Chapter 15 Postthrombotic Syndrome
Historical Background
Postthrombotic syndrome (PTS) remains an important health care problem in United States. A population-based study showed that the incidence of venous ulcers currently approaches 18 per 100,000 habitants per year.1 The same study identified that PTS is responsible for economic expenses estimated at least $200 million.1
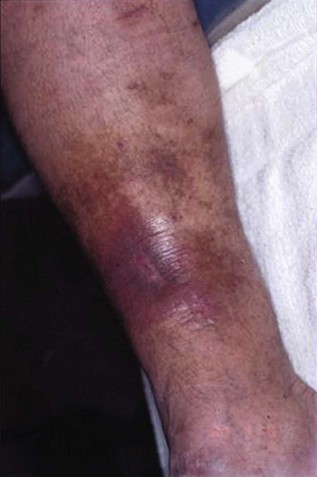
Diagnosis of PTS is based on history and clinical presentation. The syndrome consists of signs and symptoms of heaviness, intolerance to exercises, pain, leg edema, paresthesia, cramps, pruritus that may evolve to skin damage such as hyperpigmentation, lipodermatosclerosis, and ulcers (Fig. 15-1). The severity of PTS is measured by different scales and scores assigning points for the presence of each sign and symptom. Although there is a good association with PTS severity, further refinement and validation are necessary.2–5
Attention to differential diagnosis should be outlined. Trauma, congenital venous disease (i.e., venous malformations, valve aplasia) and other causes of ulcerative disease such as rheumatologic (lupus, scleroderma, rheumatoid arthritis), oncologic (squamous and basal cell carcinoma), or infectious disorders (syphilis, lymphangitis) are potential causes of misdiagnosis and inappropriate treatment.6,7 Table 15-1 shows the location and cause of non–venous-related ulcers.
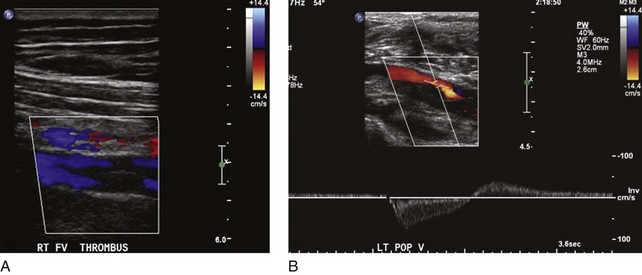
Not all patients who have had a documented episode of DVT sustain PTS. Recovery with no signs or symptoms of PTS occurs in two-thirds of the patients, and only the rest will eventually develop PTS with variable spectrum and severity.8 It appears that other factors are involved in the disease process, rendering some patients more vulnerable than others to PTS changes. Predisposing factors for PTS are age, sex, hypercoagulable state, obesity, immobility, and, most important, ipsilateral recurrent DVT. The latter is deemed to be the strongest factor for PTS, as several prospective studies have shown high odds for skin damage.9,10 Patients who had unprovoked DVT and are older than 65 years have a higher risk of recurrent DVT and therefore PTS.11 Residual thrombus was also reported as a cause of recurrent DVT12 (Fig. 15-2). In addition, patients who had DVT in more than one site, popliteal valve insufficiency, or a calf DVT associated with a proximal DVT also have an increased risk of recurrent DVT.13–15 Notably, the recurrence of DVT is likely to affect the proximal veins and is related to inadequate duration of anticoagulation.9 In a recent review, the limitations of studies on recurrent DVT are discussed.16 Most of the studies used a nonstandardized ultrasound analysis, giving limited or inaccurate information on the incidence of fatal pulmonary embolism (PE) as well as having poor documentation of anticoagulation and its monitoring. The effects of thrombolytic therapy and its socioeconomic and quality of life impact are also questions that remain unanswered.16 These are important because ipsilateral recurrent DVT has a great impact on the development of PTS.
Biomarkers have been used to estimate the odds of developing PTS. In a recent study of 305 patients with PTS, persistent elevated levels of D-dimer in the course of DVT were investigated. Patients with elevated levels of D-dimer at 4 months following DVT after stopping anticoagulation showed a fourfold risk of PTS.17
Progression of secondary cardiovascular disease (CVD) evolving to PTS has been demonstrated. A study of 73 limbs with secondary CVD showed overall progression in clinical CEAP classes in 31% of the limbs. Skin damage (C4-C6) rate strikingly rose from 4% at the first year to 25% in the 5-year follow-up. Other studies also investigated secondary CVD and clinical course of PTS.12 In an Italian cohort of 355 patients, the incidence of PTS increased from 17% after the first year to 29% at 8 years of follow-up.12 Development of reflux alone has been associated with PTS.10–17 However, a combination of reflux and obstruction is associated with more severe PTS than reflux or obstruction alone.10
Patients with a hypercoagulable state such as carriers of factor V Lieden (FVL), prothrombin gene mutation, or proteins C and S have been investigated for occurrence of PTS. In a recent study, 667 patients with DVT who sustained hypercoagulable state demonstrated no association with increased risk of PTS. Furthermore, heterozygosis for FVL was even less associated with PTS than in noncarriers.18 Another study of 387 patients tested for thrombophilia found no increased risk of PTS in carriers of FVL or prothrombin gene mutation.15 The same study also showed that the intensity of persistent signs and symptoms in the first month after the episode of acute DVT predicted the incidence of PTS in a dose-dependent manner in the first 2 years.15
Nonoperative Treatment
PTS generates high expenses for government and insurance companies due to disability premiums, loss of labor force, and need for rehabilitation and wound care. Thus, prevention should be always contemplated to minimize the socioeconomic impact of the disease. DVT prophylaxis is mandatory in different settings such as major surgeries, critically ill patients, and those with significant risk factors for DVT.19
Multiple reports have shown that elastic compression applied in patients with acute DVT may decrease the incidence of PTS by 50% at long term.20,21 This was evident in two review papers that analyzed all the relevant prospective studies.22,23 However, most of these trials had an inadequate sample size to resolve this conclusively. Currently, there is an ongoing randomized multicentre trial (SOX trial) that will provide data on the effectiveness of compression stockings on the prevention and severity of PTS, VTE recurrence, quality of life, and cost-effectiveness.24 It will also prospectively evaluate the predictive role of biomarkers in the development of PTS.
In patients with established PTS, a few treatments are available. Initial treatment for all patients with PTS is nonoperative, with compression to the legs in different levels and intensity. The use of compression stockings in 387 patients could not predict the progression or lack of PTS. However, patients who did use compression stockings had a risk of 20% of developing PTS compared with 47% who did not use compression stockings.12 The Cochrane collaboration review of 39 randomized control trials concluded that compression was more effective than no compression on ulcer healing.25 It was also shown that multicomponent systems were more effective in ulcer healing compared with single-component systems.
The shortcoming of compression therapy is that up to 20% of patients are noncompliant, and compliance with stockings does not change regardless of the symptoms of PTS.26 Complementary to the compression therapy, wound care is important to improve ulcer healing rates and patients’ quality of life. Many techniques have been applied in different settings varying from local care to more extensive surgical debridement.
More recently, in a small prospective randomized study of patients with PTS, exercise training for 6 months was associated with improvement in quality of life and improvement in scores on the Villalta scale.27 The authors concluded that these results should be confirmed in a large prospective randomized trial.
Interventional Treatment
Surgical and endovascular procedures are indicated when deep venous reflux or obstruction is present with or without superficial vein involvement in patients with skin damage or disabling symptoms. Prior to the endovenous techniques, obstruction used to be treated with bypass grafts. More recently, chronic venous obstruction is treated with balloon angioplasty and stenting. Bypass grafting is reserved for cases in which the endovenous approach is unsuccessful. However, the endovenous treatment for chronic venous obstruction is discussed extensively in Chapter 14. This chapter concentrates on the bypass grafting.
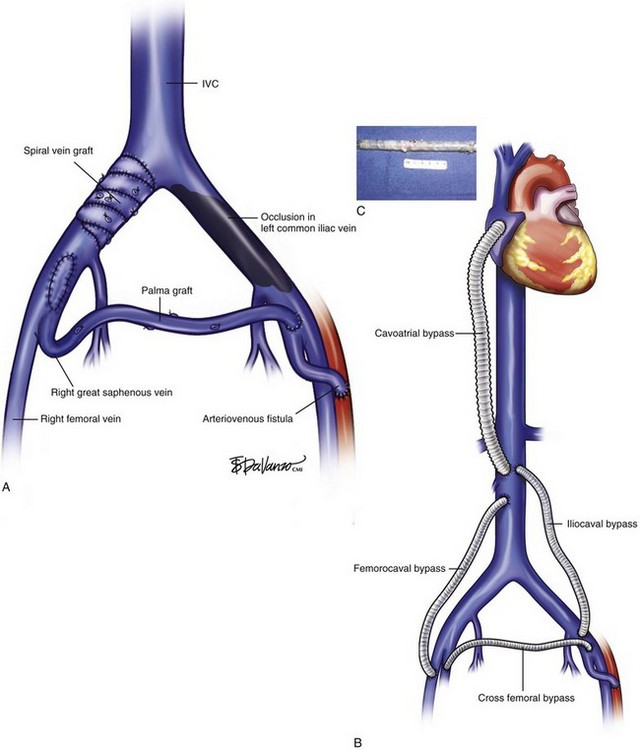
The treatment of proximal occlusion, including iliocaval segment, differs from that for femoropopliteal thrombotic occlusive disease. The higher velocity, significant blood volume, and better outflow of common femoral, iliac veins, and inferior vena cava (IVC) create a more amenable setting for treatment with either vein or prosthetic bypass grafts. A study of 44 venous reconstructions for nonmalignant iliofemoral and IVC occlusion was conducted at the Mayo Clinic.28 A wide variety of possible options for IVC and iliofemoral segment reconstruction were used, including great saphenous vein (GSV) crossover grafts (Palma procedure), supported expanded polytetrafluoroethylene (ePTFE), spiral vein grafts, and femoral vein patch angioplasty (Fig. 15-3). The overall primary and secondary patency at 3 years was 54% and 62%, respectively. Conversely, lower primary and secondary patency rates were found for iliocaval and femorocaval bypasses when analyzed separately, reaching only 38% and 54% at 2-year follow-up. The lower patency found in those bypasses was deemed to be related to the ePTFE bypass grafts. A small number of procedures failed to show statistical significance, although a trend toward higher patency rates was shown for GSV bypass grafts. Highest patency was also achieved with GSV crossover grafts, reaching 77% in a 4-year period, similar to other reports in the literature.28,29
Several open surgical techniques have been used to correct venous reflux reconstructing the valves. The main procedures described are internal or external (transcommissural) valvuloplasty, axillary vein transfer, vein transplantation, or valve transplantation.26,30,31 Overall, the results vary based on the technique used, the surgeon’s expertise, and postprocedure care. A few specialized centers have been currently conducting open venous reconstruction or valvuloplasty.
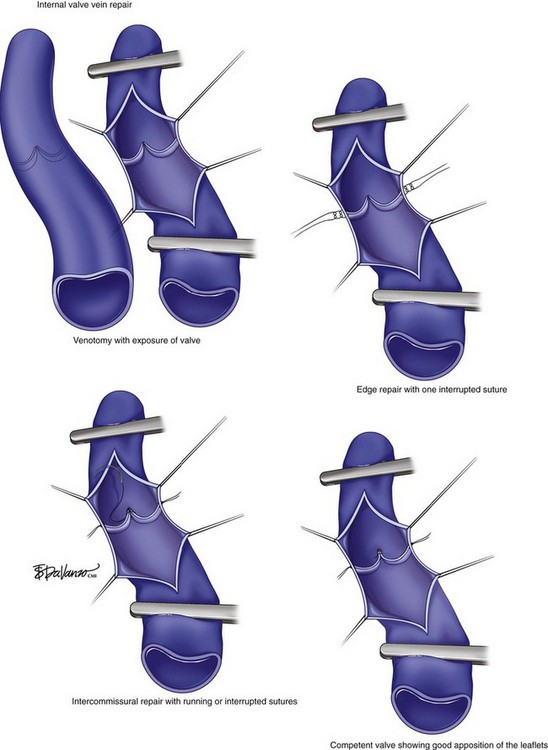
The internal valve vein repair is carried out by performing a venotomy to expose the incompetent valve to reapproximate the valve leaflets (Fig. 15-4). In a study of 42 patients (52 limbs), Cheattle and Perrin reported an 85% valve competency and 9% ulcer recurrence rate at 1-year follow-up.32 A long-term follow-up is provided in another study of 51 cases reported by Masuda and Kistner.33 In the latter, the authors found better results in patients sustaining primary valve incompetency than secondary CVD and an overall clinical success of 60% in 10-year follow-up. The drawback is that the majority of patients sustained primary valve insufficiency and, therefore, the technique may rarely be used for patients with PTS due to possible chronic degenerative changes of the venous wall and valve system damage. In addition, the series comprises a small number of patients. A few other disadvantages of the internal technique include challenging limbs with small narrowed veins and multiple venotomies required in long diseased segments, increasing the operative time and complexity of the repair.
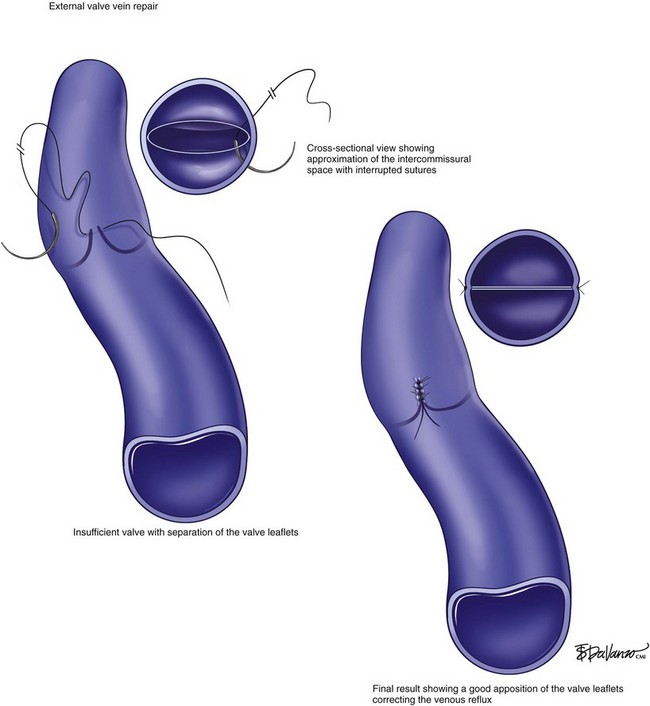
The external valve vein repair is another option that may expedite the operative procedure and simplify the repair (Fig. 15-5). The advantage of this technique is that multiple valves can be repaired in a single procedure. Despite the argument against blind suturing to tighten up the loose intercommissural space, the technique has shown decent results.34,35 A large experience applying external or so-called transcommissural repair was reported in a study of 141 limbs. The ulcer-free rate when this technique was performed was 63% at 30-month follow-up.34 Overall early complication rate was 9%, including a low early vein thrombosis rate of 3%. In contrast to internal valve repair studies,33,36 the durability achieved with transcommissural valve repair was found to be the same for patients with either “primary” or secondary CVD.35
The use of an external sleeve of Dacron or PTFE wrapped around the incompetent valve has been also applied to correct reflux.37 It has also been used around axillary vein transplant procedures to prevent dilation of the valve and subsequent reflux.38 Only a few reports exist with the external sleeve providing short-term data. In patients with PTS, this procedure will constrict the vein as there are no functioning valves; the reflux may be reduced but the effect of the obstruction is of concern.
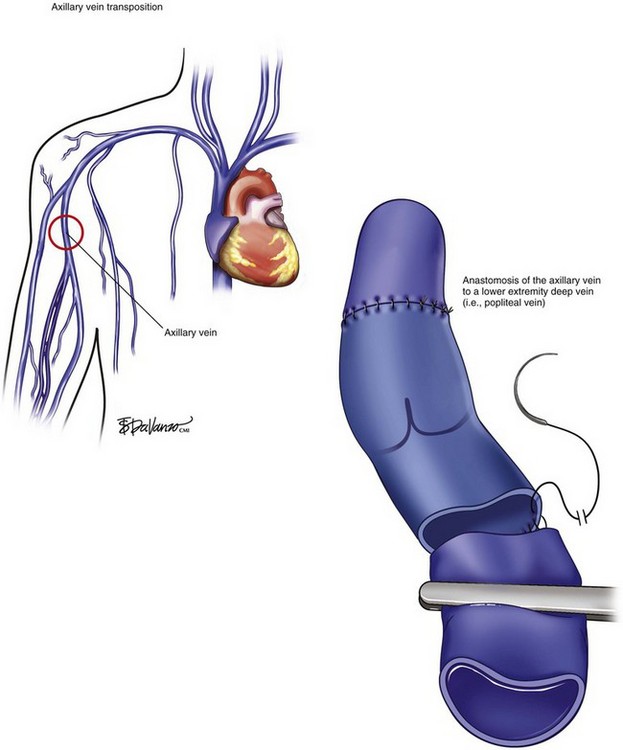
The transfer or transplantation of venous segment with competent valves is a third alternative. The first autologous valved graft transplant used was the ipsilateral GSV.39–41 The shortcomings of either in situ or transposition of a segment of GSV to the femoral vein are vein mismatch size, degenerative changes with vein incompetence secondary to vein wall dilatation, and the fact that isolated deep vein reflux with competent superficial vein is rare, limiting the use of GSV. Conversely, the axillary vein transfer (AVT) was proposed as another potential conduit to restore the directional venous outflow. The axillary vein is preferred instead of other deep veins because of the quality of the valves, good size match, and low degree of anatomic variation in that region (Fig. 15-6). Furthermore, the upper extremity blood return is mainly done by the superficial veins and therefore not being significantly impaired after axillary vein harvesting. However, a strip test is advocated to assess the axillary vein valve competency prior to transfer. Some authors reported up to 40% of axillary vein incompetency and advise in favor of valve repair in a bench or in situ procedure.35 The spatial orientation of the anastomosis with precise trimming of the vein must also be accomplished in order to avoid reflux. Several authors have reported their experience with valved vein graft transfer.31,36,42,43 Results are variable, ranging from 18% to up to 70% of recurrence of ulcers based upon follow-up time (1- to 5-year follow-up) and type of the conduit.31,36,42,43 A more recent series of AVT reported long-term patency greater than 80% and ulcer-free recurrence greater than 60% at 10-year follow-up.35
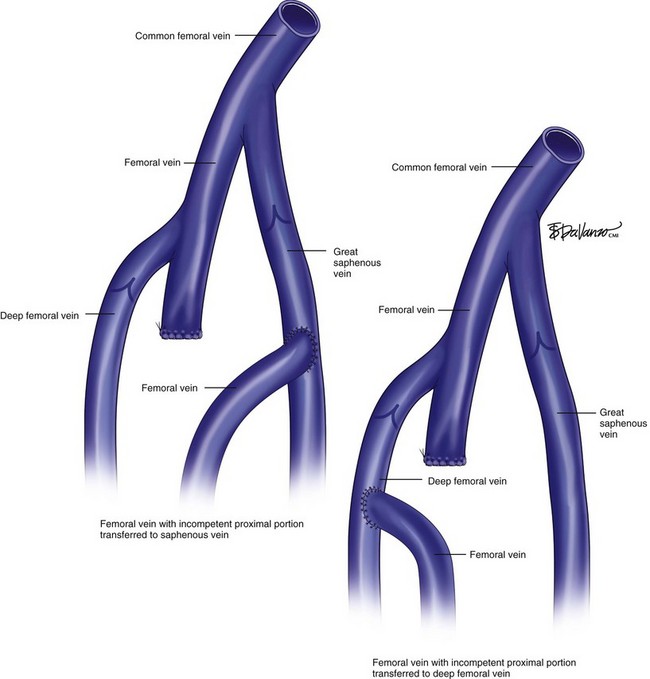
Another technique of transfer was described by Ferris and Kistner in 1982.44 It consists of ligation of the femoral vein below the level of valve incompetency and redirection of the flow to either the femoral or profunda vein (Fig. 15-7). Contraindications of the segmental femoral vein transfer are possible dilation of the recipient vein secondary to the increased rerouted flow and, subsequently, reflux involving the femoral and the target vein.
Allied to complex surgical reconstructions or vein transfer, a temporary or long-term arteriovenous fistula (AVF) is often necessary to increase the blood flow in order to keep the repair patent. The drawbacks of constructing a distal AVF are the potential risk of venous dilation and valve incompetency generating venous hypertension and ultimately skin damage. In addition, anticoagulation has been advocated in order to improve the patency rate. Mandatory life-long anticoagulation therapy is also a concern based on risks of bleeding and its morbidity and mortality. Other disadvantages of the open procedures are low patency rates and short-term and midterm follow-up with only few long-term retrospective series, frequently with small sample size.28
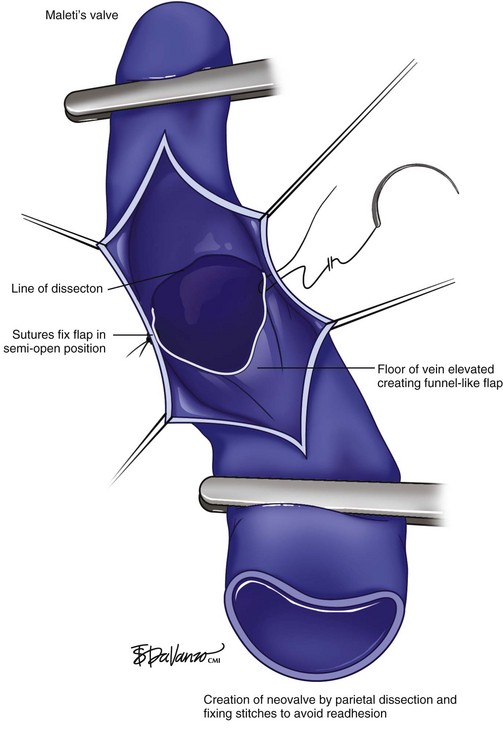
Experimental research has been carried out including neovalve construction, cryopreserved allograft, and prosthetic vein valve implants.45–48 Some promising results of a multicenter phase I trial with cryopreserved allograft valves,45 the “Maleti valve,”47 and other neovalve implants46 have been published. In preparation for neovalve reconstruction, endophlebectomy of trabeculated segments is recommended. The technique consists of longitudinal incision of the vein and resection of the fibrous septa with a microsurgery scissor or ophthalmic scalpel to the level of the intima layer. Relief of the obstruction in trabeculated veins is recommended and feasible prior to an axillary vein segment transfer or neovalve creation.45,46
A neovalve creation procedure has been developed.49 The operation encompasses endophlebectomy of the venous segment and dissection of the intima layer, creating a flap that is positioned as a monocuspid or bicuspid valve with subsequent venorrhaphy in transverse fashion (Fig. 15-8). The largest experience reported is the Italian experience of 40 neovalve creations in 36 patients with recalcitrant ulcers.46 Six valves failed in the first 19 procedures (phase I), but no failures were documented in the last 21 (phase II). No PE or major complications were reported, and overall recurrence of ulcers occurred in 8%. Mostly, the results were based on short-term and midterm follow-up (24 to 48 months for cumulative curves of competency rates). Nevertheless, to obtain good results of operative techniques for infrainguinal deep vein reflux, further investigation is necessary because of small number of patients, experience restricted to a very few centers, and scant long-term follow-up.
Endovascular Horizon
Endovenous therapy for PTS has emerged as the first option mainly for iliofemoral and IVC reconstruction because of its minimally invasive approach, lower morbidity, and promising results.26,50 Indications and results of IVC, iliac, and femoral angioplasty and stenting are discussed in Chapter 16.
A few experimental implantable endovascular valves have been investigated. The idea of implanting valves via the endovascular approach was first done using an external jugular vein with a competent valve mounted in a Z-stent (Cook Medical, Bloomington, IN) that was deployed in either the external (n = 4) or common iliac vein (n = 1) in dogs.51 One of five valves implanted was patent, competent, and with minimal inflammatory surrounding reaction. Interestingly, the normal-appearing valve was deployed in the common iliac vein, which has a higher flow pattern.51 Different valve materials varying from glutaraldehyde-preserved bovine vein to a collagen tissue model of porcine small intestine submucosa (SIS) have been initially studied in animal models.52,53 The former was made with thrombogenic material and leads to higher endothelial proliferation rate and therefore carries a higher risk of early occlusion of the valve.54 The latter, which was built with SIS, has a nonthrombogenic and nonimmunogenic surface.53 Both prototypes are mounted on Z-stents or, onto a nitinol-based stent.52–56
There were only two phase I trials reported with a total of five patients in the United States. The first enrolled two patients who underwent percutaneous insertion of a glutaraldehyde-fixed bovine venous valve via right internal jugular access.57 Both patients were anticoagulated postprocedure, but the second patient had postimplant valve thrombosis. Despite catheter direct thrombolysis, the valve was incompetent but patent. The first patient had a patent and competent valve at 12-months follow-up. Clinical improvement was achieved in both patients, although aggressive compressing therapy and other superficial vein ablation or stripping procedures were required during the follow-up.57
The second phase I trial comprised three patients and was performed using the SIS bioprosthetic venous valve model.58 The patients had percutaneous implantable valves in the proximal (n = 2) and distal (n = 1) portions of the femoral vein and were anticoagulated. All three implanted SIS valves were patent but with either some degree of valve leak in the first two cases or valve tilting in the third. At 12-month follow-up, all patients but one had clinical improvement.58 A second generation of the SIS percutaneous venous valve stents with more precise spatial configuration to prevent tilting was already tested in animal model.53
1 Prandoni P, Villalta S, Polistena P, et al. Symptomatic deep-vein thrombosis and the post-thrombotic syndrome. Haematologica. 1995;80(2 Suppl):42-48.
2 Kahn SR, Kearon C, Julian JA, et al. Predictors of the post-thrombotic syndrome during long-term treatment of proximal deep vein thrombosis. J Thromb Haemost. 2005;3:718-723.
3 Jost CJ, Gloviczki P, Cherry KJJr, et al. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33:320-327. discussion 27-28
4 Raju S, Fredericks RK, Neglen PN, et al. Durability of venous valve reconstruction techniques for “primary” and postthrombotic reflux. J Vasc Surg. 1996;23:357-366. discussion 366-367
5 Maleti O. Venous valvular reconstruction in post-thrombotic syndrome. A new technique. J Mal Vasc. 2002;27:218-221.
1 Heit JA, Rooke TW, Silverstein MD, et al. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25-year population-based study. J Vasc Surg. 2001;33:1022-1027.
2 Prandoni P, Villalta S, Polistena P, et al. Symptomatic deep-vein thrombosis and the post-thrombotic syndrome. Haematologica. 1995;80(2 Suppl):42-48.
3 Widmer LK, Zemp E, Widmer MT, et al. Late results in deep vein thrombosis of the lower extremity. VASA. Zeitschrift fur Gefasskrankheiten. 1985;14:264-268.
4 Ashrani AA, Heit JA. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis. 2009;28:465-476.
5 Ginsberg JS, Turkstra F, Buller HR, et al. Postthrombotic syndrome after hip or knee arthroplasty: a cross-sectional study. Arch Intern Med. 2000;160:669-672.
6 Mekkes JR, Loots MA, Van Der Wal AC, et al. Causes, investigation and treatment of leg ulceration. Br J Dermatol. 2003;148:388-401.
7 Labropoulos N, Manalo D, Patel NP, et al. Uncommon leg ulcers in the lower extremity. J Vasc Surg. 2007;45:568-573.
8 Tran NT, Meissner MH. The epidemiology, pathophysiology, and natural history of chronic venous disease. Semin Vasc Surg. 2002;15:5-12.
9 Kahn SR, Kearon C, Julian JA, et al. Predictors of the post-thrombotic syndrome during long-term treatment of proximal deep vein thrombosis. J Thromb Haemost. 2005;3:718-723.
10 Labropoulos N, Gasparis AP, Tassiopoulos AK. Prospective evaluation of the clinical deterioration in post-thrombotic limbs. J Vasc Surg. 2009;50:826-830.
11 Labropoulos N, Jen J, Jen H, et al. Recurrent deep vein thrombosis: long-term incidence and natural history. Ann Surg. 2010;251:749-753.
12 Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
13 Fink AM, Mayer W, Steiner A. Extent of thrombus evaluated in patients with recurrent and first deep vein thrombosis. J Vasc Surg. 2002;36:357-360.
14 Prandoni P, Frulla M, Sartor D, et al. Vein abnormalities and the post-thrombotic syndrome. J Thromb Haemost. 2005;3:401-402.
15 Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698-707.
16 Labropoulos N, Spentzouris G, Gasparis AP, et al. Impact and clinical significance of recurrent venous thromboembolism. Br J Surg. 2010;97:989-999.
17 Latella J, Desmarais S, Miron MJ, et al. Relation between D-dimer level, venous valvular reflux, and the development of the post-thrombotic syndrome after deep vein thrombosis. J Thromb Haemost. 2010;8:2169-2175.
18 Spiezia L, Campello E, Giolo E, et al. Thrombophilia and the risk of post-thrombotic syndrome: retrospective cohort observation. J Thromb Haemost. 2010;8:211-213.
19 Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(Suppl):454S-545S.
20 Brandjes DP, Buller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759-762.
21 Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
22 Giannoukas AD, Labropoulos N, Michaels JA. Compression with or without early ambulation in the prevention of post-thrombotic syndrome: a systematic review. Eur J Vasc Endovasc Surg. 2006;32:217-221.
23 Kakkos SK, Daskalopoulou SS, Daskalopoulos ME, et al. Review on the value of graduated elastic compression stockings after deep vein thrombosis. Thromb Haemost. 2006;96:441-445.
24 Kahn SR, Shbaklo H, Shapiro S, et al. Effectiveness of compression stockings to prevent the post-thrombotic syndrome (the SOX Trial and Bio-SOX biomarker substudy): a randomized controlled trial. BMC Cardiovasc Disord. 2007;7:21.
25 O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev 2009:CD000265.
26 Meissner MH, Eklof B, Smith PC, et al. Secondary chronic venous disorders. J Vasc Surg. 2007;46(Suppl S):68S-83S.
27 Kahn SR, Shrier I, Shapiro S, et al. Six-month exercise training program to treat post-thrombotic syndrome: a randomized controlled two-centre trial. CMAJ. 2011;183:37-44.
28 Jost CJ, Gloviczki P, Cherry KJJr, et al. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33:320-327. discussion 327-328
29 Harris JP, Kidd J, Burnett A, et al. Patency of femorofemoral venous crossover grafts assessed by duplex scanning and phlebography. J Vasc Surg. 1988;8:679-682.
30 Neglen P, Raju S. Venous reflux repair with cryopreserved vein valves. J Vasc Surg. 2003;37:552-557.
31 Taheri SA, Elias SM, Yacobucci GN, et al. Indications and results of vein valve transplant. J Cardiovasc Surg. 1986;27:163-168.
32 Cheatle TR, Perrin M. Venous valve repair: early results in fifty-two cases. J Vasc Surg. 1994;19:404-413.
33 Masuda EM, Kistner RL. Long-term results of venous valve reconstruction: a four- to twenty-one-year follow-up. J Vasc Surg. 1994;19:391-403.
34 Raju S, Berry MA, Neglen P. Transcommissural valvuloplasty: technique and results. J Vasc Surg. 2000;32:969-976.
35 Raju S, Fredericks RK, Neglen PN, et al. Durability of venous valve reconstruction techniques for “primary” and postthrombotic reflux. J Vasc Surg. 1996;23:357-366. discussion 366-367
36 Eklof BG, Kistner RL, Masuda EM. Venous bypass and valve reconstruction: long-term efficacy. Vasc Med (Lond Engl). 1998;3:157-164.
37 Jessup G, Lane RJ. Repair of incompetent venous valves: a new technique. J Vasc Surg. 1988;8:569-575.
38 Raju S, Fredericks R. Valve reconstruction procedures for nonobstructive venous insufficiency: rationale, techniques, and results in 107 procedures with two- to eight-year follow-up. J Vasc Surg. 1988;7:301-310.
39 Cardon JM, Cardon A, Joyeux A, et al. Use of ipsilateral greater saphenous vein as a valved transplant in management of post-thrombotic deep venous insufficiency: long-term results. Ann Vasc Surg. 1999;13:284-289.
40 Eriksson I, Almgren B. Surgical reconstruction of incompetent deep vein valves. Uppsala J Med Sci. 1988;93:139-143.
41 Kistner RL, Ferris EB3rd, Randhawa G, et al. The evolving management of varicose veins. Straub Clinic experience. Postgrad Med. 1986;80:51-53. 56-59
42 Sottiurai VS. Surgical correction of recurrent venous ulcer. J Cardiovasc Surg. 1991;32:104-109.
43 Nash T. Long term results of vein valve transplants placed in the popliteal vein for intractable post-phlebitic venous ulcers and pre-ulcer skin changes. J Cardiovasc Surg. 1988;29:712-716.
44 Ferris EB, Kistner RL. Femoral vein reconstruction in the management of chronic venous insufficiency. A 14-year experience. Arch Surg. 1982;117(12):1571-1579.
45 Dalsing MC, Raju S, Wakefield TW, et al. A multicenter, phase I evaluation of cryopreserved venous valve allografts for the treatment of chronic deep venous insufficiency. J Vasc Surg. 1999;30:854-864.
46 Lugli M, Guerzoni S, Garofalo M, et al. Neovalve construction in deep venous incompetence. J Vasc Surg. 2009;49:156-162. 162 e1-2; discussion 162
47 Maleti O, Lugli M. Neovalve construction in postthrombotic syndrome. J Vasc Surg. 2006;43:794-799.
48 Taheri SA, Schultz RO. Experimental prosthetic vein valve. Long-term results. Angiology. 1995;46:299-303.
49 Maleti O. Venous valvular reconstruction in post-thrombotic syndrome. A new technique. J Mal Vasc. 2002;27:218-221.
50 Raju S, Neglen P. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg. 2009;50:360-368.
51 Dalsing MC, Sawchuk AP, Lalka SG, et al. An early experience with endovascular venous valve transplantation. J Vasc Surg. 1996;24:903-905.
52 Gomez-Jorge J, Venbrux AC, Magee C. Percutaneous deployment of a valved bovine jugular vein in the swine venous system: a potential treatment for venous insufficiency. J Vasc Interv Radiol. 2000;11:931-936.
53 Pavcnik D, Kaufman J, Uchida B, et al. Second-generation percutaneous bioprosthetic valve: a short-term study in sheep. J Vasc Surg. 2004;40:1223-1227.
54 de Borst GJ, Teijink JA, Patterson M, et al. A percutaneous approach to deep venous valve insufficiency with a new self-expanding venous frame valve. J Endovasc Ther. 2003;10:341-349.
55 Pavcnik D, Uchida B, Timmermans H, et al. Square stent: a new self-expandable endoluminal device and its applications. Cardiovasc Interv Radiol. 2001;24:207-217.
56 Pavcnik D, Uchida BT, Timmermans HA, et al. Percutaneous bioprosthetic venous valve: a long-term study in sheep. J Vasc Surg. 2002;35:598-602.
57 Gale SS, Shuman S, Beebe HG, et al. Percutaneous venous valve bioprosthesis: initial observations. Vasc Endovasc Surg. 2004;38:221-224.
58 Pavcnik D, Machan L, Uchida B, et al. Percutaneous prosthetic venous valves: current state and possible applications. Techn Vasc Interv Radiol. 2003;6:137-142.