CHAPTER 11 PREHOSPITAL FLUID RESUSCITATION: WHAT TYPE, HOW MUCH, AND CONTROVERSIES
Fluid resuscitation is a vital treatment in the care of hypotensive trauma patients. Restoration of effective circulating blood volume improves oxygen delivery, thereby diminishing the untoward effects of shock at the cellular and organ level. However, fluid resuscitation, in and of itself, is not a panacea. Whereas restoration of effective circulating blood volume is essential, the method of supplying fluid is more controversial and complicated by several confounding factors. The inability to deliver definitive care in the field, the heterogeneity of patient populations, the variability in mechanism of injury, and the level of in-field hemorrhage control make precise study of the topic challenging. Therefore, the debate persists concerning the type, the amount, and the timing of fluid administration. The purpose of this chapter is to provide insight in the use of fluid resuscitation of trauma patients in the prehospital setting.
EPIDEMIOLOGY
If trauma is the leading cause of civilian death in Americans aged less than 45 years and the fourth leading cause of death in the United States for all ages,1 hemorrhagic shock is the primary physiologic defect leading to death. Volume deficits develop not only as a result of blood loss, but also due to diffuse capillary-endothelial leak and fluid shifts from the intravascular to the interstitial space.2 These deficits, and the attendant hypoperfusion, potentially lead to multiple organ dysfunction, failure, and death.2
Aggressive fluid administration has been mainstay therapy in trauma patients for over 40 years. Estimates of the numbers of trauma patients in the United Kingdom given prehospital intravenous fluid range from 8.6 to 65 patients per 100,000 population per year.3 However, for the last 15 years this practice, especially in the setting of uncontrolled hemorrhage, has been questioned.
CLASSES OF HEMORRHAGIC SHOCK
Hemorrhage is the most common cause of shock in the injured patient.4 Shock is defined as the presence of inadequate organ perfusion and tissue oxygenation.4 In the presence of inadequate oxygen for normal aerobic metabolism, anaerobic metabolism occurs leading to lactic acidosis. If this process continues, cellular membranes lose their integrity leading to cellular swelling, progressive cellular damage, and ultimately, cellular death.4
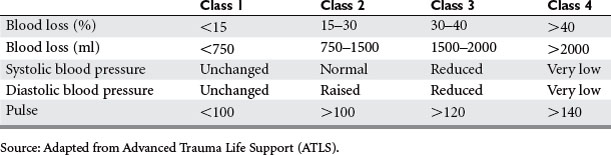
Hemorrhage, an acute loss of circulating blood volume, is classified based on the percentage of blood volume loss. Specific hemodynamic, respiratory, central nervous system, urinary, and integumentary changes occur given the degree of shock (Table 1). Whereas class I hemorrhage is associated with minimal clinical symptoms and requires little, if any, volume replacement, class IV hemorrhage is immediately life-threatening, necessitates blood transfusion, and usually calls for surgical intervention to halt ongoing bleeding.4
MANAGEMENT
Access
The basic management principles to follow in hemorrhagic shock are to stop the bleeding and replace the volume loss. Establishing a patent airway with adequate ventilatory exchange and oxygenation is the first priority. Supplemental oxygen is supplied while external bleeding is controlled. Two large-caliber (minimum of 16-gauge) peripheral intravenous catheters are inserted, preferably in the antecubital veins.4 Intravenous access should not delay transport of the patient to the trauma center.
Types of Fluid
Crystalloid
A crystalloid is a solution of small nonionic or ionic particles. They are freely permeable to the vascular membrane and are distributed mainly in the interstitial space. As such, only one-third of the volume of crystalloid infused expands the intravascular space. This accounts for the need to provide at least three times more volume of crystalloid than the volume of blood lost. Because of decreased colloid osmotic pressure secondary to decreased serum protein concentration from hemorrhage, capillary leaks, and crystalloid replacement, this ratio of volume of crystalloid infused to blood volume lost may even approach 7–10:1.5
Depletion of both the interstitial fluid volume and the intravascular space following severe injury may be a reason to use crystalloids for fluid resuscitation, which restore volume to both spaces.6 Animal and human studies demonstrating improved survival from shock when utilizing isotonic fluid and blood versus blood transfusion alone support this view. Other advantages of crystalloid use in prehospital fluid resuscitation include its negligible cost in comparison to other resuscitative fluids, immediate availability, and long-term storage capacity.
Given the predilection of crystalloid to primarily fill the interstitial space, tissue edema is common and may have deleterious effects. In head-injured patients, increased brain edema may adversely affect outcome. Gas exchange may be impaired secondary to pulmonary edema. Endothelial and red cell edema impair microcirculation and tissue oxygen exchange, potentially contributing to multiple organ dysfunction.5
According to Advanced Trauma Life Support (ATLS) guidelines, fluid resuscitation of the trauma patient begins with a 2-L bolus of crystalloid, usually lactated Ringer’s (LR) solution. LR is an isotonic fluid that contains L-lactate and D-lactate in a 50:50 mixture. The L-lactate is metabolized in the liver to bicarbonate, thereby providing additional buffer. Although the D-lactate isomer is thought to be a cause of acidosis, studies have shown that resuscitation with LR does not lead to increased lactic acid levels.7 However, normal saline (NS), another isotonic crystalloid, can induce a hyperchloremic acidosis when given in large volumes because of its concentration of chloride ions (154 mEq/l).6 Healey et al.7 suggest, in their animal model of massive hemorrhage, increased survival rate in animals resuscitated with LR and blood relative to those animals that received NS and blood. This difference was thought to be secondary to the profound acidosis occurring in the NS/blood group.
Because LR has a lower osmolality than plasma (273 mOsm/l vs. 285–295 mOsm/l), large volumes of LR can reduce serum osmolality and contribute to cerebral edema. For this reason, NS may be the preferred resuscitative fluid in head-injured patients.6
Hypertonic sodium chloride (HS) in concentrations ranging from 3% to 7.5% has been used for the treatment of hypovolemic shock.2 Because of its elevated osmolality (2400 mOsm/l in 7.5%), HS produces an increase in intravascular volume that far exceeds the infused volume6 (Table 2). The cardiovascular effects of HS include improved myocardial contractility, decreased systemic and pulmonary vascular resistance, mobilization of tissue edema into the blood compartment, and reduction in venous capacitance.2,6 These effects are transient, however, so HS has been mixed with colloids (dextran or hydroxyethyl starch) to prolong its efficacy, especially when used for small volume resuscitation.
Table 2 Effect on Plasma Volume Expansion of Various Solutions
| Volume Infused (ml) | Fluid Infused | Plasma Volume Expansion (ml) |
|---|---|---|
| 1000 | D5W | 100 |
| 1000 | Lactated Ringer’s | 250 |
| 250 | 7.5% hypertonic saline | 1000 |
| 500 | 5% albumin | 375 |
| 100 | 25% albumin | 450 |
| 500 | 6% hetastarch | 750 |
Modified from Rizoli SB: Crystalloids and colloids in trauma resuscitation: a brief overview of the current debate. J Trauma 54:S82–S88, 2003.
HS decreases intracranial pressure (ICP), primarily in areas of the brain with an intact blood-brain barrier.8 Cooper et al.,9 in a double-blind, randomized controlled trial of hypotensive patients with severe traumatic brain injury, studied the effects of prehospital resuscitation with hypertonic saline versus Ringer’s lactate on neurological outcome. These investigators did not find a significant difference in 3- or 6-month extended Glasgow Outcome Scores between the two groups.
Immunomodulatory effects of HS, either immunostimulatory or immunosuppressive depending on the concentration, have been described. HS affects nuclear activation, protein synthesis and proliferation, polymorphonuclear leukocyte function, and cytoskeleton polymerization. In animal models of hemorrhage, these effects have been associated with reduced organ dysfunction and improved survival.8
Despite its benefits, a meta-analysis evaluating the effect of HS compared to isotonic crystalloid on 30-day outcome in trauma patients failed to show a survival advantage.1 As such, the role of HS in prehospital fluid resuscitation has yet to be defined.
Colloid
Nonbiologically active
Colloids seemingly have many advantages as resuscitative fluids over crystalloids. Their ability to effectively expand plasma volume exceeds that of crystalloids. Endpoints of resuscitation are met using smaller volumes of colloid, which in turn reduce tissue edema.5 However, some investigators suggest that colloids potentiate tissue edema. The capillary-endothelial cell leak that develops after severe injury may allow the colloid to pass into the interstitium and exacerbate swelling.6
Albumin, a natural colloid, is synthesized in the liver and is responsible for 80% of the oncotic pressure of the plasma.5 The molecular weight of albumin is approximately 69 kD. Infusion of the 25% solution expands plasma volume four to five times the volume infused5 (see Table 2). Derived from pooled human plasma, its risk of transmitting infectious diseases is low because of stringent heating and sterilization. Aside from its volume replacing properties, albumin also possesses a transport function for drugs and endogenous substances and may have a beneficial effect on membrane permeability secondary to free radical scavenging. These theoretical effects have not been proven clinically.2
Disadvantages of albumin include its cost, short supply, and potential disease transmission.5 Additionally, albumin’s use for the resuscitation of critically ill patients has demonstrated either a trend toward or a significant increase in mortality.5 Therefore, the use of albumin can not be recommended as a resuscitative fluid for hypotensive trauma patients.
Synthetic colloids include dextran, hydroxyethyl starch (HES), and mixtures of dextran and HES with hypertonic saline solutions. Dextran is a glucose polymer available as 6% dextran 70 (70 kD) and 10% dextran 40 (40 kD) solutions. Increase of plasma volume after infusion of 1000 ml of dextran 70 ranges from 600 to 800 ml.2 Dextran reduces blood viscosity, reduces platelet adhesiveness, and enhances fibrinolysis,6 resulting in increased bleeding tendency.2 Severe, life-threatening anaphylactic reactions are also well described.2,6 The use of dextran as an exclusive fluid resuscitant is limited by these side effects.
As mentioned previously, dextran has been added to hypertonic saline (HSD) to extend its intravascular presence. Its use as a resuscitative fluid was compared with isotonic crystalloid and analyzed via a meta-analysis of several randomized controlled trials of hypotensive trauma patients. Although HSD was safe, demonstrated higher increases in blood pressure, and decreased early fluid and blood requirements, no statistically significant survival benefit was attributed to its use.1 Conversely, in a study by Wade et al.,10 survival to discharge was significantly improved in patients resuscitated with 250 ml of HSD that sustained penetrating torso trauma requiring surgical intervention. This suggests a subset of trauma patients may benefit from HSD in the prehospital setting.
HES solutions are modified natural polymers of amylopectin.6 The pharmacokinetic properties of each formulation are determined by its molecular weight, the pattern of hydroxyethylation, and the ratio of C2:C6 hydroxyethylation.2,5,6 These properties influence the plasma expansion, degradation, and side effect profile of HES.
Side effects associated with HES include pruritis and increased bleeding due to reduction of factor VIII and von Willebrand factor.6 However, most recent studies using modern HES preparations demonstrated no impairment of hemostasis or increased bleeding propensity.2
Still, despite the apparent advantages of colloids, meta-analyses suggest a trend toward increased mortality when they are used for the resuscitation of trauma patients.5 Although the methodology of these studies can be questioned, until better designed clinical trials provide irrefutable evidence suggesting improved outcome with the use of colloids for fluid resuscitation, these agents cannot be recommended.
Biologically active
When considering an ideal resuscitative fluid in hemorrhagic shock, its properties would include volume expansion, oxygen-carrying capacity, universal compatibility, immediate availability, long-term storage capacity, and the absence of vasoactive properties and disease transmission.11 Although blood transfusion effectively improves volume deficits and provides oxygen delivery, its use in the prehospital setting is limited by expense, short shelf-life, short supply, risk of disease transmission, and need for cross-matching. Allogenic red blood cells (RBCs) may have adverse immunoinflammatory effects that increase the risk of postinjury multiple organ failure (MOF).12
Hemoglobin-based oxygen carriers (HBOC) are attractive in the prehospital setting, then, for several reasons. Because HBOC can be heat treated, their risk of disease transmission is low. They have a shelf-life of up to 3 years and have oxygen-carrying, as well as volume-expansion properties. They are universally compatible, thus eliminating the need for cross-matching.13 Phase II clinical trials, as well as in vitro and in vivo work, suggest that resuscitation with a HBOC—in lieu of stored RBCs—attenuates the systemic inflammatory response invoked in the pathogenesis of MOF.12
Clinical trials with HBOC have shown mixed results. When diaspirin cross-linked hemoglobin (DCLHb) was studied against normal saline in a U.S. multicenter trial for the treatment of severe traumatic hemorrhagic shock, the 28-day mortality was 46% for DCLHb compared to 17% for NS. An increase in systemic and pulmonary vascular resistance leading to decreased cardiac output was felt to be responsible for the higher mortality rate.12 However, polymerized hemoglobin solutions have shown more promise. In a prospective, randomized trial comparing the therapeutic benefits of Poly-Heme with that of allogenic RBCs in the treatment of acute blood loss, the Poly-Heme group demonstrated similar total hemoglobin concentration after infusion as the RBC group with less RBC transfusion required through the first day of treatment and without serious or unexpected adverse consequences resulting from Poly-Heme.11 In time, it is possible that one or more HBOC may be routinely used in the resuscitation of hemorrhagic shock.
Resuscitation Targets
Delayed
Studies have begun to scrutinize the potential detrimental effects of raising the blood pressure during uncontrolled hemorrhage.14–18 Whereas early work utilizing controlled hemorrhage models was used to support the use of fluid resuscitation of post-traumatic hemorrhage, these models of resuscitation do not mimic the actual life situation of uncontrolled bleeding and concurrent treatment.16 In the setting of uncontrolled hemorrhage, fluid administration may disrupt thrombus formation, induce coagulopathy by diluting clotting factors, and lead to increased bleeding.14 In 1918, Cannon observed increased bleeding induced by rapid fluid infusion prior to hemorrhage control.19 More recently, in a study of penetrating torso trauma, hypotensive patients were randomized to immediate versus delayed fluid resuscitation with isotonic crystalloid.14 Prehospital fluid resuscitation was started in the immediate group, but held in the delayed group until control of hemorrhage in the operating room. Compared to patients in the delayed group, patients in the immediate resuscitation group had higher mortality and higher rates of postoperative complications. Although the results of this study have been argued,16 the study rekindled interest and stimulated thought concerning approaches of management for the treatment of uncontrolled hemorrhage.
Hypotensive
This strategy of resuscitation attempts to maintain adequate vital organ perfusion while minimizing further bleeding.20 A mean arterial pressure (MAP) of 60 mm Hg has been used as a resuscitation target. It is regarded as the lowest safe level because it is the lowest MAP of active autoregulation of cerebral blood flow.19 No lower limit of hypotensive resuscitation, however, has been firmly established.
Using blood pressure as a guideline simulates prehospital scenarios in which this variable is one of the only hemodynamic parameters available.19 Dutton et al.18 randomized hypotensive blunt and penetrating trauma patients to a systolic blood pressure (SBP) of 70 mm Hg (hypotensive) or more than 100 mm Hg (normotensive). Crystalloid or blood products were administered to maintain the intended SBP for each group. There was no difference in survival between the two cohorts. This was partly attributed to the difficulty in maintaining the targeted blood pressures. The average SBP for the hypotensive and normotensive groups were 100 mm Hg and 114 mm Hg, respectively. This response suggests spontaneous reduction of bleeding due to inherent hemostatic mechanisms and may validate use of this resuscitation strategy in certain scenarios of uncontrolled hemorrhage.18
Normotensive
The traditional approach to the resuscitation of trauma patients in hemorrhagic shock has been to normalize blood pressure by administering large volumes of crystalloid followed by transfusion of blood products. This method of resuscitation developed from animal models of controlled hemorrhage. Restoration of vital organ perfusion improved survival, whereas untreated animals developed organ dysfunction and succumbed. In situations of uncontrolled hemorrhage, animal studies revealed decreased splanchnic perfusion and greater blood loss.21 In situations in which bleeding has spontaneously resolved, the standard approach to resuscitation is reasonable. It is difficult to predict, however, whether bleeding has spontaneously ceased or may be exacerbated by aggressive resuscitation.
MORBIDITY AND COMPLICATIONS
Hypothermia develops commonly after traumatic shock and is exacerbated with the administration of cold fluids. Adverse consequences of hypothermia include impaired coagulation function, reduction of oxygen delivery, and increased rate of infection.22 The importance of administering warm fluids to avoid the untoward effects of hypothermia cannot be overstated.
1 Wade CE, Kramer GC, Grady JJ, et al. Efficacy of hypertonic 7.5% saline and 6% dextran-70 in treating trauma: a meta-analysis of controlled clinical studies. Surgery. 1997;122:609-616.
2 Boldt J. Fluid choice for resuscitation of the trauma patient: a review of the physiological, pharmacological, and clinical evidence. Can J Anesth. 2004;51:500-513.
3 Dretzke J, Sandercock J, Bayliss S, Burls A. Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients. Health Technol Assess. 2004;8(23):1-103.
4 American College of Surgeons Committee on Trauma. Advanced Trauma Life Support Manual. Chicago: American College of Surgeons, 2001.
5 Rizoli SB. Crystalloids and colloids in trauma resuscitation: a brief over-view of the current debate. J Trauma. 2003;54:S82-S88.
6 Nolan J. Fluid resuscitation for the trauma patient. Resuscitation. 2001;48:57-69.
7 Healey MA, Davis RE, Liu FC, et al. Lactated Ringer’s is superior to normal saline in a model of massive hemorrhage and resuscitation. J Trauma. 1998;45:894-899.
8 Hoyt DB. Fluid resuscitation: the target from an analysis of trauma systems and patient survival. J Trauma. 2003;54:S31-S35.
9 Cooper DJ, Myles PS, McDermott FT, et al. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury. JAMA. 2004;291:1350-1357.
10 Wade CE, Grady JJ, Kramer GC. Efficacy of hypertonic saline dextran fluid resuscitation for patients with hypotension from penetrating trauma. J Trauma. 2003;54:S144-S148.
11 Gould SA, Moore EE, Hoyt DB, et al. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg. 1998;187:113-122.
12 Moore EE, Johnson JL, Cheng AM, et al. Insights from studies of blood substitutes in trauma. Shock. 2005;24:197-205.
13 Philbin N, Rice J, Gurney J, et al. A hemoglobin-based oxygen carrier, bovine polymerized hemoglobin (HBOC–201) versus hetastarch (HEX) in a moderate severity hemorrhagic shock swine model with delayed evacuation. Resuscitation. 2005;66:367-378.
14 Bickell WH, Wall MJ, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105-1109.
15 Martin RR, Bickell WH, Pepe PE, et al. Prospective evaluation of preoperative fluid resuscitation in hypotensive patients with penetrating truncal injury: a preliminary report. J Trauma. 1992;33:354-362.
16 Fowler R, Pepe P. Prehospital care of the patient with major trauma. Emerg Med Clin North Am. 2003;20:953-974.
17 Pepe P, Mosesso VN, Falk JL. Prehospital fluid resuscitation of the patient with major trauma. Prehosp Emerg Care. 2002;6:81-91.
18 Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52:1141-1146.
19 Friedman Z, Berkenstadt H, Preisman S, et al. A comparison of lactated Ringer’s solution to hydroxyethyl starch 6% in a model of severe hemorrhagic shock and continuous bleeding dogs. Anesth Analg. 2003;96:39-45.
20 Revell M, Greaves I, Porter K. Endpoints for fluid resuscitation in hemorrhagic shock. J Trauma. 2003;54:S63-S67.
21 Varela JE, Cohn SM, Diaz I, et al. Splanchnic perfusion during delayed, hypotensive, or aggressive fluid resuscitation from uncontrolled hemorrhage. Shock. 2003;20:476-480.
22 Gentiello LM. Advances in the management of hypothermia. Surg Clin North Am. 1995;75:243-256.