Chapter 4 Practice standards and guidelines
INTRODUCTION
As discussed in Chapter 3 pharmacy practice standards and guidelines have been developed to express certain values, goals, and aspirations about the role of pharmacists. As these documents reference contemporary practice, they provide the framework to maintain and improve the quality of pharmaceutical services, forming an important facet in the regulation of the profession. The major pharmacy organisations in Australia, the Pharmaceutical Society of Australia (PSA), the Pharmacy Guild of Australia (PGA) and the Society of Hospital Pharmacists of Australia (SHPA), have assumed critical roles in the development of these practice standards and guidelines.
Where there is litigation, either of a disciplinary or civil nature, the first line of defence for the pharmacist would be to demonstrate that their operating procedures were current and in accordance with contemporary standards and guidelines. If the matter related to community pharmacy practice, the standards and guidelines applicable would be those included in accreditation programs, for example the PGA’s Quality Care Pharmacy Program (QCPP).
Standards and guidelines are intended to be used as tools to achieve consistency and uniformity in service delivery and to implement continuous quality improvement to reduce the risk of misadventure.[1] Implementing the standards and guidelines enhances the development of systems to assist in the prevention of errors and improves the quality of the service provided. As the pharmacist’s role has changed significantly and pharmacists now accept a greater role in patients’ medication management, it is particularly important that pharmacists follow current standards, both to benefit the patient and to protect them against potential litigation.
PRACTICE STANDARDS
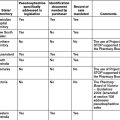
Each standard comprises the following elements:
In addition to the pharmacy-specific standards and guidelines, various other health service’s standards and guidelines also exist that apply to health service providers. At a national level, the Australian Commission on Safety and Quality in Health Care (ACSQHC) has the role to recommend nationally agreed standards for safety and quality improvement. Of specific relevance to pharmacists is that the ACSQHC has taken on a coordinating role in the improvement and safety of medication usage in Australia. One of the commission’s main achievements has been the development of a national inpatient medication chart to standardise medication ordering in all public hospitals. This chart has been utilised in most Australian public hospitals since June 2006.[2]
Various state and territory bodies are also responsible for the development, implementation and monitoring of health care standards and guidelines. For example, in Queensland, the Health Quality and Complaints Commission (HQCC) has developed clinically focussed standards (review of hospital-related deaths, management of acute myocardial infarction on admission and following discharge, and surgical safety) as well as provider-related standards (hand hygiene, credentialing and scope of clinical practice, complaints management).[3] The monitoring of the implementation of these standards is currently at a hospital level although consideration may be given to expand monitoring to other service providers in the future.
DISPENSING
Dispensing is that component of pharmacy practice related to the preparation and/or provision of medicine by a pharmacist. It is a core service provided by pharmacists with prescription medicines comprising an average of almost 75% of total community pharmacy sales.[4] Dispensing has become more complex over the years, expanding from a mainly technical function to a process involving many cognitive aspects. The dispensing standard is defined as:
The pharmacist ensures that dispensing occurs accurately, reflects the prescriber’s intentions, and is consistent with the needs and safety of the consumer.[1]
Conversely, with expanded areas of pharmacy practice, the requirement to identify an interaction or to give advice regarding potential side-effects of medication provides greater scope for error. These activities are less task-oriented, and often require professional judgment specific to the individual patient’s needs.[5, 6] These cognitive functions therefore may pose a different challenge in determining a pharmacist’s liability. For example, cases involving a failure to identify clinically significant medication interactions or medication-disease interactions, or failure to detect an overdose, have a substantial discretionary element.
GENERIC SUBSTITUTION
The Guidelines for Pharmacists on Pharmaceutical Benefits Scheme (PBS) Brand Substitution provide general advice in regard to brand substitution on PBS prescriptions. Substitution is permitted where a medicine is shown to have brand equivalence in the Schedule of Pharmaceutical Benefits and both the prescriber and patient have agreed to the substitution. It should be noted that there is no readily identifiable and authorised source of bioequivalence that would reliably support brand substitution involving a medicine dispensed as a private prescription.[7]
The increased utilisation of generic medicines has the potential to increase the risk for patients unless guidelines are rigorously adhered to. This is particularly the case with medicines with a narrow therapeutic index. Additionally, the inactive ingredients or excipients, including diluents, binders, fillers, surfactants, lubricants, coatings and dyes, can differ from brand to brand and may have an impact on patient tolerability.[8] The need to discuss generic substitution with the patient in a meaningful way is paramount, and goes far beyond merely asking the patient whether they ‘would like the cheaper brand’.
The National Prescribing Service (NPS) recommends substitution should occur only after consultation with the patient, with their informed consent, and after considering the following:[9]
NPS also identified the following points that should be discussed and clarified with patients. These are:[9]
Generic substitution should be discussed with patients, which places an increased demand on pharmacy staff. Pharmacists are required to use professional judgment and not supply a generic brand if there is any doubt that it will be in the patient’s best interest. The patient’s health outcome should be the prime consideration in any brand substitution decision. Pharmacists could be placed in a difficult legal position if it was found that they based substitution decisions solely on cost. Other issues, such as the potential for patient confusion, must also be considered in addition to the appropriateness of non-active ingredients (such as colouring agents) for patients with specific food allergies.
BARCODE SCANNERS
More than 50% of dispensing errors reported to Pharmaceutical Defence Limited (PDL), the major indemnity insurer for pharmacists in Australia, relate to human error.[10] With regard to most pharmacy dispensing errors, PDL has identified the two most frequent causes as involving the selection of the incorrect strength of a medicine, or the selection of the incorrect product.[11] These ‘slip/lapse’ errors often occur when different products have similar packaging or names that look or sound alike.
It is believed a significant reduction in dispensing errors could be achieved through the appropriate use of barcode scanners during the dispensing process.[12] These scan the unique barcode that appears on the outer packaging of all medicines marketed in Australia and compare the product (the barcode represents) with the product entered into the computer by the dispensing pharmacist. A discrepancy between the two data elements will trigger an alert to the pharmacist. The use of barcode scanners has indeed been identified by PDL as a risk management tool for the reduction in dispensing errors.[10] Additionally, at the time of writing all pharmacy registering authorities, except Queensland, mandate the use of barcode scanners.
THE PROVISION OF ADVICE
Consumer Medicine Information (CMI) leaflets
The Medicines Information to Consumers program was an initiative under the Third Guild Government Agreement, which provided pharmacists with an incentive to use CMI leaflets in practice. The pharmacist was paid a small fee when a CMI was provided and this amount was subsequently included in the dispensing fee. Pharmacists are now automatically paid to provide CMI leaflets; the assumption being that CMI leaflets are provided according to the guidelines and pharmacists assume responsibility to ensure CMI leaflets are issued when appropriate.
CMI leaflets have been criticised as not being user friendly, with research indicating that patients often misinterpret the information about the side-effects of medicine.[13] Notwithstanding the results of this research, the PSA, following receipt of a legal opinion, has advised pharmacists against withholding CMI leaflets.[14] Such recommendations emphasise the importance of keeping proper records regarding the supply of CMI leaflets to protect pharmacists from potential legal action.
The role of pharmacists in advising on both over-the-counter (OTC) and prescription medicines has increased with the changes in pharmacy practice. Pharmacists are often the first health professional to be approached by patients for health advice. In addition, patients on chronic medication have their prescriptions filled on a regular basis and often have more contact with their pharmacist than with their doctor. This increased reliance by the public on health and medication advice from pharmacists makes a pharmacist’s role in disseminating medication information crucial.[15]
Pharmacists have a legal and professional obligation to ensure patients have the information they need to enable them to make informed decisions about their medicines. However, the provision of information may not be necessary each time a product is supplied or dispensed. Pharmacists therefore need to apply professional judgment regarding the individual requirements of each patient or carer as this, in a significant way, must influence the nature and scope of the advice, how the counselling is conducted and the supportive tools to be used.[16] In addition, the appropriate counselling of patients or carers not only provides an opportunity to promote the quality use of medicines, but also the opportunity for pharmacists to undertake a final check of the dispensed medicine.
THE SUPPLY OF PHARMACIST ONLY AND PHARMACY MEDICINES
MEDICATION REVIEWS
Medication Management Reviews (MMRs) provide an opportunity for pharmacists to work closer with prescribers in the management of patients’ medication. MMRs involve the delivery of structured medication reviews to patients in aged care facilities, referred to as Residential Medication Management Reviews (RMMRs), or reviews involving patients in domiciliary settings, referred to as Home Medicines Review (HMRs). The payment for these services by the government to pharmacists approved under section 90 of the National Health Act 1953 (Cth) demonstrates the government’s recognition of the important role that pharmacists play in patients’ medication management. The pharmacist undertaking the review must be accredited with the Australian Association of Consultant Pharmacy (AACP) to provide the service. To become AACP accredited pharmacists need to complete specified training, a practice portfolio and pass an assessment. Once accredited, pharmacists need to undergo annual re-accreditation.[17]
A separate standard covers the provision of Services to Residential Care Facilities:
Medication reviews involve accredited pharmacists making recommendations to general practitioners regarding patients’ medication management. The process not only provides commercial and professional opportunities, but also creates new responsibilities. The introduction of government remuneration for pharmacists for the provision of medication reviews represents both a significant step towards recognising the role of the pharmacist in the medication management process, and the imposition of greater professional obligations on, and thereby potential risks for, pharmacists.
COMPOUNDING/EXTEMPORANEOUS DISPENSING
In Australia, as is the case internationally, there has been a trend by some pharmacists to focus on extemporaneous compounding and to market these services. It should be noted that some concerns have been raised regarding safety and efficacy issues involving some of the products. In the Medical Journal of Australia it was reported that there were three cases of women who developed endometrial cancer after using ‘bioidentical’ hormone replacement therapy.[18]
The practice of ‘compounding chemists’ has caused some regulatory difficulties as the traditional differentiation between ‘compounding’ and ‘manufacturing’ began to blur. While extemporaneous dispensing is the preparation and supply of a single ‘unit of issue’ of product which is intended for immediate use by a specific patient, extemporaneous manufacturing is the production of a batch of a product which results in a number of units of use intended for supply over a period of time.[1] In an attempt to introduce regulatory consistency, the Therapeutic Goods Administration (TGA), through the National Coordinating Committee on Therapeutic Goods (NCCTG), in April 2008 issued a discussion paper with a proposed regulatory framework.[19] This paper suggested a three-class structure for pharmacies and pharmacists:
HARM MINIMISATION SERVICES
Australia has a harm minimisation policy which aims to reduce the adverse consequences of drug use for both the individual and society.[1] Pharmacists can make an important contribution to harm minimisation through the provision of various educational and supply services. These include the participation in opioid substitution and needle and syringe programs. The standard applicable to participating in a needle and syringe program is defined as:
Opioid substitution therapy with methadone, buprenorphine or a buprenorphine/naloxone combination is used in the treatment of patients with heroin dependence. Methadone is responsible for more adverse effects and diversion problems than buprenorphine or the buprenorphine/naloxone combination. The Tasmanian coroner, in investigating the death of two men who died of drug overdoses involving methadone, benzodiazepines and other drugs, specifically raised concerns about the use of methadone due to its poor safety profile.[20]
The PSA developed guidelines for pharmacists to follow regarding the provision of opioid substitution services. Pharmacists should use professional judgment when adapting these guidelines to the specific circumstances within their community pharmacy. The guidelines address the main objectives of: providing opioid substitution services; staff requirements; necessary equipment and private dosing areas; guidelines for dispensing and dosing; record-keeping; and a contract outlining pharmacist and client responsibilities.
GUIDELINES FOR PHARMACISTS ISSUING MEDICAL CERTIFICATES
The legislation limits the ability of registered health practitioners to issue certificates only in respect of the area of practice in which the practitioner is registered or licensed. Pharmacists should therefore limit the provision of medical certificates primarily in relation to:[21]
THE SOCIETY OF HOSPITAL PHARMACISTS OF AUSTRALIA (SHPA) STANDARDS AND GUIDELINES
SHPA Standards of Practice for the Provision of Consumer Medicine Information by Pharmacists in Hospitals
SHPA Standards of Practice for Parenteral Therapy in Home Health Care
Home Health Care (HHC) involves the provision of products and services to patients in their home environment and is aimed at minimising the psychosocial stress of illness or disability. The standards refer to home infusion therapy and other injectable drug therapy including nutrition therapy. The standards include the extent and operation of the service, policies and procedures, resources, staffing structure and levels, training and education, and a quality system.
SUPPLY PROTOCOLS
There are three PSA supply protocols addressing issues with particular medicines. These are:
Pharmaceutical Society of Australia. Australian Pharmaceutical Formulary and Handbook, 20th edn. Deakin, Act: Pharmaceutical Society of Australia, 2006.
Pharmaceutical Defence Limited & Australian Journal of Pharmacy. Guide to Good Dispensing. Online. Available: www.pdl.org.au/mediacentre/dispensing_guide
1 Pharmaceutical Society of Australia. Australian Pharmaceutical Formulary and Handbook, 20th edn. Deakin ACT: Pharmaceutical Society of Australia; 2006.
2 Australian Commission on Safety and Quality in Health Care. National inpatient medication chart. 2008. Online. Available: www.safetyandquality.gov.au/[accessed 28 October 2008]
3 Queensland Health Quality and Complaints Commission. Quality of Health Services — Duty of Provider. 2007. Online. Available: www.hqcc.qld.gov.au/home/default.asp [accessed 28 October 2008]
4 Berbatis C.G., Sunderland V.B., Joyce A., Bulsara M., Mills C. Characteristics of Australia’s community pharmacies: National Pharmacy Database Project. International Journal of Pharmacy Practice. 2007 December;15:265-271.
5 Dwyer P. A pharmacist’s duty to warn. Australian Health Law Bulletin. 2003;12(8):103-104.
6 Sweet BV, Tatro DS, Whitsett TL. Pharmacy law digest. Facts and Comparisons part of Wolters Kluwer Health, 2004
7 Pharmaceutical Defence Limited. Generic Brand Substitution. Melbourne: Pharmaceutical Defence Limited; 2007.
8 McLachlan A. Frequently asked questions about generic medicines. Australian Prescriber. April 2007;30(2):41-43.
9 Smith A., McLachlan A. Generic Medicines: Same Difference?, February 2006 edn. Canberra: National Prescribing Service; 2006.
10 Guild Insurance/Guildwatch. Liability & Dispensing Guide for Pharmacists. Guild Insurance; 2006.
11 Pharmaceutical Defence Limited. List of similar names. Pharmaceutical Defence Limited Annual Report, 2004.
12 Poon E.G., Cina J.L., Churhill W., Patel N., Featherstone E., Rothschild J.M., et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Annals of Internal Medicine. 19 September 2006;145(6):426-434.
13 Berry D.C., Knapp P., Raynor D.K. Provision of information about drug side-effects to patients. The Lancet. 9 March 2002;359:853-854.
14 Pharmaceutical Society of Australia. Consumer Medicine Information and the Pharmacist. Pharmaceutical Society of Australia; 2007. 1–3
15 Dwyer P. The legal note. A vital tradition takes novel form. Australian Pharmacist. May 1999;18(4):251-252.
16 Low J. Criteria for Counselling. Pharmaceutical Society of Australia (Qld branch); April/May 2004. 6
17 Australian Association of Consultant Pharmacy. Accreditation. 2008. Online. Available: www.aacp.com.au/[accessed 26 February 2008]
18 Eden J.A., Hacker N.F., Fortune M. Three cases of endometrial cancer associated with ‘bioidentical’ hormone replacement therapy. Medical Journal of Australia. 20 August 2007;187(4):244-245.
19 National Coordinating Committee on Therapeutic Goods (NCCTG). A discussion paper on regulation of extemporaneously prepared medicines in non-hospital pharmacies. April 2008.
20 ABC News. Coroner wants methadone rethink. 2007. Online. Available: www.abc.net.au/news/stories/2007/04/06/1891474.htm [accessed 28 October 2008]
21 Pharmaceutical Society of Australia & The Pharmacy Guild of Australia. Guidelines for Pharmacists Issuing Medical Certificates. January 2008
22 The Society of Hospital Pharmacists of Australia. Committee of Specialty Practice in Oncology. Standards of practice for the provision of oral chemotherapy for the treatment of cancer. Journal of Pharmacy Practice and Research. 2007;37(2):149-152.