Chapter 45 Practical Aspects of Hematologic Stem Cell Harvesting and Mobilization
Choice of Hematologic Stem Cell Product for Transplantation
Table 45-1 Randomized Studies Comparing Marrow and PBSC as HSC Sources*
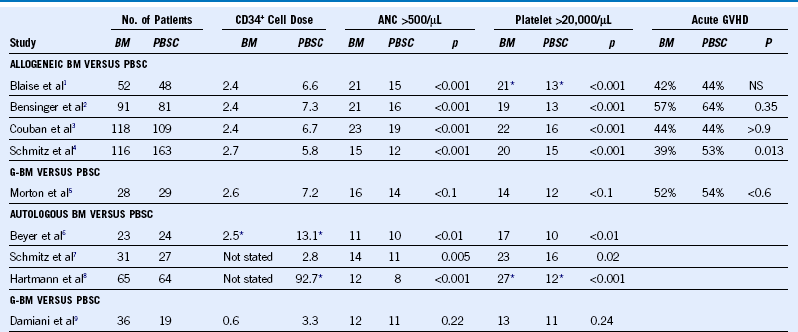
ANC, Absolute neutrophil count; BM, bone marrow, G-BM, granulocyte colony-stimulated bone marrow; NS, not significant; PBSC, peripheral blood stem cell.
Evaluation of the Marrow or Peripheral Blood Stem Cell Donor
• Education of donor, including education regarding procedures, risks, alternatives, and possible future collections
• Medical history, including special attention to history of autoimmune disorders, arthritis, cardiac and vascular diseases, and history of cancer
• History of high-risk behaviors, such as recent tattoos, body piercing, sexual practices, and travel
• Physical examination, including vein assessment (PBSC donors) and oral examination (marrow donors undergoing inhalational anesthesia)
• Laboratory studies, including verification HLA typing, ABO typing, CBC, chemistry panel, infectious disease panel, urinalysis, ECG, CXR
• Consent for collection procedures and the release of protected health information to the stem cell recipient
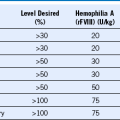
Table 45-2 Relationship Among Mobilization Therapy, Dose of Progenitor Cells, and Engraftment Kinetics for Autologous Peripheral Blood Stem Cell Transplantation
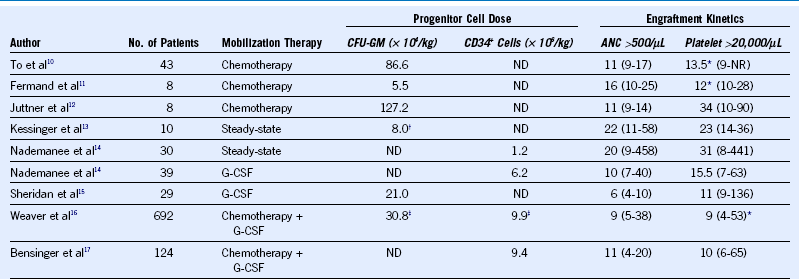
Shown are mean values for progenitor cell quantities infused and median times to achieve the particular endpoint of engraftment. ANC, Absolute neutrophil count; G-CSF, granulocyte colony-stimulating factor; ND, not measured; NR, not reached.
* Time to achieve >50,000 platelets/µL
† Colony-forming unit granulocyte-macrophage (CFU-GM) cultures performed on thawed cells.
Mobilization and Collection of Peripheral Blood Stem Cell for Autologous Transplantation
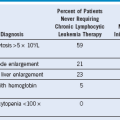
Table 45-3 Incidence of Somatic Complaints for Normal Donors Treated With Granulocyte Colony-Stimulating Factor
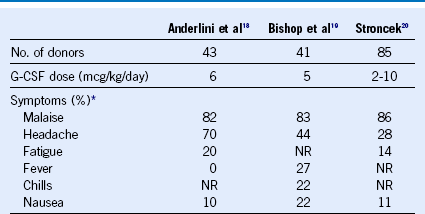
G-CSF, Granulocyte colony-stimulating factor; NR, particular complaint not reported in this series.
* Shown are reported proportions of donors experiencing the listed somatic complaint.
1 Beyer J, Schwella N, Zingsem J, et al. Hematopoietic rescue after highdose chemotherapy using autologous peripheral-blood progenitor cells or bone marrow: A randomized comparison. J Clin Oncol. 1995;13:1328.
2 Schmitz N, Linch DC, Dreger P, et al. Randomised trial of filgrastimmobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353.
3 Hartmann O, Le Corroller AG, Blaise D, et al. Peripheral blood stem cell and bone marrow transplantation for solid tumors and lymphomas: Hematologic recovery and costs. A randomized, controlled trial. Ann Intern Med. 1997;126:600.
4 Blaise D, Kuentz M, Fortanier C, et al. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: A report from the Societe Francaise de Greffe de Moelle. J Clin Oncol. 2000;18:537.
5 Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175.
6 Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525.
7 Schmitz N, Beksac M, Hasenclever D, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100:761.
8 Morton J, Hutchins C, Durrant S. Granulocyte-colony-stimulating factor (G-CSF)-primed allogeneic bone marrow: Significantly less graftversus-host disease and comparable engraftment to G-CSF-mobilized peripheral blood stem cells. Blood. 2001;98:3186.
9 Damiani D, Fanin R, Silvestri F, et al. Randomized trial of autologous filgrastim-primed bone marrow transplantation versus filgrastimmobilized peripheral blood stem cell transplantation in lymphoma patients. Blood. 1997;90:36.
10 To LB, Roberts MM, Haylock DN, et al. Comparison of haematological recovery times and supportive care requirements of autologous recovery phase peripheral blood stem cell transplants, autologous bone marrow transplants and allogeneic bone marrow transplants. Bone Marrow Transplant. 1992;9:277.
11 Fermand J-P, Levy Y, Gerota J, et al. Treatment of aggressive multiple myeloma by high-dose chemotherapy and total body irradiation followed by blood stem cells autologous graft. Blood. 1989;73:20.
12 Juttner CA, To LB, Ho JQK, et al. Early lympho-hemopoietic recovery after autografting using peripheral blood stem cells in acute nonlymphoblastic leukemia. Transplant Proc. 1988;20:40.
13 Kessinger A, Armitage JO, Landmark JD, et al. Autologous peripheral hematopoietic stem cell transplantation restores hematopoietic function following marrow ablative therapy. Blood. 1988;71:723.
14 Nadamanee A, Sniecinski I, Schmidt GM, et al. High-dose therapy followed by autologous peripheral-blood stem-cell transplantation for patients with Hodgkin’s disease and non-Hodgkin’s lymphoma using unprimed and granulocyte colony-stimulating factor-mobilized peripheral-blood stem cells. J Clin Oncol. 1994;12:2176.
15 Sheridan WP, Begley CG, To LB, et al. Phase II study of autologous filgrastim (G-CSF)-mobilized peripheral blood progenitor cells to restore hemopoiesis after high-dose chemotherapy for lymphoid malignancies. Bone Marrow Transplant. 1994;14:105.
16 Weaver CH, Hazelton B, Birch R, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961.
17 Bensinger W, Appelbaum F, Rowley S, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547.
18 Anderlini P, Przepiorka D, Seong D, et al. Clinical toxicity and laboratory effects of granulocyte-colony-stimulating factor (filgrastim) mobilization and blood stem cell apheresis from normal donors, and analysis of charges for the procedure. Transfusion. 1996;36:590.
19 Bishop MR, Tarantolo SR, Jackson JD, et al. Allogeneic-blood stem-cell collection following mobilization with low-dose granulocyte colonystimulating factor. J Clin Oncol. 1997;15:1601.
20 Stroncek DF, Clay ME, Petzoldt ML, et al. Treatment of normal individuals with granulocyte-colony stimulating factor: Donor experiences and the effects on peripheral blood CD34+ cell counts and on the collection of peripheral blood stem cells. Transfusion. 1996;36:601.