Am. 2003;32:313-337.
117 van Kasteren YM, Schoemaker J. Premature ovarian failure: A systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:583-592.
118 Taylor A, Adams J, Mulder J, et al. A randomized, controlled trial of estradiol replacement therapy in women with hypergonadotropic amenorrhea. J Clin Endocrinol Metab. 1996;81:3615-3621.
119 Lieman H, Santoro N. Premature ovarian failure: A modern approach to diagnosis and treatment. Endocrinologist. 1997;7:314-321.
120 Sauer MV, Paulson RJ, Macaso TM, et al. Oocyte and pre-embryo donation to women with ovarian failure: An extended clinical trial. Fertil Steril. 1991;55:39-43.
121 Orshan SA, Furniss KK, Forst C, Santoro N. The lived experience of premature ovarian failure. JOGNN. 2001;30:202-208.
122 Conway GS. Premature ovarian failure. Curr Opin Obstet Gynecol. 1997;9:202-206.
123 Pouilles JM, Tremolliers F, Bonneu M, Ribot C. Influence of early age at menopause on vertebral bone mass. J Bone Min Res. 1994;9:311-315.
124 Joakimsen O, Bonaa KH, Stensland BE, Jacobsen BK. “Population-based study of age at menopause and ultrasound assessed carotid atherosclerosis: The Tromso study. J Clin Epidemiol. 2000;53:525-530.
125 Shepard JE. Effects of estrogen on cognition, mood and degenerative brain diseases. J Am Pharmaceut Assoc. 2001;41:221-228.
126 Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998;8:229-235.
127 Snowdown DA, Kane RL, Beeson WL, Burke GL, et al. Is early natural menopause a biological marker of health and aging? Am J Public Health. 1989;79:709-714.