Chapter 134 Posttraumatic and Idiopathic Syringomyelia
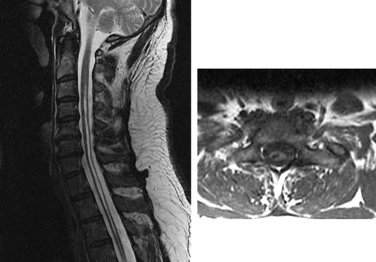
Syringomyelia denotes a collection of heterogeneous pathologic conditions characterized by abnormal, longitudinally oriented, fluid-filled cavities within the spinal cord (Fig. 134-1). Syringomyelia may be the result of congenital, traumatic, or neoplastic processes and therapy is directed at correcting the underlying pathology. However, in many cases the etiology of the condition remains unknown, and treatment results are usually unsatisfactory.1 This chapter offers guidance for diagnosis and management of patients with posttraumatic and idiopathic syringomyelia and provides an update on current research.
Although Charles Estienne first described the condition in 1546, the term syringomyelia was suggested by Olivier d’Angers in 1827 from the Greek syrinx, meaning “pipe,” “tube,” or “channel,” and myelos, meaning “marrow.”2,3 The term is restricted to this condition and should not be used to describe similar entities such as proteinaceous cysts or a terminal ventricle. The term hydromyelia refers to cystic dilation of the ependyma-lined central canal by cerebrospinal fluid (CSF; Fig. 134-2). However, the syrinx may dissect into the parenchyma of the spinal cord and its original connection with the central canal may disappear. Furthermore, the type of cellular lining is not a reliable criterion to distinguish between syringomyelia and hydromyelia. Because of these difficulties, the term syringohydromyelia has been used in the literature to refer to both entities.4–7 In this chapter, syringomyelia is used to denote all abnormal fluid-filled cavities in the spinal cord.8
Epidemiology
Syringomyelia affects primarily children and young adults, presenting on average before the third decade of life. Before the widespread availability of MRI, a prevalence of 9 per 100,000 and an incidence of approximately 0.44 cases per year were cited in the literature.9–13 Approximately 50% of patients presenting with syringomyelia have a Chiari malformation, whereas 25% present with a history of spinal cord trauma or arachnoiditis.9 The incidence of syringomyelia appears to be similar after quadriplegia or paraplegia. There is a statistically significant male preponderance in posttraumatic syringomyelia (80%), which follows the gender distribution in spinal cord injury.6,10,12 Of patients with a spinal cord injury investigated between 1 and 30 years after the initial insult, 21% to 28% have a syrinx and 30% to 50% have some degree of spinal cystic change. However, symptomatic syringomyelia is reported in only 1% to 9% of the spinal cord injury population.8,14–18
Classification
A distinction based on pathologic and MRI findings is made between communicating and noncommunicating syringomyelia.19–21 Communicating syrinx cavities are central canal dilations in continuity with the fourth ventricle and are often associated with hydrocephalus. They occur in children and young adults with conditions that obstruct CSF outflow from the fourth ventricle, such as Chiari II malformations or Dandy-Walker cysts. Only 10% of the lesions are of the communicating type.19
Noncommunicating syringomyelia can be further classified into central canal and extracanalicular syringomyelia.19,20 Noncommunicating central canal syrinx cavities are associated with Chiari I malformations, cervical spinal stenosis, spinal arachnoiditis, and basilar impression. The cavities are dilations of the central canal and are partially or completely lined by ependymal cells. Noncommunicating extracanalicular syrinx cavities are associated with spinal trauma, infarction, hemorrhage, or transverse myelitis. Most extracanalicular syrinx cavities are found in the vascular watershed zones of the central and dorsolateral gray matter. Extracanalicular syrinx cavities are irregular in shape and commonly have microglia, hemosiderin-containing macrophages, and gliosis of the cyst wall.20 In addition, the perivascular spaces may be enlarged and direct communication with the subarachnoid space at the dorsal nerve root entry zones or the ventromedian fissure can occur.20 Posttraumatic syrinx cavities are usually near the injury site and extend rostrally in 81%, caudally in 4%, and in both directions in 15% of cases.8,20,21
Etiology
The exact pathophysiologic process underlying posttraumatic syrinx formation is not clear, and various theories have been advanced. Two popular explanations are the hydrodynamic theory and a theory that assumes a differential between intracranial and spinal pressure caused by a valvelike effect at the foramen magnum.8 The second of these theories does not adequately account for the occurrence of noncommunicating syrinx cavities. The hydrodynamic theory proposes that fluid flows into the central canal from the fourth ventricle because of a “water-hammer–like” transmission of arterial or respiratory pressure. These theories have concentrated on canalicular syringomyelia associated with Chiari malformations, frequently at the expense of ignoring posttraumatic cases.22–24
Historically, the explanation for syrinx cavities isolated from the fourth ventricle was a secondary obstruction of the rostral central canal. However, this conclusion is difficult to reconcile with the observation that the central canal in humans is obstructed by segmental occlusions by the end of the third decade of life, and that there is a clear anatomic distinction between the central canal and syrinx cavity in most cases of posttraumatic syringomyelia.19,20,25 The initial formation of a spinal cord cavity may involve different mechanisms than the subsequent enlargement of the cavity to form a syrinx. Inflammatory responses to traumatic injury in the central nervous system result in localized edema and may lead to cyst formation.26–28
It has been reported that an intramedullary hematoma after spinal cord trauma increases the likelihood of developing a syrinx.6 Natural dissolution and absorption of the hematoma can leave a cystic cavity that can predispose the patient to syrinx formation. Furthermore, posttraumatic syrinx cavities are usually found in vascular watershed regions in the spinal cord.6,19 This finding implicates ischemia during the primary injury or subsequent inflammatory response as a contributor to syrinx formation.6,8,19
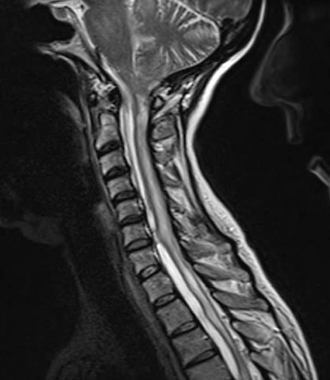
Spinal cord injury results in multiple pathologic conditions that can disturb CSF flow and potentially contribute to syrinx enlargement. Subarachnoid adhesions, spinal deformity, or stenosis is often seen in patients with spinal cord injury who subsequently develop syringomyelia. It has been shown that the presence of uncorrected kyphosis correlates with syrinx formation and symptom severity and appears to correlate with disruption of CSF flow in the subarachnoid space17,18,29–31 (Fig. 134-3). The pathophysiology of posttraumatic syringomyelia remains uncertain, but ongoing work with MRI and mathematical modeling supported by studies in animal models continues to shed light on this debilitating condition.
Clinical Presentation
Posttraumatic syringomyelia has been reported to develop anywhere between 3 months and 34 years after the initial injury to the spinal cord.14,20,21,29,32 Although sudden neurologic decline due to hemorrhage into a syrinx cavity has been described, symptomatic progression is usually gradual.6 Common initial symptoms include segmental pain and sensory loss. The pain is usually dull or burning, indicating injury to the spinothalamic tracts,6,29 and is usually at or above the level of injury. It may be exacerbated by coughing, sneezing, straining, or sitting.6,29 Characteristic dissociated pain and temperature sensation loss with preservation of light touch and proprioception in ascending segments are more common than is complete loss of sensation.6,29 After the onset of sensory symptoms, patients generally report a gradual loss of motor function above the level of previous injury that is characterized by progressive asymmetrical weakness. Associated symptoms may include hyperhidrosis, autonomic dysreflexia, Horner syndrome, cardiorespiratory dysfunction, and an asymmetrical reduction in reflexes.6,29,32 In rare cases, the syrinx cavity can extend rostrally into the brainstem and result in bulbar symptoms.8
Diagnosis
Syringomyelia was difficult to diagnose before the advent of modern neuroimaging. CT is at best unreliable for diagnostic purposes because of signal degradation by surrounding bony elements, and CT myelography misses approximately 50% of syrinx cavities.33,34 MRI is the imaging modality of choice for the diagnosis of posttraumatic syringomyelia, and demonstration of a syrinx cavity in the spinal cord should prompt an MRI examination of the entire neuraxis.33–35 However, MRI scans rarely enable differentiation of hydromyelia and syringomyelia because both entities are characterized on MRI by the presence of a longitudinally oriented fluid collection in the spinal cord. Congenital or posttraumatic syringohydromyelia does not show contrast enhancement.33–35 Phase-contrast cine MRI may accurately localize subarachnoid space obstruction and demonstrate normalization of CSF flow after surgery, and may also be used to help confirm spinal cord tethering and communication of spinal cord cysts with the subarachnoid space.8,33–35
Management
Posttraumatic syringomyelia continues to be a difficult condition to manage because it is characterized by slow clinical progression, and the syrinx cavities may exist for years without becoming symptomatic.8 Despite reports of neurologic recovery after surgery, in up to 80% of cases 5- and 10-year follow-up studies demonstrate stabilization of symptoms only regardless of the mode of treatment or degree of radiologic improvement achieved.36 The goal of surgical management is to correct the assumed underlying mechanism. Surgical options include correction of deformity, alleviation of compression, shunting procedures, arachnolysis with or without duraplasty, and cord transection. When feasible, correction of a deformity or compressive lesion is usually preferable to a shunting procedure.18 Syrinx cavities usually shrink after decompression of the spinal cord or correction of deformity. This has the added benefit of avoiding intradural surgery and, hence, postoperative arachnoid scarring.18 Shunting of the syrinx to the subarachnoid space should be performed only in cases where these surgical measures fail or are not an option. Many authors advocate surgical treatment only for patients with progressive neurologic decline or extreme pain.6,30,37,38 In patients managed nonoperatively, close clinical observation for progressive neurologic deficits is essential. Serial MRI studies for anatomic progression of the syrinx at regular intervals should be considered.
Gardner W.J., Angel J. The cause of syringomyelia and its surgical treatment. Cleve Clin Q. 1958;25:4-8.
Milhorat T.H., Johnson R.W., Milhorat R.H., et al. Clinicopathological correlations in syringomyelia using axial magnetic resonance imaging. Neurosurgery. 1995;37:206-213.
Oldfield E.H., Muraszko K., Shawker T.H., et al. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils: implications for diagnosis and treatment. J Neurosurg. 1994;80:3-15.
Perrouin-Verbe B., Lenne-Aurier K., Robert R., et al. Post-traumatic syringomyelia and post-traumatic spinal canal stenosis: a direct relationship: review of 75 patients with a spinal cord injury. Spinal Cord. 1998;36:137-143.
Sgouros S., Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1-10.
Tator C.H., Fehlings M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15-26.
1. Lotbiniere A.C.J. Historical considerations. In: The AANS Publications Committee, Anson J.A., Benzel E.C., Awad I.A. Syringomyelia and the Chiari malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1997:1-26.
2. Estienne C. La dissection des parties du corps humain divisée en trois livres. Paris: Simon de Collines; 1546. p 405
3. Olivier d’Angers C.P. Traité des maladies de la moelle épinière et de ses maladies: contenant l’histoire anatomique, physiologique et pathologique de ce centre nerveux chez l’homme, ed 2. Paris: Méquignon-Marvis père et fils; 1827. pp 178–183
4. Hoffman H.J., Neill J., Crone K.R., et al. Hydrosyringomyelia and its management in childhood. Neurosurgery. 1987;21:347-351.
5. Ballentine H.T., Ojemann R.G., Drew J.H. Syringohydromyelia. Prog Neurol Surg. 1971;4:227-245.
6. Edgar R., Quail P. Progressive post-traumatic cystic and non-cystic myelopathy. Br J Neurosurg. 1994;8:7-22.
7. Barnett H.J.M., Botterell E.H., Jousse A.T., et al. Progressive myelopathy as a sequel to traumatic paraplegia. Brain. 1966;89:159-174.
8. Brodbelt A.R., Stoodley M.A. Post-traumatic syringomyelia: a review. J Clin Neurosci. 2003;10:401-408.
9. Moriwaka F., Tashiro K., Tachibana S., et al. Epidemiology of syringomyelia in Japan: the nationwide survey. Rinsho Shinkeigaku. 1995;35:1395-1397.
10. Ferrero Arias J., Pilo Martin I. Prevalence of several neurological diseases in the central provinces of the Iberian Peninsula in eighteen-year-old males. Neurologia. 1991;6:89-94.
11. Brewis M., Poskanzer D.C., Rolland C., et al. Neurological disease in an English city. Acta Neurol Scand. 1966;42:1-89.
12. Hertel G., Ricker K. A geomedical study on the distribution of syringomyelia in Germany [in German]. In: den Hartog Jager W.A., Bruyn A.P., Heijstee A.P.J., editors. Neurology: proceedings of the 11th World Congress of Neurology. Amsterdam: Excerpta Medica; 1978:353-365.
13. Gudmundsson K.R. The prevalence of some neurological diseases in Iceland. Acta Neurol Scand. 1968;44:57-69.
14. Backe H.A., Betz R.R., Mesgarzadeh M., et al. Post-traumatic spinal cord cysts evaluated by magnetic resonance imaging. Paraplegia. 1991;29:607-612.
15. Williams B. Pathogenesis of post-traumatic syringomyelia. Br J Neurosurg. 1992;6:517-520.
16. Wang D., Bodley R., Sett P., et al. A clinical magnetic resonance imaging study of the traumatised spinal cord more than 20 years following injury. Paraplegia. 1996;34:65-81.
17. Perrouin-Verbe B., Lenne-Aurier K., Robert R., et al. Post-traumatic syringomyelia and post-traumatic spinal canal stenosis: a direct relationship: review of 75 patients with a spinal cord injury. Spinal Cord. 1998;36:137-143.
18. Abel R., Gerner H.J., Smit C., et al. Residual deformity of the spinal canal in patients with traumatic paraplegia and secondary changes of the spinal cord. Spinal Cord. 1999;37:14-19.
19. Milhorat T.H., Capocelli A.L.Jr., Anzil A.P., et al. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg. 1995;82:802-812.
20. Milhorat T.H. Classification of syringomyelia. Neurosurg Focus. 2000;8:1-6.
21. Milhorat T.H., Johnson R.W., Milhorat R.H., et al. Clinicopathological correlations in syringomyelia using axial magnetic resonance imaging. Neurosurgery. 1995;37:206-213.
22. Oldfield E.H., Muraszko K., Shawker T.H., et al. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils: implications for diagnosis and treatment. J Neurosurg. 1994;80:3-15.
23. Gardner W.J., Angel J. The cause of syringomyelia and its surgical treatment. Cleve Clin Q. 1958;25:4-8.
24. Williams B. Pathogenesis of syringomyelia. Lancet. 1972;2:969-970.
25. Milhorat T.H., Kotzen R.M., Anzil A.P. Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J Neurosurg. 1994;80:716-722.
26. Tator C.H., Fehlings M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15-26.
27. Guizar-Sahagun G., Grijalva I., Madrazo I., et al. Development of post-traumatic cysts in the spinal cord of rats-subjected to severe spinal cord contusion. Surg Neurol. 1994;41:241-249.
28. Fischbein N.J., Dillon W.P., Cobbs C., et al. The ‘‘presyrinx’’ state: a reversible myelopathic condition that may precede syringomyelia. AJNR Am J Neuroradiol. 1999;20:7-20.
29. Schurch B., Wichmann W., Rossier A.B. Post-traumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. J Neurol Neurosurg Psychiatry. 1996;60:61-67.
30. Schaller B., Mindermann T., Gratzl O. Treatment of syringomyelia after posttraumatic paraparesis or tetraparesis. J Spinal Disord. 1999;12:485-488.
31. Klekamp J., Batzdorf U., Samii M., et al. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86:233-240.
32. Rossier A.B., Foo D., Shillito J., et al. Posttraumatic cervical syringomyelia: incidence, clinical presentation, electrophysiological studies, syrinx protein and results of conservative and operative treatment. Brain. 1985;108:439-461.
33. Schenk M., Ruggieri P.M. Imaging of syringomyelia and the Chiari malformations. In: The AANS Publications Committee, Anson J.A., Benzel E.C., Awad I.A. Syringomyelia and the Chiari malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1997:41-56.
34. Davis C.H., Symon L. Mechanisms and treatment in post-traumatic syringomyelia. Br J Neurosurg. 1989;3:669-674.
35. Schwartz E.D., Falcone S.F., Quencer R.M., et al. Posttraumatic syringomyelia: pathogenesis, imaging, and treatment. AJR Am J Roentgenol. 1999;173:487-492.
36. Sgouros S., Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1-10.
37. El Masry W.S., Biyani A. Incidence, management, and outcome of post-traumatic syringomyelia. Neurosurg Psychiatry. 1996;60:141-146.
38. Hida K., Iwasaki Y., Imamura H., et al. Posttraumatic syringomyelia: its characteristic magnetic resonance imaging findings and surgical management. Neurosurgery. 1994;35:886-891.