Practical Biomarkers for Female Genital Tract Lesions
Introduction
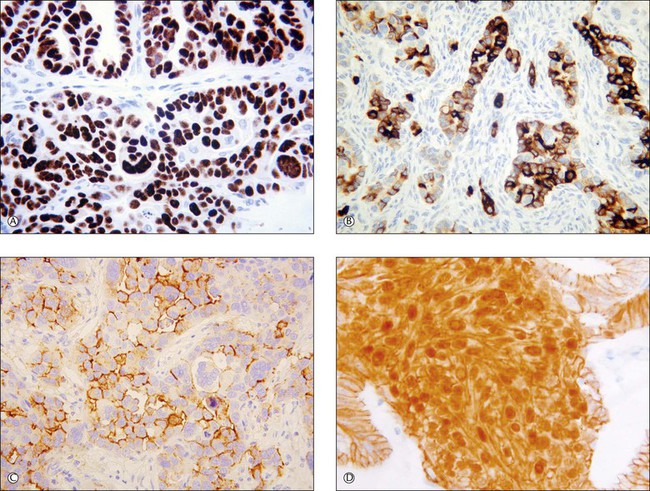
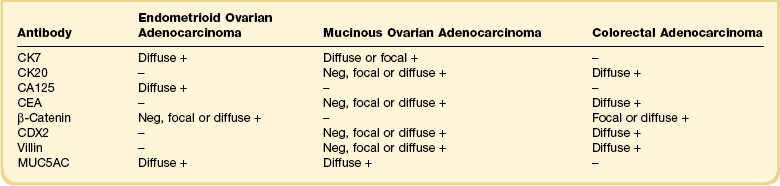
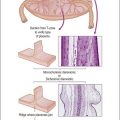
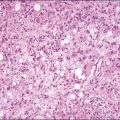
Recent years have witnessed a marked expansion in the use of immunohistochemical markers in gynecologic pathology.1,2 Most relate to the use of antibodies in the diagnosis of gynecologic neoplasms but some markers have prognostic or predictive value. In general when immunohistochemistry is used diagnostically, panels of markers provide better information than reliance on a single antibody. As anticipated historically, most antibodies, although initially thought specific for a given tumor, later have proven to have a broader range of reactivity than is initially suspected, with reactivity in a more diverse set of tumor types. This chapter provides an overview of the antibodies commonly used in the diagnosis of gynecologic lesions grouped as to function or type. The value of markers as prognostic or predictive factors is discussed where appropriate, understanding only a handful are sufficiently informative to be used in routine practice. Different markers result in different staining patterns, for example nuclear, membranous, cytoplasmic, or a combination (Figure 36.1), and knowledge of the expected staining patterns is essential when interpreting immunohistochemical slides.
Broad Spectrum Differentiation Markers
Epithelial Markers
Cytokeratins
Cytokeratins (CKs) belong to the group of intermediate filament proteins that are intermediate between microfilaments and microtubules. They constitute the cytoskeletal structure of virtually all epithelial cells, both benign and malignant. Some nonepithelial cell types and tumors derived from these may also express CKs. The cytokeratin family of proteins, which are coded by different genes, has been numbered (numbers 1–20).3 The expression of the various CKs in cells and tumors depends on their embryonic origin and also the degree of cellular differentiation.3 One broad group of CKs, type I (CK9–20), has an acidic isoelectric point. The other group, type II (CK1–8), has a basic neutral isoelectric point. Antibodies against CKs help confirm the epithelial lineage of a neoplasm. In this regard, monoclonal antibodies, such as AE1/3 and CAM 5.2, are available that recognize multiple members of the CK family. AE1/3 reacts against almost all of the CK family of proteins (AE1 recognizes most of the type 1 CKs whereas AE3 reacts against most of the type II CKs) while CAM 5.2 reacts against CK8 and CK18. Additionally, antibodies are available that react against specific CKs, for example, CK7, CK20, or CK5/6. The following sections detail the use of various anti-CK antibodies in the diagnosis of female genital tract lesions.
Broad Spectrum Cytokeratins
Broad spectrum anti-CK antibodies, such as AE1/3, often prove of value in confirming the epithelial lineage of a neoplasm. For example, in distinguishing a poorly differentiated carcinoma from sarcoma, melanoma, or lymphoma, reactivity with AE1/3, especially if widespread, favors a diagnosis of carcinoma. However, anti-CK antibodies, such as AE1/3, occasionally react with tumors of melanocytic, mesenchymal, and lymphoid origin.4 Smooth muscle tumors may also react with anti-CK antibodies.5 This may result in diagnostic difficulties, especially if dealing with an epithelioid smooth muscle neoplasm, and underscores the necessity of using panels of antibodies.
Endometrial stromal neoplasms may also be CK positive.6 Broad spectrum anti-CK antibodies are reactive with trophoblastic cells and may be useful in distinguishing intermediate trophoblast from decidua, thus confirming the presence of a placental site.
CK7 and 20
A combination of antibodies against CK7 and 20 (differential CK staining) has been widely used in ovarian and peritoneal pathology to distinguish between a primary ovarian or peritoneal adenocarcinoma and a metastatic adenocarcinoma, especially of colorectal origin.1,2,7–9 In general, primary ovarian carcinomas of serous, endometrioid, and clear cell type exhibit diffuse CK7 reactivity and are negative with CK20. Primary ovarian mucinous neoplasms exhibit a more variable immunophenotype. In general, they are diffusely reactive with CK7 and nonreactive or focally reactive with CK20. However, there are exceptions with occasional primary ovarian mucinous neoplasms, especially those of intestinal type, being diffusely CK20 positive. In the distinction between a primary ovarian endometrioid adenocarcinoma and a metastatic colorectal adenocarcinoma with an endometrioid appearance, differential CK staining is very useful alone or as part of a larger panel (Table 36.1). Endometrioid adenocarcinoma is usually diffusely CK7 reactive and CK20 negative while colonic carcinoma generally exhibits the opposite immunophenotype. In the case of an ovarian mucinous neoplasm, differential CK staining is not uncommonly difficult to interpret since many primary ovarian mucinous neoplasms may be CK20 reactive and mucinous colorectal adenocarcinoma may be focally CK7 positive. Rectal adenocarcinomas may also be CK7 positive. Additionally mucinous tumors arising in a teratoma often exhibit a large intestinal immunophenotype with diffuse CK20 immunoreactivity. In this regard, other antibodies (discussed in the following sections) are sometimes of value.
Differential CK staining is of limited value in distinguishing between a primary ovarian carcinoma and a metastatic adenocarcinoma from other organs, since many of these tumors exhibit a CK7-positive/CK20-negative or focally positive immunophenotype (Table 36.2). However, dual CK7 and CK20 reactivity raises the possibility of a primary neoplasm in the stomach, pancreas, biliary tree, or urinary bladder.1,2,7–9 Breast, pulmonary, endometrial, and endocervical adenocarcinomas are most commonly CK7 positive and CK20 negative.
Table 36.2
Typical Differential Cytokeratin Reaction Patterns in Tumors
| CK7 | CK20 | |
| Mucinous ovarian adenocarcinoma | + | − or + |
| Non-mucinous ovarian adenocarcinoma | + | − |
| Colorectal adenocarcinoma | − | + |
| Cervical adenocarcinoma | + | − |
| Endometrial adenocarcinoma | + | − |
| Pancreatic/biliary adenocarcinoma | + | + or − |
| Gastric adenocarcinoma | + | + or − |
| Renal cell carcinoma | − | − |
| Bladder adenocarcinoma | + | + or − |
| Breast adenocarcinoma | + | − |
| Pulmonary adenocarcinoma | + | − |
| Mesothelioma | + | − |
CK7 and CK20 staining also helps to confirm that most cases of pseudomyxoma peritonei in women are of appendiceal (or more rarely colorectal) origin rather than originating from a ruptured ovarian mucinous neoplasm.10 In cases of pseudomyxoma peritonei with coexistent appendiceal and ovarian mucinous neoplasms, the epithelial elements in all locations, i.e., the appendix, ovary, and peritoneum, are usually diffusely CK20 positive and negative or focally positive with CK7, in keeping with an intestinal origin.
CK7 may be of value in the vulva in confirming a diagnosis of Paget disease and excluding mimics such as malignant melanoma and mycosis fungoides. The cells of primary vulvar Paget disease are usually intensely CK7 positive,11,12 a feature that may assist in assessment of margins. Strong CK20 reactivity should result in consideration of secondary Paget disease, from either the colorectum or urinary tract.11,12
Ck5/6
CK5/6 is often reactive in mesothelial cells (benign or malignant) and is helpful to distinguish a mesothelial from a serous epithelial proliferation (benign, borderline, or malignant); the latter is usually negative.13 In this regard, CK5/6 should be used as part of a panel that may include Ber-EP4 (an epithelial marker reactive in most epithelial lesions and generally negative in mesothelial lesions). Other antibodies generally reactive in mesothelial lesions are calretinin, HBME1, thrombomodulin, D2-40, and CD44H (generally negative in epithelial lesions).13,14 CK5/6 is more likely to be positive in squamous than glandular neoplasms and this may be useful in diagnosis.
Other Cytokeratins
Although the remaining specific CKs have found little place in diagnostic gynecologic pathology, there are a few exceptions. Staining with the high molecular weight CK34β E12 may assist in highlighting the basal cell layer in ectopic prostatic tissue within the cervix.15 CK18 is especially likely to be positive in undifferentiated endometrial carcinoma and this marker may be useful in distinguishing undifferentiated endometrial carcinoma from undifferentiated sarcoma.16
Epithelial Membrane Antigen and Ber-EP4
Epithelial membrane antigen (EMA), a glycoprotein found in human milk fat globule membranes, and Ber-EP4, an epithelial-specific antigen to a membrane-bound glycoprotein, help to confirm that a neoplasm has an epithelial lineage. Trophoblast and trophoblastic neoplasms are also reactive. Both markers are commonly used in panels to distinguish an ovarian adenocarcinoma (reactive) from a sex cord–stromal tumor (negative).1,2,17 Ber-EP4 is useful in distinguishing a serous proliferation of the ovary or peritoneum (reactive) from mesothelial derived lesions (negative). Although EMA reactivity is rare in ovarian sex cord–stromal tumors, focal immunoreactivity has been found in 50% of a small series of juvenile granulosa cell tumors.18 EMA is generally negative in female adnexal tumor of wolffian origin (FATWO). This is diagnostically useful since FATWO may be confused with an epithelial neoplasm, which is usually EMA reactive. EMA is often positive in undifferentiated endometrial carcinoma and may be useful in distinguishing this from undifferentiated sarcoma.16
Mesenchymal Cell Markers
Vimentin
In the cervix, vimentin may be used as an aid to distinguish between tuboendometrial metaplasia and endometriosis (usually vimentin reactive) and adenocarcinoma in situ (AIS) (usually vimentin negative).19 Vimentin may also be useful in differentiating between an endometrial adenocarcinoma of endometrioid type and an endocervical adenocarcinoma of usual type.20 The former usually exhibits diffuse vimentin reactivity whereas endocervical adenocarcinomas are generally negative.
Vimentin may help distinguish between a microglandular variant of endometrioid or mucinous adenocarcinoma of the endometrium (usually vimentin reactive) and cervical microglandular hyperplasia (vimentin negative).21
Smooth Muscle Markers
The three most common smooth muscle markers are alpha smooth muscle actin (α-SMA), desmin, and h-caldesmon. These are helpful in several diagnostic scenarios, especially in confirming smooth muscle differentiation within a neoplasm within the female genital tract. Some smooth muscle neoplasms, especially malignant and epithelioid variants, are negative or only focally reactive.22 h-Caldesmon is the most specific, but is less sensitive than desmin. However, it should be kept in mind that some tumors that may mimic smooth muscle neoplasms, such as gastrointestinal stromal tumor (GIST), may be h-caldesmon positive.23 Desmin is not a specific smooth muscle marker, as it also stains skeletal muscle.
In the uterus, the main value of smooth muscle markers is in establishing a diagnosis of a smooth muscle neoplasm, either benign or malignant. An antibody panel composed of desmin, h-caldesmon, and CD10 (discussed later) helps distinguish cellular and other morphologically problematic leiomyomatous neoplasms from endometrial stromal neoplasms24,25 (Table 36.3). In general, leiomyomatous neoplasms are diffusely reactive with desmin and h-caldesmon. CD10 is usually negative or focally reactive, although some cellular leiomyomatous neoplasms and leiomyosarcomas may be diffusely reactive. Endometrial stromal neoplasms are usually diffusely CD10 reactive and desmin and h-caldesmon usually negative. Sometimes, desmin and h-caldesmon, are focally positive, although occasional examples are diffusely positive, especially with desmin. α-SMA is of limited value since many endometrial stromal neoplasms are diffusely reactive. Uterine tumor resembling ovarian sex cord tumor, sex cord-like areas within endometrial stromal neoplasms, and uterine perivascular epithelioid cell tumor (PEComa) are also variably reactive with smooth muscle markers.26
Table 36.3
Typical Reaction Patterns in Endometrial Stromal and Smooth Muscle Neoplasm
| Antibody | Smooth Muscle Neoplasm | Endometrial Stromal Neoplasm |
| Desmin | Diffuse + | − or focal + |
| α-SMA | Diffuse + | Neg, focal or diffuse + |
| h-Caldesmon | Diffuse + | − |
| CD10 | Neg, focal or diffuse + | Diffuse + |
| Oxytocin receptor | Diffuse + | − |
In the cervix, α-SMA may be useful in distinguishing normal endocervical glands or non-neoplastic endocervical glandular lesions from the well-differentiated glands of adenoma malignum. The presence of many α-SMA reactive stromal cells suggests a desmoplastic response to tumor.27
In the vulvovaginal region, many of the wide range of relatively site-specific mesenchymal neoplasms such as angiomyofibroblastoma, aggressive angiomyxoma, and superficial myofibroblastoma of the lower female genital tract react with smooth muscle markers, especially desmin.28 Thus, none of these markers are of value in confirming that a mesenchymal lesion represents a leiomyomatous neoplasm. However, negative staining with smooth muscle markers is of value in diagnosing cellular angiofibroma which, in contrast to most other neoplasms in the differential diagnosis, does not usually react.28
Another tumor that commonly shows reactivity with desmin is intra-abdominal desmoplastic small round cell tumor (IADSRCT; in females this may clinically mimic a primary ovarian neoplasm), usually with paranuclear dot-like immunoreactivity.29 This is useful in diagnosis, especially in differentiating this neoplasm from the wide range of ‘small blue cell tumors’ that may involve the ovary and peritoneum. Desmin sometimes assists in the distinction between benign and malignant mesothelial proliferations. Benign mesothelial cells are usually desmin reactive while the cells of malignant mesothelioma are generally negative, although there is significant overlap.
Skeletal Muscle Markers
A variety of skeletal muscle markers are available, including myoglobin, myogenin, myoD1, and sarcomeric actin. These assist in confirming rhabdomyosarcomatous differentiation within a neoplasm such as carcinosarcoma. Embryonal rhabdomyosarcomas are rare in general in the female genital tract and are most common in the vagina where the differential diagnosis usually includes the ‘small blue cell tumors of childhood.’ Rhabdomyosarcomas of embryonal, alveolar, and pleomorphic types rarely arise in the cervix, uterine corpus, or ovary. Myogenin and myoD1 are markers which have been generated against intranuclear transcription factors.30 They are relatively specific nuclear markers of skeletal muscle and are the antibodies of choice in demonstrating skeletal muscle differentiation within a neoplasm, having superseded myoglobin and sarcomeric actin.
Endometrial Stromal Markers
CD10
CD10 is useful in diagnosing an endometrial stromal neoplasm, since most endometrial stromal nodules and endometrial stromal sarcomas (low-grade endometrial stromal sarcomas) exhibit diffuse intense reactivity, although fibrous variants may be negative.24,25 In the distinction between an endometrial stromal and a smooth muscle neoplasm, CD10 should be used as part of a panel (Table 36.3), since conventional uterine smooth muscle tumors may be focally reactive and it is not uncommon for cellular and highly cellular leiomyomas (which are not infrequently mistaken for endometrial stromal neoplasms) and leiomyosarcomas to be diffusely reactive.
CD10 is also characteristically reactive in mesonephric glandular lesions within the female genital tract. Mesonephric remnants throughout the female genital tract usually exhibit luminal CD10 reactivity.31,32 CD10 reactivity in a benign cervical glandular lesion is good evidence of a mesonephric origin,31 although so-called ectopic prostatic tissue may also be reactive.15 However, CD10 is of limited value in confirming a mesonephric origin for an adenocarcinoma since many cervical and endometrial adenocarcinomas are also reactive.31 FATWO may be CD10 reactive.33
Other uses of CD10 staining in gynecologic pathology include the distinction between a metastatic renal clear cell carcinoma involving the ovary (CD10 reactive)34 and a primary ovarian clear cell carcinoma (usually CD10 negative). In addition, most trophoblastic cell populations and trophoblastic neoplasms are reactive.32 A wide range of other gynecologic neoplasms may be CD10 reactive, including leiomyosarcoma, carcinosarcoma, undifferentiated uterine sarcoma, ovarian sex cord–stromal tumors, uterine tumors resembling ovarian sex cord tumor, and mixed tumor of the vagina.35 However, CD10 immunoreactivity in these neoplasms is inconsistent and unlikely to be of diagnostic value. In summary, CD10 is expressed in a much wider range of gynecologic neoplasms than was originally appreciated and, when used as an aid to diagnosis, should always be part of a panel, which will depend on the differential diagnoses under consideration.
Mesothelial Markers
Calretinin
Calretinin is a 29 kDa calcium-binding protein, best known for its role in the diagnosis of mesothelioma. In the distinction between a mesothelioma and an adenocarcinoma, calretinin should be used as part of a panel. Calretinin and Ber-EP4 are the two most useful antibodies to distinguish between a serous epithelial and a mesothelial proliferation.14 Most serous proliferations are Ber-EP4 reactive and calretinin negative; the converse is the rule for mesothelial lesions. Nuclear reactivity with calretinin is more specific than cytoplasmic staining for mesothelial cells.14
Calretinin is also expressed in most ovarian sex cord–stromal tumors, being more sensitive but less specific than inhibin.36,37 Calretinin is more likely to be reactive in an ovarian fibroma than inhibin.
In general, neoplasms reactive for inhibin also show reactivity with calretinin. Other gynecologic neoplasms that may show calretinin reactivity include FATWO, uterine tumor resembling ovarian sex cord tumor, sex cord-like areas within endometrial stromal neoplasms, and adenomatoid tumor. Mesonephric lesions, both benign and malignant, within the cervix and elsewhere in the female genital tract may show reactivity.31
Blood Vessel Markers
CD34
CD34, a single chain transmembrane glycoprotein, leukocyte differentiation antigen, is expressed by hematopoietic progenitor cells, endothelial cells, and connective tissue cells, such as skin fibroblasts. CD34 is variably expressed in several vulvovaginal mesenchymal lesions, including aggressive angiomyxoma, cellular angiofibroma, and superficial myofibroblastoma of the lower female genital tract.28 Solitary fibrous tumors rarely occur at various sites within the female genital tract and are CD34 reactive. Endometrial stromal neoplasms are usually CD34 negative, which may be of use in differential diagnosis in that many mimics are reactive.38 Metastatic GIST in the ovary is usually CD34 positive,23 as are rare primary GISTs arising in the rectovaginal septum or elsewhere in the female genital tract.39
Narrow Spectrum Differentiation Markers
Trophoblastic Markers
β-hCG
β-hCG reacts against syncytiotrophoblast but not cytotrophoblast. Choriocarcinoma shows the strongest and most diffuse reactivity. Placental site trophoblastic tumor (PSTT) and epithelioid trophoblastic tumor are less reactive. Trophoblastic elements in mixed germ cell tumors show reactivity, as do isolated syncytiotrophoblast cells in neoplasms such as dysgerminoma and endometrial carcinoma. β-hCG may be reactive on occasions in a variety of nontrophoblastic neoplasms, such as cervical squamous carcinoma.40
Mel-CAM (CD146)
Mel-CAM is expressed in implantation site intermediate trophoblastic cells.41 Chorion-type intermediate trophoblastic cells are usually negative or focally reactive. Placental site trophoblastic tumor and exaggerated placental site, lesions of implantation site intermediate trophoblast, express Mel-CAM, whereas placental site nodule and epithelioid trophoblastic tumor, lesions of chorion-type intermediate trophoblast, are usually negative. In distinguishing placental site trophoblastic tumor from exaggerated placental site, double immunohistochemical staining with Mel-CAM and MIB1 is of value.41 In exaggerated placental site, the MIB1 index in intermediate trophoblastic cells is close to zero whereas it is significantly elevated (14 ± 6.9%) in placental site trophoblastic tumor.41
HLA-G
HLA-G is expressed in all known trophoblastic tumors, including choriocarcinoma, placental site trophoblastic tumor, and epithelioid trophoblastic tumor, as well as in benign trophoblastic lesions, such as placental site nodule and exaggerated placental site.42 HLA-G is generally negative in nontrophoblastic uterine neoplasms.64 HLA-G reactivity has been demonstrated in some ovarian carcinomas.43
Melanocytic Markers
HMB45
HMB45 is probably the most specific marker of malignant melanoma, and it is melanosome associated. HMB45 reactivity is useful to confirm the diagnosis of malignant melanoma at any site within the female genital tract, most commonly in the vulva or vagina. Metastatic melanoma in the ovary can assume an unusual array of morphologic appearances and easily fool the pathologist if there is no history of melanoma. HMB45 may assist in this regard. However, occasional ovarian steroid cell tumors, which may mimic melanoma, are HMB45 reactive.44 Another neoplasm characteristically reactive with HMB45 is PEComa.26 This is an uncommon neoplasm, which in the female genital tract most often involves the myometrium.41 Uterine epithelioid leiomyosarcomas with a clear cell appearance may also express HMB45.45
Melan-A (MART-1)
Melan-A, also known as MART-1, is another melanocytic marker of value in the diagnosis of malignant melanoma. Ovarian sex cord–stromal tumors are also commonly reactive.46
Neuroendocrine Markers
There are various neuroendocrine markers in widespread use, including chromogranin, CD56, synaptophysin, and PGP9.5. These vary in their specificity and sensitivity. For example, chromogranin is a highly specific but poorly sensitive marker while CD56 is sensitive but lacks specificity. Reactivity with neuroendocrine markers is not necessary to establish a diagnosis of a small cell neuroendocrine carcinoma since many of these are sparsely granulated and negative with neuroendocrine markers. In contrast, reactivity with neuroendocrine markers is a prerequisite for a diagnosis of large cell neuroendocrine carcinoma. Rarely paraganglioma and typical and atypical carcinoid occur within the female genital tract and are reactive. A high percentage of ovarian sex cord–stromal tumors are CD56 positive.47 Undifferentiated uterine carcinomas may be focally positive with neuroendocrine markers.16
Lymphoid Markers
Several markers may assist in the diagnosis of a low-grade endometritis, which usually depends on the morphologic identification of plasma cells that may be difficult to visualize with H&E when few in number. In the normal endometrium, most lymphoid cells are of T-cell or natural killer (NK) cell lineage. B-lymphocytes account for less than 1% of endometrial leukocytes, and are mainly located in lymphoid aggregates. In endometritis, the number of T-lymphocytes and NK cells does not differ from controls. However, the use of B-lymphoid markers, such as CD20 and CD79a, reveals substantially increased numbers of B-cells in unusual locations such as beneath the surface epithelium and intraepithelially.48 Markers against plasma cells, such as syndecan and VS38, may also assist in diagnosing endometritis.49
Markers of Altered Function in Disease States
Tumor Markers
Carcinoembryonic Antigen
Monoclonal CEA helps distinguish non-mucinous ovarian adenocarcinomas (usually negative) from colorectal adenocarcinoma (usually reactive), when used as part of a panel.1,2 Primary ovarian mucinous neoplasms are often reactive. Adenocarcinomas from other organs, such as pancreas and stomach, are variably reactive.
CEA is useful as part of a panel to help distinguish endometrioid-type endometrial adenocarcinoma from endocervical adenocarcinoma of usual type.20 Endocervical adenocarcinomas are usually, but not always, diffusely reactive with CEA. Primary endometrioid adenocarcinomas of the corpus are negative or focally reactive, although the associated squamous elements may be diffusely reactive. CEA staining patterns of primary mucinous adenocarcinoma of the endometrium are not well studied, but at least a proportion are reactive. CEA is usually reactive in cervical AIS and negative in benign endocervical glandular lesions.50
CA125 (Oc125)
Immunohistochemical staining with CA125 helps to distinguish between a primary and a metastatic ovarian adenocarcinoma and in the evaluation of a disseminated peritoneal tumor in a female.1,2 In general, primary ovarian (or peritoneal) adenocarcinomas of serous, endometrioid, and clear cell types exhibit diffuse CA125 reactivity. Primary ovarian mucinous carcinomas are usually negative, as are colorectal adenocarcinomas. In distinguishing primary ovarian adenocarcinoma from a metastatic colorectal adenocarcinoma, CA125 should be used in a panel (Table 36.2) that could include CK7, CK20, estrogen receptor (ER), CDX2, and CEA. CA125 reactivity is not specific for an ovarian adenocarcinoma, as primary adenocarcinomas of many other organs, including pancreas, breast, lung, cervix, and uterine corpus exhibit reactivity in a proportion of cases. Mesotheliomas are commonly reactive, as are benign mesothelial cells.51
Inhibin
Inhibin is a dimeric 32 kDa peptide hormone composed of an α- and a β-subunit and produced by ovarian granulosa and theca cells. Individual antibodies are available against each subunit. Most ovarian sex cord–stromal tumors show focal to diffuse cytoplasmic reactivity with inhibin (antibody against α-subunit), although fibroma, poorly differentiated Sertoli–Leydig, and sarcomatoid granulosa cell tumors are sometimes negative.52,53 Since ovarian sex cord–stromal neoplasms may be morphologically confused with a wide range of neoplasms, especially endometrioid carcinomas, immunohistochemical evaluation with inhibin (and other sex cord–stromal markers such as calretinin) may be extremely useful in primary diagnosis and also when confirming a metastatic neoplasm, which may occur years or decades later. In distinguishing between a sex cord–stromal tumor and an endometrioid carcinoma, the former is almost always negative with EMA17 (Table 36.4). Ovarian sex cord–stromal tumors are usually negative with CK7 but may be focally positive with broad spectrum anti-CK antibodies. Most carcinomas are negative with inhibin, although occasional tumors are focally reactive. Activated ovarian stromal cells that occur in association with and at the periphery of any ovarian neoplasm may be reactive with sex cord–stromal markers, so close attention must be paid to the cellular morphology of the particular clusters that are immunohistochemically reactive.
Table 36.4
Antibodies of Value in Distinguishing between Ovarian Endometrioid Adenocarcinoma and Sex Cord–Stromal Tumor
| Antibody | Endometrioid Adenocarcinoma | Sex Cord–Stromal Tumor |
| CK7 | + | − |
| EMA | + | − |
| α-Inhibin | − | + |
| Calretinin | − or + | + |
| Steroidogenic factor 1 | − | + |
| Broad spectrum CKs | Diffuse + | − or focal + |
Inhibin staining may also be of use in the evaluation of aspirates of ovarian cysts.54 Reactivity of the cells in an aspirate with inhibin and negative staining with EMA helps confirm the presence of granulosa cells, indicating a follicular rather than an epithelial lined cyst. Inhibin may also help to demonstrate luteinized stromal cells in cases of ovarian stromal hyperthecosis, or in association with ovarian neoplasms of non-sex cord–stromal type that have resulted in androgenic or estrogenic manifestations.55
Other gynecologic neoplasms that are variably reactive with inhibin include FATWO, cervical mesonephric adenocarcinoma, uterine tumor resembling ovarian sex cord tumor, and sex cord-like areas within endometrial stromal neoplasms.31,56 Inhibin also stains some trophoblastic cell populations, syncytiotrophoblast, and some intermediate trophoblastic cells showing reactivity while cytotrophoblast is negative.57 Choriocarcinoma and other trophoblastic neoplasms, such as placental site trophoblastic tumor and epithelioid trophoblastic tumor, may be inhibin reactive. β-Inhibin is less useful diagnostically than α-inhibin since many ovarian and extraovarian carcinomas are reactive.58
OCT3/4
OCT3/4 is an octamer binding transcription factor expressed in both mouse and human embryonic stem and germ cells. Nuclear reactivity is present in ovarian dysgerminoma and embryonal carcinoma and in the germ cell component of gonadoblastoma.59 Most ovarian epithelial and sex cord–stromal tumors are unreactive, although occasional clear cell carcinomas are positive.59 As clear cell carcinoma and dysgerminoma often superficially resemble each other, OCT3/4 reactivity must be assessed with caution.
HIK1083
HIK1083, a monoclonal antibody against gastric/pyloric gland mucins, is reactive in cervical minimal deviation adenocarcinoma of mucinous type (adenoma malignum) and primary cervical adenocarcinoma of gastric type.60,61 Focal reactivity may be present in ordinary endocervical adenocarcinomas and less well-differentiated areas in adenoma malignum may be negative.
The benign endocervical glandular lesion lobular endocervical glandular hyperplasia, which can mimic adenoma malignum and which is also considered to exhibit gastric differentiation, may also be reactive with HIK1083.60
CDX-2
CDX-2 is a gene that encodes for a transcription factor involved in the development and differentiation of the small and large intestines. Colorectal adenocarcinomas usually exhibit diffuse nuclear reactivity with antibodies against CDX-262 and this may be useful, as part of a panel, in distinguishing between a primary ovarian adenocarcinoma and a metastatic colorectal adenocarcinoma. However, occasional primary ovarian endometrioid adenocarcinomas exhibit CDX-2 reactivity, as do some primary ovarian mucinous tumors, chiefly those of intestinal type.62 CDX-2 is also commonly expressed in intestinal type AIS in the cervix and in some cervical adenocarcinomas, including those of intestinal type.63 CDX-2 has been shown to be almost invariably expressed in squamous morules in endometrioid proliferative lesions of the uterus or ovary.64 CDX-2 positivity in the cells of vulvar Paget disease suggests secondary Paget disease from a primary colorectal adenocarcinoma.
Alpha-Fetoprotein
Alpha-fetoprotein (α-FP) is a glycoprotein composed of 590 amino acid residues present in yolk sac tumors and some cases of hepatocellular carcinoma. In the female genital tract, α-FP helps to establish a diagnosis of yolk sac tumor. Primary hepatoid carcinomas of the ovary, metastatic hepatocellular carcinoma, and metastatic hepatoid carcinomas from other organs may also be reactive. Some Sertoli–Leydig cell tumors in the ovary express α-FP and are associated with an elevated α-FP serum level.65
Hep-PAR1
Hep-PAR1 is expressed in most ovarian hepatoid yolk sac tumors, primary ovarian hepatoid carcinomas, and hepatoid carcinomas metastatic to the ovary.66 Hep-PAR1 is of no value in distinguishing any of these tumors from each other. Occasional cervical carcinomas, of either glandular or squamous type, also express Hep-PAR1.67
Muc Antibodies
Mucins are high molecular weight glycoproteins. Several mucin genes have been identified or cloned (MUC1–MUC12) and monoclonal antibodies to these are available. Expression of the mucin gene MUC5AC helps distinguish colonic adenocarcinoma metastatic to the ovary (nonreactive) from a primary ovarian adenocarcinoma (reactive).7,68 Appendiceal and pancreatic adenocarcinomas typically express MUC5AC.7,68 Colorectal adenocarcinomas express MUC2. MUC2 expression in vulvar Paget’s disease favors an underlying colorectal adenocarcinoma.69 MUC2 reactivity is also useful to confirm that pseudomyxoma peritonei is of appendiceal origin.71 MUC6 (along with HIK1083) is often positive in so-called primary cervical adenocarcinomas of gastric type.60,61
CD99
In the female genital tract, CD99 helps establish the diagnosis of a tumor in the Ewing family or peripheral primitive neuroectodermal tumor, which has rarely been described in the ovary, uterus, cervix, and vulva.72 The reactivity should be membranous. Cytoplasmic reactivity is less specific. Ovarian sex cord–stromal tumors also commonly exhibit membranous CD99 reactivity, as may uterine tumor resembling ovarian sex cord tumor and sex cord-like areas within endometrial stromal neoplasms.73
Thyroid Transcription Factor 1
Thyroid transcription factor 1 (TTF1) is a 38 kDa nuclear protein member of the NKx2 family of homeodomain transcription factors, which is positive in thyroid neoplasms and in primary pulmonary adenocarcinomas and neuroendocrine neoplasms. It may be useful in the ovary (in combination with thyroglobulin) in diagnosing unusual morphologic variants of struma ovarii. It may also be useful in helping to confirm a diagnosis of a metastatic pulmonary adenocarcinoma. In the female genital tract, TTF1 is often positive in cervical small cell and large cell neuroendocrine carcinomas, sometimes with diffuse immunoreactivity,74 and is of no value in distinguishing these from a lung metastasis. Studies have shown that a not insignificant proportion of primary gynecologic adenocarcinomas of uterine, cervical, and ovarian origin may be TTF1 positive, including some with diffuse immunoreactivity.75 Cervical mesonephric adenocarcinomas may also be positive.76 This illustrates that TTF1 nuclear immunoreactivity in an adenocarcinoma is not always indicative of a pulmonary primary. Some normal epithelia in the female genital tract may be TTF1 positive.77
Prostatic-Specific Antigen and Prostatic Acid Phosphatase
Prostatic-specific antigen and prostatic acid phosphatase are positive in cases of so-called ectopic prostatic tissue in the cervix and in vaginal tubulosquamous polyp.15,78 It is probable that these are part of the same spectrum of lesions and derived from misplaced periurethral Skene glands.79 The latter are the female equivalent of prostatic glands in the male and may exhibit immunoreactivity with prostatic markers.
Glypican 3
Glypican 3 has emerged as a useful marker of yolk sac tumor or yolk sac areas in a mixed gonadal or extragonadal germ cell tumor.80 Positive staining is cytoplasmic and membranous. Glypican 3 appears a more sensitive and specific marker of yolk sac tumor than α-FP. Positive staining is restricted to areas of yolk sac tumor, all patterns of yolk sac tumor are positive, staining is more widespread than with α-FP, and there is less background staining. Some ovarian clear cell carcinomas (44% in one study) are also glypican 3 positive.81
SALL 4
SALL 4 is a marker of primitive gonadal and extragonadal germ cell tumors.82 There is nuclear immunoreactivity in yolk sac tumor, embryonal carcinoma, dysgerminoma, and some immature teratomas. SALL 4 is a more sensitive marker of yolk sac tumor than α-FP; all patterns of yolk sac tumor are positive, staining is more widespread, and there is less background staining.
Steroidogenic Factor 1
Steroidogenic factor 1 (SF-1) is a sensitive and useful nuclear marker of ovarian sex cord–stromal tumors with all morphologic subtypes positive while most mimics, such as endometrioid adenocarcinoma and carcinoid tumor, are negative.83
ALK1
Occasional inflammatory myofibroblastic tumors of the uterus have been described and these are ALK1 positive with cytoplasmic staining.84
HER-2/NEU
Human epidermal growth factor receptor 2 (HER-2/NEU) is a 185 kDa transmembrane tyrosine kinase receptor with close homology to the epidermal growth factor receptor. Many cases of primary vulvar Paget disease overexpress HER-2/NEU, and anti- HER-2/NEU antibodies such as trastuzumab may be a treatment option.85 Similarly some uterine serous carcinomas overexpress HER-2/NEU,86 as well as some ovarian carcinomas, especially of mucinous type.87
Tumor Suppressor Genes
WT1
Antibodies are available against both the C terminal and the N terminal of WT1 and nuclear staining is regarded as positive. Nonspecific cytoplasmic staining may be seen in many diverse neoplasms as well as in endothelial cells and mesenchymal tissues. IADSRCT, in most cases, is reactive with antibodies against the C terminal rather than the N terminal. This may be useful in diagnosis and in the distinction of IADSRCT from the other small blue cell tumors that rarely may involve the ovary and peritoneum.88
Primary ovarian, peritoneal, and tubal serous carcinomas are usually WT1 positive (antibody against N terminal).89,90 In a poorly differentiated ovarian carcinoma, nuclear WT1 reactivity, especially when diffuse, favors a serous neoplasm since most endometrioid, clear cell, and mucinous carcinomas are negative. Transitional and undifferentiated carcinomas of the ovary are also commonly WT1 reactive, providing evidence that many are variants of high-grade serous carcinoma.91 With a disseminated serous carcinoma involving more than one site, diffuse reactivity with WT1 favors an ovarian, peritoneal, or tubal primary. Most uterine serous carcinomas are unreactive or only focally reactive, although the literature is somewhat contradictory and some cases are positive.89,90,92
Ovarian small cell carcinoma of hypercalcemic type is usually reactive with an antibody against the N terminal of WT1 (Table 36.5).88 As this small cell tumor can morphologically resemble adult or juvenile granulosa cell tumor, a panel of antibodies to WT1, inhibin, and EMA may be of value. However, it is cautioned that some ovarian granulosa cell tumors of adult and juvenile type are WT1 positive.
Table 36.5
Antibodies of Value in Distinguishing between Ovarian Small Cell Carcinoma of Hypercalcemic Type and Juvenile Granulosa Cell Tumor
| Antibody | Ovarian Small Cell Carcinoma of Hypercalcemic Type | Juvenile Granulosa Cell Tumor |
| WT1 (N terminal) | Diffuse + (intense) | − or focal + (weak) |
| α-Inhibin | − | Diffuse or focal + |
| EMA | Focal + | − or focal + |
Other neoplasms involving the female genital tract that may be WT1 reactive include malignant mesothelioma, adenomatoid tumor, endometrial stromal neoplasms, leiomyomatous tumors, and a variety of ovarian sex cord–stromal tumors.93
WT1 is commonly positive with nuclear immunoreactivity in müllerian smooth muscle neoplasms and may be useful in distinguishing between a müllerian and non-müllerian smooth muscle tumor.94
DPC4
Deleted in Pancreatic Cancer, locus 4 (DPC4; also known as SMAD4), is a tumor suppressor gene that is inactivated by allelic loss in approximately 50% of pancreatic cancers. Such cases lack immunohistochemical staining.7 In contrast, primary ovarian, colorectal, and appendiceal carcinomas are usually DPC4 reactive since there is no allelic loss.7 Since pancreatic adenocarcinoma metastatic to the ovary may closely mimic a primary ovarian mucinous neoplasm, evaluation of DPC4 reactivity is helpful diagnostically.
p53
p53 is a tumor suppressor gene, located on the short arm of chromosome 17, which encodes a 35 kDa nuclear protein involved in regulating cell growth. Mutated p53 gene (TP53 mutation) is among the most commonly detected genetic abnormalities in human neoplasia. Mutations result in a conformational change of the protein, which becomes stabilized, thus allowing for immunohistochemical detection. The most widely used anti-p53 antibody is D07. Usually, but not always, diffuse intense nuclear reactivity is found whenever TP53 mutation occurs. It has also been shown that totally absent p53 staining may be associated with underlying TP53 mutation (usually nonsense mutation, in contrast to missense mutation, which results in diffuse staining) and that it is this ‘all or nothing’ staining that is aberrant and of diagnostic importance and associated with TP53 mutation.95,96 In contrast, ‘wild-type’ staining with a focal, weak, and heterogeneous pattern occurs in many tumors and in normal tissues and is not associated with TP53 mutation.
Diffuse intense nuclear p53 reactivity or total absence of staining is characteristic of uterine serous carcinoma.1,2,97 p53 may also help identify the presumed precursor lesion of serous carcinoma, namely serous endometrial intraepithelial carcinoma (serous EIC), which exhibits diffuse or totally absent staining. This is often a subtle lesion that not uncommonly involves an endometrial polyp. p53 helps distinguish serous carcinoma and serous EIC from benign papillary endometrial proliferations and metaplasias, a problem most likely to be encountered in small endometrial biopsies. Diffuse p53 reactivity is much more common in uterine leiomyosarcomas than benign leiomyomatous neoplasms, including symplastic or atypical leiomyoma.98
In ovarian carcinomas, aberrant p53 function (diffuse p53 reactivity or total absence of staining) is more common in high-grade serous and undifferentiated carcinomas than in other morphologic subtypes.99–101 TP53 mutation occurs early in the evolution of high-grade ovarian serous carcinoma, having been identified in microscopic ovarian or tubal high-grade serous carcinomas, and in its putative precursor lesion, serous tubal intraepithelial carcinoma (serous TIC).102
In the vulva, p53 helps distinguish classic (undifferentiated, usual) vulvar intraepithelial neoplasia (VIN; ‘wild-type’ staining) from differentiated (simplex) VIN. Strong p53 reactivity extending above the basal cell layer favors differentiated VIN,103 although p53 reactivity may also be seen in the basal cell layers in lichen sclerosus in the absence of differentiated VIN.104
p63
The p63 gene is a transcription factor that belongs to the p53 family. The p63 protein has six isoforms, three each classified into two groups, designated TA and Δ N p63.105 Cytotrophoblast expresses the Δ N p63 isoform whereas chorion-type intermediate trophoblast in the fetal membranes, placental site nodule, and epithelioid trophoblastic tumor express the TA p63 isoform.105 Intermediate trophoblast in the implantation site and placental site trophoblastic tumor do not express p63 . Most commercially available antibodies react against all p63 isoforms. In the cervix, p63 is preferentially expressed in immature cells of squamous lineage and in basal and reserve cells.106
p63 helps distinguish cervical small cell neuroendocrine carcinoma (negative or focally reactive) from poorly differentiated and small cell nonkeratinizing squamous carcinoma (positive).106 However, in one study a small number of cervical neuroendocrine carcinomas were p63 positive.74
In ovarian neoplasms, p63 reactivity is largely confined to transitional neoplasms, including benign, borderline, and some malignant Brenner tumors.107
PTEN
PTEN (phosphatase and tensin homolog deleted on chromosome 10) is a tumor suppresser gene mutated in a high percentage of endometrioid adenocarcinomas of the endometrium.108 PTEN mutation is generally associated with loss of PTEN immunohistochemical staining.109 PTEN is also mutated in some endometrioid adenocarcinomas of the ovary.110 PTEN mutation occurs early in the development of endometrioid-type endometrial adenocarcinoma since mutation with associated absence of staining has been found in 66% of the cases of the precursor lesion, termed endometrial intraepithelial neoplasia (EIN) in one classification scheme and atypical endometrial hyperplasia in another. PTEN-null glands also occur not uncommonly in normal cyclical endometrium109 and therefore a lack of reactivity cannot be used to diagnose EIN or atypical endometrial hyperplasia. Furthermore, not all cases of EIN exhibit an absence of PTEN reactivity and in other cases loss of expression precedes the development of morphologic features of EIN. The most widely used anti-PTEN antibody is 6H2.1. The PTEN-null rate in endometrial adenocarcinoma varies by tumor subtype, ranging from a low of 13% of serous cancers to 83% of those endometrioid tumors preceded by an EIN lesion.109
Proto-Oncogenes
Bcl-2
In proliferative endometrium, bcl-2 is diffusely expressed in the glandular cytoplasm. The activity is reduced in the glands of both atypical hyperplasia and endometrioid-type adenocarcinomas.111 In the cervix, bcl-2 is normally expressed in the basal cell layer of the squamous epithelium. Normal fallopian tube epithelium is bcl-2 reactive,112 as are foci of ciliated metaplasia involving the ovarian surface epithelium and the epithelium of cortical inclusion cysts.112
Tuboendometrial metaplasia and endometriosis in the cervix generally exhibit diffuse cytoplasmic reactivity, which helps distinguish them from AIS, which is generally negative.113 Endometrial stromal neoplasms are commonly bcl-2 reactive, but this is of limited value since many other tumors included in the differential diagnosis are also reactive.38 A subset of uterine leiomyosarcomas overexpressed bcl-2 may be associated with a good prognosis.114
CD117 (C-Kit)
CD117, a transmembrane tyrosine kinase receptor, is expressed in metastatic GIST within the ovary23 and in rare primary GISTs arising in the rectovaginal septum, uterine corpus, or elsewhere in the female genital tract.39 Some gynecologic sarcomas, including leiomyosarcoma,115,116 may focally express CD117, as occasionally do other tumors, including uterine carcinosarcoma and ovarian serous carcinoma. Dysgerminoma commonly exhibits diffuse membranous immunoreactivity.
Cell Cycle and Nuclear Proliferation
Ki-67 (MIB1) and Proliferating Cell Nuclear Antigen
Cervical squamous intraepithelial lesions exhibit an increased MIB1 proliferation index.117 In normal squamous epithelium, MIB1 reactivity is largely confined to the basal and parabasal layers, with substantial to nearly full thickness reactivity in high-grade squamous intraepithelial lesion (HSIL). Its major use is to distinguish between HSIL and benign mimics such as atrophic squamous epithelium, transitional metaplasia, and immature squamous metaplasia.
Similarly in endocervical AIS the proliferation index is in excess of 30%, in comparison with its benign mimics such as tuboendometrial metaplasia, endometriosis, and microglandular hyperplasia, which usually exhibit a proliferation index <10%.113 Because there may be quantitative overlap at the lower end of the AIS and upper end of the benign spectrum, MIB1 should form part of a panel including p16.
In the vulva, MIB1 has some utility in evaluating squamous lesions.118 Human papillomavirus (HPV) infection of the vulva is associated with clusters of MIB1 reactive cells in the middle and upper thirds of the epithelium,118 which helps to definitively categorize a lesion in which an equivocal diagnosis of condyloma might be made. The cells of high-grade VIN of classic type express MIB1 throughout much of the full epithelial thickness, which helps distinguish high-grade VIN from atrophic squamous epithelium.
Proliferation markers have been used in the assessment of trophoblastic lesions. In chorionic villi, reactivity with proliferation markers is largely confined to villous cytotrophoblast. MIB1 aids in distinguishing PSTT from an exaggerated placental site.41 In the former, the MIB1 index is significantly elevated (14 ± 7%) whereas it is nearly zero in the latter.41 Care should be taken to exclude reactivity in small lymphocytes. MIB1 also helps distinguish PSTT from choriocarcinoma, since the latter exhibits a much higher proliferation index.41
p16
p16, also known as cyclin-dependent kinase-4 inhibitor (CD K4-I), is the product of the INK4-A gene and specifically binds to cyclin D-CDK4/6 complexes to control the cell cycle at the G1–S interphase. In the cervix, diffuse p16 staining usually, but not always, correlates with the presence of high-risk HPV; in other words, diffuse p16 staining is a surrogate for the presence of high-risk HPV.119,120 Thus, there is diffuse p16 expression (usually a combination of nuclear and cytoplasmic staining) in most HSILs.119,120 In cervical squamous lesions, p16 may help identify small focal areas of HSIL and distinguish immature squamous metaplasia from HSIL involving immature metaplastic squamous epithelium.120
p16 may be useful in diagnosing endocervical AIS, which is usually diffusely reactive, and distinguishing this from mimics such as tuboendometrial metaplasia and endometriosis, which are either negative or focally reactive.113,121 In this regard, p16, MIB1, and bcl-2 can be used as part of a panel (Table 36.6).
Table 36.6
Antibodies of Value in Distinction between Cervical Tuboendometrial Metaplasia and Endometriosis and AIS
| Antibody | Tuboendometrial Metaplasia/Endometriosis | Cervical AIS |
| MIB1 | <30% | >50% |
| Bcl-2 | Diffuse + | − |
| p16 | − or focal + | Diffuse + |
p16 helps distinguish endocervical adenocarcinoma of usual type, which exhibits diffuse reactivity, from endometrial adenocarcinoma of endometrioid type, which is generally negative or focally reactive.122 The squamous elements in endometrial adenocarcinomas may be strongly reactive. Some cases of uterine corpus endometrioid adenocarcinoma will exhibit diffuse reactivity with p16 but even in these cases there are usually admixed positive and negative areas, a so-called mosaic staining pattern. Many endometrial and ovarian high-grade serous carcinomas also react diffusely with p16.123
p16 is useful to distinguish metastatic cervical adenocarcinoma in the ovary (p16 reactive) from primary ovarian endometrioid or mucinous adenocarcinoma (usually, but not always, p16 negative).124 In the vulva, p16 reactivity is characteristic of classic VIN, since this disease is associated with HPV infection. Differentiated VIN is usually p16 negative since there is no association with HPV. Similarly, HPV-associated vulvar squamous carcinomas are p16 reactive while those not associated with HPV are negative.125 p16 is much more strongly expressed in uterine leiomyosarcomas than leiomyomas, including morphologically problematic variants, and this may be useful in diagnosis.97
p57
p57, also known as Kip2, is a cell cycle inhibitor of cell proliferation and tumor suppressor encoded by a strongly paternally imprinted, maternally expressed gene. p57 is only expressed when maternal DNA is present. A particular use is in the distinction of complete hydatidiform mole from partial hydatidiform mole and hydropic abortion. p57 is expressed in the nuclei of the cytotrophoblast and villous mesenchyme in the normal placenta, hydropic abortion, and partial mole since all have a maternal component.126,127 In contrast, p57 is absent in the complete mole, since these villi are paternally derived and lack maternal DNA. Positive reactivity in decidua and extravillous trophoblast (it is not known why extravillous trophoblast reacts) acts as an internal positive control.
Cyclin D1
Cyclin D1 is positive in normal endocervical glands while staining is typically lost in premalignant and malignant endocervical glandular lesions.128 However, there may be focal nuclear immunoreactivity in adenocarcinomas, especially at the invasive front.129 A similar staining pattern is seen in some endometrial adenocarcinomas and it has been suggested that cyclin D1 is upregulated in areas of epithelial–mesenchymal transition at the advancing edge of endometrial and cervical adenocarcinomas. In the former, these often correspond to glands exhibiting a microcystic, elongated, and fragmented (MELF) pattern of myometrial invasion.130
Cyclin E
Cyclin E is useful in the distinction between an epithelioid trophoblastic tumor and a placental site nodule, two lesions derived from the intermediate trophoblast of the chorion laeve (chorionic-type intermediate trophoblast).131 There is much higher cyclin E immunostaining in epithelioid trophoblastic tumor than in placental site nodule.131
ProExC
ProExC is a cocktail of antibodies against minichromosome maintenance protein 2 (MCM2) and topoisomerase II-α, which are key proteins overexpressed in the nucleus during aberrant S-phase induction of the cell cycle. ProExC is useful in the evaluation of premalignant cervical squamous lesions (reliable marker of HSIL) and glandular lesions (useful in distinction between AIS and benign glandular mimics).132,133 It may also be useful in the diagnosis of high-grade VIN of classic type. Positive staining is limited to the basal and parabasal layers of normal cervical and vulvar squamous epithelium but extends higher in the squamous epithelium in accordance with the degree of cervical intraepithelial neoplasia or VIN.134
Hormone Receptors
Estrogen Receptor and Progesterone Receptor
Most of the vulvovaginal mesenchymal lesions react with ER and PR, including aggressive angiomyxoma, angiomyofibroblastoma, cellular angiofibroma, superficial myofibroblastoma of the lower female genital tract, and smooth muscle neoplasms.28,135 Therefore, ER and PR do not assist in distinguishing between these neoplasms, most of which are thought to arise from the zone of hormone receptor-positive subepithelial cells that extend from the cervix to the vulva. The fact that aggressive angiomyxoma is often hormone receptor positive is the rationale for treating these neoplasms with gonadotropin-releasing hormone agonists, especially recurrent neoplasms and those tumors not amenable to surgical resection.136
Endometrial cancers of endometrioid type are commonly ER and PR reactive, whereas serous and clear cell carcinomas are often negative. However, there is considerable immunophenotypic overlap and a significant percentage of serous carcinomas are ER and/or PR positive, especially ER.92 In spite of this, diffuse strong nuclear reactivity with ER and PR favors an endometrioid adenocarcinoma whereas negative staining or focal reactivity suggests a serous carcinoma. In practice it is useful to combine ER and PR with p53, the latter usually being diffusely reactive or totally negative in serous carcinomas and exhibiting ‘wild-type’ staining in endometrioid cancers. Similarly, a combination of ER, PR, and p53 may help distinguish problematic benign papillary proliferations from a small uterine serous carcinoma or serous EIC. Endometrial metaplasias usually exhibit a weak heterogeneous pattern of p53 staining whereas serous EIC generally exhibits diffuse intense reactivity. Many other uterine neoplasms may be ER and PR positive, including endometrial stromal and smooth muscle neoplasms, both benign and malignant.
ER, as part of a panel (Table 36.7), helps differentiate endometrioid adenocarcinoma of the endometrium from endocervical adenocarcinoma.20 This may be a difficult distinction to make when tumor is present in both endometrial and cervical biopsies or in a hysterectomy specimen where tumor involves both the corpus and cervix. Endometrial adenocarcinomas of endometrioid type are generally diffusely ER reactive while endocervical adenocarcinomas are negative or at most focally reactive, although occasional well-differentiated cervical adenocarcinomas are diffusely positive. In a panel with vimentin, monoclonal CEA, and p16,20 endometrioid-type endometrial adenocarcinomas are usually vimentin reactive, CEA negative, or focally reactive and p16 negative, or focally reactive. In contrast, endocervical adenocarcinomas are usually vimentin negative and diffusely reactive with CEA and p16. Squamous elements in endometrioid adenocarcinomas of the uterus may be both CEA and p16 reactive. Molecular techniques, such as in situ hybridization or polymerase chain reaction, to demonstrate HPV may also be of value. Endometrioid adenocarcinoma of the corpus is HPV negative, whereas endocervical adenocarcinoma of usual type is usually positive.137
Table 36.7
Typical Reaction Patterns of Endometrial Adenocarcinoma of Endometrioid Type and Endocervical Adenocarcinoma
| Antibody | Endometrioid Type Endometrial Adenocarcinoma | Endocervical Adenocarcinoma |
| ER | Diffuse + | − or focal + |
| Vimentin | Diffuse + | − |
| Monoclonal CEA | − or focal + | Diffuse or focal + |
| p16 | − or focal + | Diffuse + |
Androgen Receptor
In both the cervix and vagina, androgen receptor is reactive in mesonephric remnants and ectopic prostatic tissue.15 Normal endocervical glands are usually negative, although the expression of androgen receptor in the wide range of benign endocervical glandular lesions has not been extensively studied. Normal cervical and vaginal stromal fibroblasts are androgen receptor positive.15 Other neoplasms in the female genital tract found to express androgen receptor in a variable percentage of cases include endometrial adenocarcinoma, endometrial stromal sarcoma, cervical mesonephric adenocarcinoma, and FATWO.138
Oxytocin Receptor
Oxytocin receptor is present in the nonpregnant uterus, in both the endometrium and myometrium. An antibody against oxytocin receptor helps to distinguish a uterine smooth muscle tumor (oxytocin receptor reactive) from an endometrial stromal neoplasm (oxytocin receptor negative).139
Cell Adhesion Markers
β-Catenin
β-Catenin may be a useful addition to the panel of antibodies employed to distinguish between a primary ovarian adenocarcinoma and a metastatic colorectal adenocarcinoma.140 Most, but not all, colorectal adenocarcinomas exhibit nuclear reactivity while the majority of primary ovarian mucinous neoplasms are negative. Primary ovarian endometrioid adenocarcinomas may exhibit nuclear reactivity since they can be associated with β-catenin gene mutation and the squamous morules are almost invariably positive, as are squamous morules in endometrioid proliferations within the uterus.64 Membranous β-catenin staining is the norm in epithelial cells and only nuclear reactivity is of diagnostic value. β-Catenin nuclear staining occurs in a significant percentage of uterine low-grade endometrial stromal sarcomas.141
E-Cadherin
There is loss of E-cadherin membranous staining at the invasive front of some uterine endometrioid adenocarcinomas exhibiting a MELF pattern of myometrial invasion and this may be a form of epithelial–mesenchymal transition. E-cadherin (but not β-catenin) nuclear staining has been demonstrated in a high percentage of ovarian adult granulosa cell tumors and in the granulosa cells of developing ovarian follicles.142
Other Markers
PAX8
PAX8 is a paired-box gene important in embryogenesis of the thyroid, müllerian, and renal/upper urinary tracts. In the female genital tract, PAX8 is typically positive with nuclear immunoreactivity in non-mucinous ovarian carcinomas, including those of serous, endometrioid, and clear cell types, and endometrial and cervical adenocarcinomas.143 It may be useful as part of a panel in the distinction between an ovarian carcinoma of serous or endometrioid type (PAX8 positive) and a breast carcinoma (PAX8 negative).143,144 It may also be useful in the distinction between a serous neoplasm (PAX8 positive) and a mesothelial proliferation (PAX8 negative).145 Cervical mesonephric adenocarcinomas are commonly positive.76
PAX2
PAX2 encodes a transcription factor necessary in the development of the wolffian and müllerian duct systems. In the cervix, PAX2 is strongly and diffusely expressed with nuclear immunoreactivity in mesonephric remnants, normal endocervical glands, and in benign glandular lesions.146 In contrast, most cervical adenocarcinomas and AIS are negative. Inactivation of the PAX2 gene with loss of expression has been demonstrated in endometrial precancers and cancers.147
HMGA2
The best known application of HMGA2 staining in surgical pathology is in the diagnosis of vulvovaginal aggressive angiomyxoma. In this lesion, rearrangements involving the HMGA2 gene at chromosome 12q15 can cause overexpression. In one study, 11 of 13 aggressive angiomyxomas exhibited nuclear positivity, usually diffuse, with this marker.148 It has also been shown that there is HMGA2 nuclear reactivity in most cases of ovarian and uterine serous carcinoma and in serous tubal intraepithelial carcinoma.149,150 Since serous EIC is also positive, HMGA2 abnormalities may be involved early in the pathogenesis of uterine serous carcinoma. Cervical mesonephric adenocarcinomas may be positive with this marker.76
Hepatocyte Nuclear Factor 1-β
Hepatocyte nuclear factor 1-beta (HNF1-β) was identified from large-scale gene expression studies as being a useful marker of ovarian and uterine clear cell carcinomas.151 Both tumor types exhibit nuclear immunoreactivity with this marker while most, but not all, of the other morphologic subtypes are negative.
FOXL2
Mutation of the FOXL2 gene is present in a high percentage of ovarian adult granulosa cell tumors and only rarely in other variants of ovarian sex cord–stromal tumors.152 Mutations have not been demonstrated in ovarian epithelial neoplasms. There is nuclear staining with FOXL2 in a high percentage (80%) of ovarian sex cord–stromal tumors, including many without the mutation.153 In one large study, all epithelial neoplasms were negative and FOXL2 staining was found to be 99% specific for a sex cord–stromal tumor.153 Occasional FATWO origin and uterine tumors resembling ovarian sex cord tumor were positive.153
IMP-3 and IMP-2
Insulin-like growth factor II mRNA-binding protein 3 (IMP-3) is an oncofetal protein highly expressed in fetal tissue and malignant tumors but rarely found in adult benign tissues. It is highly expressed in uterine serous carcinomas and, when used as part of a panel, is useful in the distinction from an endometrioid adenocarcinoma.154 It has also been shown that IMP-2 may be of value in this scenario since uterine serous carcinomas are diffusely positive while there is variable loss of staining in endometrioid adenocarcinomas.155
DOG1
Discovered on gastrointestinal stromal tumor 1 (DOG1) is a sensitive and specific marker of gastrointestinal stromal tumors and may be useful, combined with CD117, in the diagnosis of primary and metastatic gastrointestinal stromal tumor within the female genital tract, although a variety of other neoplasms are occasionally positive.70
References
1. McCluggage, WG. Recent advances in immunohistochemistry in the diagnosis of ovarian neoplasms. J Clin Pathol. 2000; 53:327–334.
2. McCluggage, WG. Recent advances in immunohistochemistry in gynaecological pathology. Histopathology. 2002; 40:309–326.
3. Heatley, MK. Cytokeratins and cytokeratin staining in diagnostic histopathology. Histopathology. 1996; 28:479–483.
4. Ben-Izhak, O, Stark, P, Levy, R, et al. Epithelial markers in malignant melanoma. A study of primary lesions and their metastases. Am J Dermatopathol. 1994; 16:241–246.
5. Norton, AJ, Thomas, JA, Isaacson, PG. Cytokeratin-specific monoclonal antibodies are reactive with tumours of smooth muscle derivation. An immunocytochemical and biochemical study using antibodies to intermediate filament cytoskeletal proteins. Histopathology. 1987; 11:487–499.
6. Adegboyega, PA, Qiu, S. Immunohistochemical profiling of cytokeratin expression by endometrial stromal sarcoma. Hum Pathol. 2008; 39:1459–1464.
7. Ji, H, Isacson, C, Seidman, JD, et al. Cytokeratins 7 and 20, DPC4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc 4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 2002; 21:391–400.
8. Ladendijk, JA, Mullink, EH, van Diest, PJ, et al. Tracing the origin of adenocarcinomas with unknown primary using immunohistochemistry. Differential diagnosis between colonic and ovarian carcinomas as primary sites. Hum Pathol. 1998; 29:491–497.
9. Chu, P, Wu, E, Weiss, LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000; 13:962–972.
10. Ronnett, BM, Shmookler, BM, Diener-West, M, et al. Immunohistochemical evidence supporting the appendiceal origin of pseudomyxoma peritonei in women. Int J Gynecol Pathol. 1997; 16:1–9.
11. Goldblum, JR, Hart, WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997; 21:1178–1187.
12. Goldblum, JR, Hart, WR. Perianal Paget’s disease—a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998; 2:170–171.
13. Cury, PM, Butcher, DN, Fisher, C, et al. Value of the mesothelium-associated antibodies thrombomodulin, cytokeratin 5/6, calretinin, and CD44H in distinguishing epithelioid pleural mesothelioma from adenocarcinoma metastatic to the pleura. Mod Pathol. 2000; 13:107–112.
14. Attanoos, RL, Webb, R, Dojcinov, SD, Gibbs, AR. Value of mesothelial and epithelial antibodies in distinguishing diffuse peritoneal mesothelioma in females from serous papillary carcinoma of the ovary and peritoneum. Histopathology. 2002; 40:237–244.
15. McCluggage, WG, Ganesan, R, Hirschowitz, L, et al. Ectopic prostatic tissue in the uterine cervix and vagina: report of a series with a detailed immunohistochemical analysis. Am J Surg Pathol. 2006; 30:209–215.
16. Silva, EG, Deavers, MT, Malpica, A. Undifferentiated carcinoma of the endometrium: a review. Pathology. 2007; 39:134–138.
17. Costa, MJ, De Rose, PB, Roth, LM, et al. Immunohistochemical phenotype of ovarian granulosa cell tumors: absence of epithelial membrane antigen has diagnostic value. Hum Pathol. 1994; 25:60–66.
18. McCluggage, WG. Immunoreactivity of ovarian juvenile granulosa cell tumours with epithelial membrane antigen. Histopathology. 2005; 46:235–236.
19. Marques, T, Andrade, LA, Vassallo, J. Endocervical tubal metaplasia and adenocarcinoma in situ: role of immunohistochemistry for carcinoembryonic antigen and vimentin in differential diagnosis. Histopathology. 1996; 28:549–550.
20. McCluggage, WG, Sumathi, VP, McBride, HA, et al. A panel of immunohistochemical stains, including carcinoembryonic antigen, vimentin and estrogen receptor aids the distinction between primary endometrial and endocervical adenocarcinomas. Int J Gynecol Pathol. 2002; 21:11–15.
21. Qiu, W, Mittal, K. Comparison of morphologic and immunohistochemical features of cervical microglandular hyperplasia with low-grade mucinous adenocarcinoma of the endometrium. Int J Gynecol Pathol. 2003; 22:261–265.
22. Oliva, E, Young, RH, Amin, MB, Clement, PB. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: a study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol. 2002; 26:403–412.
23. Irving, JA, Lerwill, MF, Young, RH. Gastrointestinal stromal tumors metastatic to the ovary: a report of five cases. Am J Surg Pathol. 2005; 29:920–926.
24. Nucci, MR, O’Connell, JT, Huettner, PC, et al. h-Caldesmon expression effectively distinguishes endometrial stromal tumors from uterine smooth muscle tumors. Am J Surg Pathol. 2001; 25:253–258.
25. McCluggage, WG, Sumathi, VP, Maxwell, P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology. 2001; 39:273–278.
26. Vang, R, Kempson, RL. Perivascular epithelioid cell tumour (PEComa) of the uterus : a subset of HMB-45 positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am J Surg Pathol. 2002; 26:1–13.
27. Mikami, Y, Kiyokawa, T, Moriya, T, Sasano, H. Immunophenotypic alteration of the stromal component in minimal deviation adenocarcinoma (‘adenoma malignum’) and endocervical glandular hyperplasia: a study using oestrogen receptor and alpha-smooth muscle actin double immunostaining. Histopathology. 2005; 46:130–136.
28. McCluggage, WG. A review and update of morphologically bland vulvovaginal mesenchymal lesions. Int J Gynecol Pathol. 2004; 24:26–38.
29. Ordonez, NG. Desmoplastic small round cell tumour. II: An ultrastructural and immunohistochemical study with emphasis on new immunohistochemical markers. Am J Surg Pathol. 1998; 22:1314–1327.
30. Morotti, RA, Nicol, KK, Parham, DM, et al. An immunohistochemical algorithm to facilitate diagnosis and subtyping of rhabdomyosarcoma: the Childrens Oncology Group experience. Am J Surg Pathol. 2006; 30:962–968.
31. McCluggage, WG, Oliva, E, Herrington, CS, et al. CD10 and calretinin staining of endocervical glandular lesions, endocervical stroma and endometrioid adenocarcinoma of the uterine corpus: CD10 positivity is characteristic of, but not specific for, mesonephric lesions and is not specific for endometrioid stroma. Histopathology. 2003; 43:144–150.
32. Ordi, J, Romagosa, C, Tavassoli, FA, et al. CD10 expression in epithelial tissues and tumors of the gynecologic tract; a useful marker in the diagnosis of mesonephric, trophoblastic and clear cell tumors. Am J Surg Pathol. 2003; 27:78–186.
33. Devouassoux-Shisheboran, M, Silver, SA, Tavassoli, FA. Wolffian adnexal tumor, so-called female adnexal tumor of probable Wolffian origin (FATWO): immunohistochemical evidence in support of a Wolffian origin. Hum Pathol. 1999; 30:856–863.
34. Cameron, RI, Ashe, P, O’Rourke, DM, et al. A panel of immunohistochemical stains assists in the distinction between ovarian and renal clear cell carcinoma. Int J Gynecol Pathol. 2003; 22:272–276.
35. Oliva, E. CD10 expression in the female genital tract: does it have useful diagnostic applications? Adv Anat Pathol. 2004; 11:310–315.
36. McCluggage, WG, Maxwell, P. Immunohistochemical staining for calretinin is useful in the diagnosis of ovarian sex cord-stromal tumours. Histopathology. 2001; 38:403–408.
37. Shah, VI, Freites, ON, Maxwell, P, McCluggage, WG. Inhibin is more specific than calretinin as an immunohistochemical marker for differentiating sarcomatoid granulosa cell tumor of the ovary from other spindle cell neoplasms. J Clin Pathol. 2003; 56:221–224.
38. Bhargava, R, Shia, J, Hummer, AJ, et al. Distinction of endometrial stromal sarcomas from ‘hemangiopericytomatous’ tumors using a panel of immunohistochemical stains. Mod Pathol. 2005; 18:40–47.
39. Lam, MM, Corless, CL, Goldblum, JR, et al. Extragastrointestinal stromal tumors presenting as vulvovaginal/rectovaginal septal masses: a diagnostic pitfall. Int J Gynecol Pathol. 2006; 25:288–292.
40. Hameed, A, Miller, DS, Muller, CY, et al. Frequent expression of beta-human chorionic gonadotropin (beta-hCG) in squamous cell carcinoma of the cervix. Int J Gynecol Pathol. 1999; 18:381–386.
41. Shih, IM, Kurman, RJ. The pathology of intermediate trophoblastic tumors and tumor-like lesions. Int J Gynecol Pathol. 2001; 20:31–47.
42. Singer, G, Kurman, RJ, McMaster, MT, Shih, IM. HLA-G immunoreactivity is specific for intermediate trophoblast in gestational trophoblastic disease and can serve as a useful marker in differential diagnosis. Am J Surg Pathol. 2002; 26:914–920.
43. Davidson, B, Elstrand, MB, McMaster, MT, et al. HLA-G expression in effusions is a possible marker of tumor susceptibility to chemotherapy in ovarian carcinoma. Gynecol Oncol. 2005; 96:42–47.
44. Deavers, MT, Malpica, A, Ordonez, NG, Silva, EG. Ovarian steroid cell tumors: an immunohistochemical study including a comparison of calretinin with inhibin. Int J Gynecol Pathol. 2003; 22:162–167.
45. Silva, EG, Deavers, MT, Bodurka, DC, Malpica, A. Uterine epithelioid leiomyosarcomas with clear cells: reactivity with HMB-45 and the concept of PEComa. Am J Surg Pathol. 2004; 28:244–249.
46. Stewart, CJR, Nandini, CL, Richmond, JA. Value of A103 (melan-A) immunostaining in the differential diagnosis of ovarian sex cord tumours. J Clin Pathol. 2000; 53:206–211.
47. McCluggage, WG, McKenna, M, McBride, HA. CD56 is a sensitive and diagnostically useful immunohistochemical marker of ovarian sex cord-stromal tumors. Int J Gynecol Pathol. 2007; 26:322–327.
48. Disep, B, Innes, BA, Cochrane, HR, et al. Immunohistochemical characterization of endometrial leucocytes in endometritis. Histopathology. 2004; 45:625–632.
49. Bayer-Garner, IB, Korourian, S. Plasma cells in chronic endometritis are easily identified when stained with syndecan-1. Mod Pathol. 2001; 14:877–879.
50. Cina, SJ, Richardson, MS, Austin, RM, Kurman, RJ. Immunohistochemical staining for Ki-67 antigen, carcinoembryonic antigen, and p53 in the differential diagnosis of glandular lesions of the cervix. Mod Pathol. 1997; 10:176–180.
51. Bateman, AC, al-Talib, RK, Newman, T, et al. Immunohistochemical phenotype of malignant mesothelioma: predictive value of CA125 and HBME-1 expression. Histopathology. 1997; 30:49–56.
52. Deavers, MT, Malpica, A, Liu, J, et al. Ovarian sex cord-stromal tumors : an immunohistochemical study including a comparison of calretinin and inhibin. Mod Pathol. 2003; 16:584–590.
53. McCluggage, WG, Maxwell, P, Sloan, JM. Immunohistochemical staining of ovarian granulosa cell tumors with monoclonal antibody against inhibin. Hum Pathol. 1997; 28:1034–1038.
54. McCluggage, WG, Patterson, A, White, J, Anderson, NH. Immunocytochemical staining of ovarian cyst aspirates with monoclonal antibody against inhibin. Cytopathology. 1998; 9:336–342.
55. McCluggage, WG. The value of inhibin staining in gynecological pathology. Int J Gynecol Pathol. 2001; 20:79–85.
56. McCluggage, WG. Uterine tumours resembling ovarian sex cord tumours: immunohistochemical evidence for true sex cord differentiation. Histopathology. 1999; 34:373–380.
57. McCluggage, WG, Ashe, P, McBride, H, et al. Localisation of the cellular expression of inhibin in trophoblastic tissue. Histopathology. 1998; 32:252–256.
58. McCluggage, WG, Maxwell, P. Adenocarcinomas of various sites may exhibit immunoreactivity with anti-inhibin antibodies. Histopathology. 1999; 35:216–220.
59. Cheng, L, Thomas, A, Roth, CM, et al. OCT4. A novel biomarker for dysgerminoma of the ovary. Am J Surg Pathol. 2004; 18:1341–1346.
60. Mikami, Y, Kiyokawa, T, Hata, S, et al. Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and adenoma malignum. Mod Pathol. 2004; 17:962–972.
61. Kojima, A, Mikami, Y, Sudo, T, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007; 31664–31672.
62. Werling, RW, Yaziji, H, Bacchi, CE, Gown, AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003; 27:303–310.
63. McCluggage, WG, Shah, R, Connolly, LE, McBride, HA. Intestinal-type cervical adenocarcinoma in situ and adenocarcinoma exhibit a partial enteric immunophenotype with consistent expression of CDX2. Int J Gynecol Pathol. 2006; 27:92–100.
64. Houghton, O, Connolly, LE, McCluggage, WG. Morules in endometrioid proliferations of the uterus and ovary consistently express the intestinal transcription factor CDX2. Histopathology. 2008; 53:156–165.
65. Gagnon, S, Tetu, B, Silva, EG, McCaughey, WT. Frequency of alpha-fetoprotein production by Sertoli-Leydig cell tumors of the ovary: an immunohistochemical study of eight cases. Mod Pathol. 1989; 2:63–67.
66. Pitman, MB, Triratanachat, S, Young, RH, Oliva, E. Hepatocyte paraffin 1 antibody does not distinguish primary ovarian tumors with hepatoid differentiation from metastatic hepatocellular carcinoma. Int J Gynecol Pathol. 2004; 23:58–64.
67. Thamboo, TP, Wee, A. Hep Par 1 expression in carcinoma of the cervix: implications for diagnosis and prognosis. J Clin Pathol. 2004; 57:48–53.
68. Albarracin, CT, Jafri, J, Montag, AG, et al. Differential expression of MUC2 and MUC5AC mucin genes in primary ovarian and metastatic colonic carcinoma. Hum Pathol. 2000; 31:672–677.
69. Kuan, SF, Montag, AG, Hart, J, et al. Differential expression of mucin genes in mammary and extramammary Paget’s disease. Am J Surg Pathol. 2001; 25:1469–1477.
70. Miettinen, M, Wang, ZF, Lasota, J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009; 33:1401–1408.
71. O’Connell, JT, Hacker, CM, Barsky, SH. MUC2 is a molecular marker for pseudomyxoma peritonei. Mod Pathol. 2002; 15:958–972.
72. McCluggage, WG, Sumathi, V, Nucci, M, et al. Ewing family of tumours involving the vulva and vagina: report of a series of four cases. J Clin Pathol. 2007; 60:674–680.
73. Loo, KT, Leung, AKF, Chan, JKC. Immunohistochemical staining of ovarian granulosa cell tumours with MIC2 antibody. Histopathology. 1995; 27:388–390.
74. McCluggage, WG, Kennedy, K, Busam, KJ. An immunohistochemical study of cervical neuroendocrine carcinomas: Neoplasms that are commonly TTF1 positive and which may express CK20 and P63. Am J Surg Pathol. 2010; 34:525–532.
75. Siami, K, McCluggage, WG, Ordonez, NG, et al. Thyroid transcription factor 1 expression in endometrial and endocervical adenocarcinomas. Am J Surg Pathol. 2007; 31:1759–1763.
76. Kenny, SL, McBride, HA, Jamison, J, McCluggage, WG. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and Hepatocyte Nuclear Factor 1-β. Am J Surg Pathol. 2012; 36:799–807.
77. Niu, HL, Pasha, TL, Pawel, BR, et al. Thyroid transcription factor-1 expression in normal gynecologic tissues and its potential significance. Int J Gynecol Pathol. 2009; 28:301–307.
78. McCluggage, WG, Young, RH. Tubulo-squamous polyp: a report of 10 cases of a distinctive hitherto uncharacterized vaginal polyp. Am J Surg Pathol. 2007; 31:1013–1019.
79. Kelly, P, McBride, HA, Kennedy, K, et al. Misplaced Skene’s glands: glandular elements in the cervix, vagina and vulva which are variably prostate marker positive and which encompass vaginal tubulosquamous polyp and cervical ectopic prostatic tissue. Int J Gynecol Pathol. 2011; 30:605–612.
80. Zynger, DL, McCallum, JC, Luan, C, et al. Glypican 3 has a higher sensitivity than alpha-fetoprotein for testicular and ovarian yolk sac tumour: immunohistochemical investigation with analysis of histological growth patterns. Histopathology. 2010; 56:750–757.
81. Maeda, D, Ota, S, Takazawa, Y, et al. Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod Pathol. 2009; 22:824–832.
82. Cao, D, Guo, S, Allan, RW, et al. SALL4 is a novel sensitive and specific marker of ovarian primitive germ cell tumors and is particularly useful in distinguishing yolk sac tumor from clear cell carcinoma. Am J Surg Pathol. 2009; 33:894–904.
83. Cao, D, Guo, S, Allan, RW, et al. Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors. Am J Surg Pathol.. 2009; 33:894–904.
84. Rabban, JT, Zaloudek, CJ, Shekitka, KM, Tavassoli, FA. Inflammatory myofibroblastic tumor of the uterus: a clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. Am J Surg Pathol. 2005; 29:1348–1355.
85. Richter, CE, Hui, P, Buza, N, et al. HER-2/NEU overexpression in vulvar Paget disease: the Yale experience. J Clin Pathol. 2010; 63:544–547.
86. Singh, P, Smith, CL, Cheetham, G, et al. Serous carcinoma of the uterus-determination of HER-2/neu status using immunohistochemistry, chromogenic in situ hybridization, and quantitative polymerase chain reaction techniques: its significance and clinical correlation. Int J Gynecol Cancer. 2008; 18:1344–1351.
87. Gilks, CB, McAlpine, J. Human epidermal growth factor 2 overexpression and amplification in mucinous tumours of ovary. Histopathology. 2011; 58:1173–1174.
88. McCluggage, WG. Ovarian neoplasms composed of small round cells. A review. Adv Anat Pathol. 2004; 11:288–296.
89. Al-Hussaini, M, Stockman, A, Foster, H, McCluggage, WG. WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing serous and ovarian endometrioid carcinoma. Histopathology. 2004; 44:109–115.
90. McCluggage, WG. WT1 is of value in ascertaining the site of origin of serous carcinomas within the female genital tract. Int J Gynecol Pathol. 2004; 23:97–99.
91. Logani, S, Oliva, E, Amin, MB, et al. Immunoprofile of ovarian tumors with putative transitional cell (urothelial) differentiation using novel urothelial markers: histogenetic and diagnostic implications. Am J Surg Pathol. 2003; 27:1434–1441.
92. Hirschowitz, L, Ganesan, R, McCluggage, WG. WT1, p53 and hormone receptor expression in uterine serous carcinoma. Histopathology. 2009; 55:478–482.
93. Sumathi, VP, Al-Hussaini, M, Connolly, LE, et al. Endometrial stromal neoplasms are immunoreactive with WT-1 antibody. Int J Gynecol Pathol. 2004; 23:241–247.
94. Patil, DT, Laskin, WB, Fetsch, JF, Miettinen, M. Inguinal smooth muscle tumors in women- a dichotomous group consisting of Mullerian-type leiomyomas and soft tissue leiomyosarcomas: an analysis of 55 cases. Am J Surg Pathol. 2011; 35:315–324.
95. Kobel, M, Reuss, A, Du Bois, A, et al. The biological and clinical value of p53 expression in pelvic high-grade serous carcinomas. J Pathol. 2010; 222:191–198.
96. Yemelyanova, A, Vang, R, Kshirsagar, M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011; 24:1248–1253.
97. Alkushi, A, Lim, P, Coldman, A, et al. Interpretation of p53 immunoreactivity in endometrial carcinoma: establishing a clinically relevant cut-off level. Int J Gynecol Pathol. 2004; 23:129–137.
98. O’Neill, CJ, McBride, HA, Connolly, LE, McCluggage, WG. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology. 2007; 50:851–858.
99. Russell, SE, McCluggage, WG. A multistep model for ovarian tumorigenesis: the value of mutation analysis in the KRAS and BRAF genes. J Pathol. 2004; 203:617–619.
100. O’Neill, CJ, Deavers, MT, Malpica, A, et al. An immmunohistochemical comparison between low grade and high grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, bcl2, HER-2/neu and C-KIT in high grade neoplasms. Am J Surg Pathol. 2005; 29:1034–1041.
101. Herrington, CS, McCluggage, WG. The emerging role of the distal fallopian tube and p53 in pelvic serous carcinogenesis. J Pathol. 2010; 220:5–6.
102. Lee, Y, Medeiros, F, Kindelberger, D, et al. Advances in the recognition of tubal intraepithelial carcinoma: applications to cancer screening and the pathogenesis of ovarian cancer. Adv Anat Pathol. 2006; 13:1–7.
103. Yang, B, Hart, WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: a clinicopathologic study including analysis of HPV and p53 expression. Am J Surg Pathol. 2000; 24:429–441.
104. Liegl, B, Regauer, S. p53 immunostaining in lichen sclerosus is related to ischaemic stress and is not a marker of differentiated vulvar intraepithelial neoplasia (d-VIN). Histopathology. 2006; 48:268–274.
105. Shih, IM, Kurman, RJ. p63 expression is useful in the distinction of epithelioid trophoblastic and placental site trophoblastic tumors by profiling trophoblastic subpopulations. Am J Surg Pathol. 2004; 28:1177–1183.
106. Wang, TY, Chen, BF, Yang, YC, et al. Histologic and immunophenotypic classification of cervical carcinomas by expression of the p53 homologue p63: a study of 250 cases. Hum Pathol. 2001; 32:479–486.
107. Liao, XY, Xue, WC, Shen, DH, et al. p63 expression in ovarian tumours: a marker for Brenner tumours but not transitional cell carcinomas. Histopathology. 2007; 51:477–483.
108. Bussaglia, E, del Rio, E, Matias-Guiu, X, Prat, J. PTEN mutations in endometrial carcinomas: a molecular and clinicopathologic analysis of 38 cases. Hum Pathol. 2000; 31:312–317.
109. Mutter, GL, Ince, TA, Baak, JP, et al. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res. 2001; 61:4311–4314.
110. Catasus, L, Bussaglia, E, Rodrguez, I, et al. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. 2004; 35:1360–1368.
111. Henderson, GS, Brown, KA, Perkins, SL, et al. bcl-2 is down-regulated in atypical endometrial hyperplasia and adenocarcinoma. Mod Pathol. 1996; 9:430–438.
112. Piek, JM, Verheijen, RH, Menko, FH, et al. Expression of differentiation and proliferation related proteins in epithelium of prophylactically removed ovaries from women with a hereditary female adnexal cancer predisposition. Histopathology. 2003; 43:26–32.
113. Cameron, RI, Maxwell, P, Jenkins, D, McCluggage, WG. Immunohistochemical staining with MIB-1, bcl2 and p16 assists in the distinction of cervical glandular intraepithelial neoplasia from tubo-endometrial metaplasia, endometriosis and microglandular hyperplasia. Histopathology. 2002; 41:313–321.
114. D’Angelo, E, Spagnoli, LG, Prat, J. Comparative clinicopathologic and immunohistochemical analysis of uterine sarcomas diagnosed using the World Health Organization classification system. Hum Pathol. 2009; 40:1571–1585.
115. Raspollini, MR, Amunni, G, Villanucci, A, et al. c-Kit expression in patients with uterine leiomyosarcomas: a potential alternative therapeutic treatment. Clin Cancer Res. 2004; 10:3500–3503.
116. Winter, WE, 3rd., Seidman, JD, Krivak, TC, et al. Clinicopathological analysis of c-kit expression in carcinosarcomas and leiomyosarcomas of the uterine corpus. Gynecol Oncol. 2003; 91:3–8.
117. Pirog, EC, Baergen, RN, Soslow, RA, et al. Diagnostic accuracy of cervical low-grade squamous intraepithelial lesions is improved with MIB1 immunostaining. Am J Surg Pathol. 2002; 26:70–75.
118. Logani, S, Cu, D, Quint, WGV, et al. Low-grade vulvar and vaginal intraepithelial neoplasia: correlation of histologic features with human papillomavirus DNA detection and MIB1 immunostaining. Mod Pathol. 2003; 16:735–741.
119. Kalof, AN, Evans, MF, Simmons-Arnold, L, et al. p16INK4A immunoexpression and HPV in situ hybridization signal patterns: potential markers of high-grade cervical intraepithelial neoplasia. Am J Surg Pathol. 2005; 29:674–679.
120. Klaes, R, Benner, A, Friedrich, T, et al. p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2002; 26:1389–1399.
121. Negri, G, Egarter-Vigi, E, Kasal, A, et al. p16 (INK4a) is a useful marker for the diagnosis of adenocarcinoma of the cervix uteri and its precursors. Am J Surg Pathol. 2003; 27:187–193.
122. McCluggage, WG, Jenkins, D. Immunohistochemical staining with p16 may assist in the distinction between endometrial and endocervical adenocarcinoma. Int J Gynecol Pathol. 2003; 22:231–235.
123. Chiesa-Vottero, AG, Malpica, A, Deavers, MT, et al. Immunohistochemical overexpression of p16 and p53 in uterine serous carcinoma and ovarian high-grade serous carcinoma. Int J Gynecol Pathol.. 2007; 26:328–333.
124. Elishaev, E, Gilks, CB, Miller, D, et al. Synchronous and metachronous endocervical and ovarian neoplasms: evidence supporting interpretation of the ovarian neoplasms as metastatic endocervical adenocarcinomas simulating primary ovarian surface epithelial neoplasms. Am J Surg Pathol. 2005; 29:281–294.
125. Quddus, MR, Xu, C, Steinhoff, MM, et al. Simplex (differentiated) type VIN: absence of p16INK4 supports its weak association with HPV and its probable precursor role in non-HPV related vulvar squamous cancers. Histopathology. 2005; 46:718–720.
126. Castrillon, DH, Sun, DQ, Weremowicz, S, et al. Discrimination of complete hydatidiform mole from its mimics by immunohistochemistry of the paternally imprinted gene product p57 (KIP2). Am J Surg Pathol. 2001; 25:1225–1230.
127. Genest, DR, Dorfman, DM, Castrillon, DH. Ploidy and imprinting in hydatidiform moles. Complementary use of flow cytometry and immunohistochemistry of the imprinted gene product p57 KIP2 to assist molar classification. J Reprod Med. 2002; 47:342–346.
128. Little, L, Stewart, CJ. Cyclin D1 immunoreactivity in normal endocervix and diagnostic value in reactive and neoplastic endocervical lesions. Mod Pathol. 2010; 23:611–618.
129. Stewart, CJ, Crook, ML, Little, L, Louwen, K. Correlation between invasive pattern and immunophenotypic alterations in endocervical adenocarcinoma. Histopathology. 2011; 58:720–728.
130. Stewart, CJ, Crook, ML, Leung, YC, Platten, M. Expression of cell cycle regulatory proteins in endometrial adenocarcinoma: variations in conventional tumor areas and in microcystic, elongated and fragmented glands. Mod Pathol. 2009; 22:725–733.
131. Mao, TL, Seidman, JD, Kurman, RJ, Shih, IEM. Cyclin E and p16 immunoreactivity in epithelioid trophoblastic tumor- an aid in differential diagnosis. Am J Surg Pathol. 2006; 30:1105–1110.
132. Pinto, AP, Schlecht, NF, Woo, TYC, et al. Biomarker (ProExC, p16, and MIB1) distinction of high-grade squamous intraepithelial lesion from its mimics. Mod Pathol. 2008; 21:1067–1074.
133. Aximu, D, Azad, A, Ni, R, et al. A pilot evaluation of a novel immunohistochemical assay for Topoisomerase II-α and minichromosome maintenance protein 2 expression (ProExC) in cervical adenocarcinoma in situ, adenocarcinoma, and benign glandular mimics. Int J Gynecol Pathol. 2009; 28:114–119.
134. Chen, H, Gonzalez, JL, Brennick, JB, et al. Immunohistochemical patterns of ProExC in vulvar squamous lesions. Am J Surg Pathol. 2010; 34:1250–1257.
135. McCluggage, WG, Patterson, A, Maxwell, P. Aggressive angiomyxoma of pelvic parts exhibits oestrogen and progesterone receptor positivity. J Clin Pathol. 2000; 53:603–605.
136. Fine, BA, Muñoz, AK, Litz, CE, Gershenson, DM. Primary medical management of recurrent aggressive angiomyxoma of the vulva with a gonadotropin-releasing hormone agonist. Gynecol Oncol. 2001; 81:120–122.
137. Staebler, A, Sherman, ME, Zaino, RJ, et al. Hormone receptor immunohistochemistry and human papillomavirus in situ hybridisation are useful for distinguishing endocervical and endometrial adenocarcinomas. Am J Surg Pathol. 2002; 26:998–1006.
138. Moinfar, F, Regitnig, P, Tabrizi, AD, et al. Expression of androgen receptors in benign and malignant endometrial stromal neoplasms. Virchows Arch. 2004; 444:410–414.
139. Loddenkemper, C, Mechsner, S, Foss, H-D, et al. Use of oxytocin receptor expression in distinguishing between uterine smooth muscle tumors and endometrial stromal sarcoma. Am J Surg Pathol. 2003; 27:1458–1462.
140. Logani, S, Oliva, E, Arnell, PM, et al. Use of novel immunohistochemical markers expressed in colonic adenocarcinoma to distinguish primary ovarian tumors from metastatic colorectal carcinoma. Mod Pathol. 2005; 18:19–25.
141. Jung, C-K, Jung, J-H, Lee, A, et al. Diagnostic use of β-catenin expression for the assessment of endometrial stromal tumors. Mod Pathol. 2008; 21:756–763.
142. Ohishi, Y, Oda, Y, Kurihara, S, et al. Nuclear localization of E-cadherin but not beta-catenin in human ovarian granulosa cell tumours and normal ovarian follicles and ovarian stroma. Histopathology. 2011; 58:423–432.
143. Laury, AR, Perets, R, Piao, H, et al. A Comprehensive Analysis of PAX8 Expression in Human Epithelial Tumors. Am J Surg Pathol. 2011; 35:816–826.
144. Nonaka, D, Chirboga, L, Soslow, RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008; 32:1566–1571.
145. Laury, AR, Hornick, JL, Perets, R. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am J Surg Pathol. 2010; 34:627–635.
146. Rabban, JT, McAlhany, S, Lerwill, MF, et al. PAX2 distinguishes benign mesonephric and mullerian glandular lesions of the cervix from endocervical adenocarcinoma, including minimal deviation adenocarcinoma. Am J Surg Pathol. 2010; 34:137–146.
147. Monte, NM, Webster, KA, Neuberg, D, et al. Joint loss of PAX2 and PTEN expression in endometrial precancers and cancer. Cancer Res. 2010; 70:6225–6232.
148. McCluggage, WG, Connolly, L, McBride, HA. HMGA2 is a sensitive but not specific immunohistochemical marker of vulvovaginal aggressive angiomyxoma. Am J Surg Pathol. 2010; 34:1037–1042.
149. McCluggage, WG, Connolly, LE, McBride, HA, et al. HMGA2 is commonly positive in uterine serous carcinomas and is a useful adjunct to diagnosis. Histopathology. 2012; 60:547–553.
150. Wei, JJ, Wu, J, Luan, C, et al. HMGA2: a potential biomarker complement to p53 for detection of early-stage high-grade papillary serous carcinoma in fallopian tubes. Am J Surg Pathol. 2010; 34:18–26.
151. Yamamoto, S, Tsuda, H, Aida, S, et al. Immunohistochemical detection of hepatocyte nuclear factor 1beta in ovarian and endometrial clear-cell adenocarcinomas and nonneoplastic endometrium. Hum Pathol. 2007; 38:1074–1080.
152. Shah, SP, Kobel, M, Senz, J, et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med. 2009; 360:2719–2729.
153. Al-Agha, OM, Huwait, HF, Chow, C, et al. FOXL2 is a sensitive and specific marker for sex cord-stromal tumors of the ovary. Am J Surg Pathol. 2011; 35:484–494.
154. Zheng, W, Yi, X, Fadare, O, et al. The oncofetal protein IMP3: a novel biomarker for endometrial serous carcinoma. Am J Surg Pathol. 2008; 32:304–315.
155. Zhang, L, Liu, Y, Hao, S, et al. IMP2 expression distinguishes endometrioid from serous endometrial adenocarcinomas. Am J Surg Pathol. 2011; 35:868–872.