Chapter 195 Postoperative Spinal Deformities
Definition
Postoperative deformity is an important complication, and a major reason for revision spine surgery. Revision rates in spine surgery have been reported variably, and the rates depend on the primary pathology treated, patient factors, methods of treatment, and choice of levels for arthrodesis.1–4 Postoperative deformity with adjacent-segment pathology is associated with significant disability and is one of the most common reasons for revision surgery.5–9 This chapter addresses the etiologies of postoperative spinal deformity and provides guidance for both avoiding and managing deformities after spine surgery.
Fusion of the Spine in Malalignment
Fusion of the spine with inadequate correction in the sagittal plane is a common cause of immediate postoperative deformity. Neutral sagittal alignment follows a vertical axis from C2, in front of T7, behind L3, and within 3 cm of the dorsal margin of the sacrum.10 There is significant normal variation of lumbar lordosis and thoracic kyphosis in maintaining overall sagittal balance.11,12 Sagittal balance is important for biomechanical optimization of forces at segmental interspaces. Sagittal plane malalignment is most often clinically significant when there is loss of normal lordosis of the lumbar and cervical spine. Excessive kyphosis across these mobile, unsupported segments increases intradiscal pressures and compromises the mechanical advantage of the erector spinae musculature.13,14 Clinically, the patient with fixed sagittal deformity presents with intractable pain, early fatigue, and a subjective sense of imbalance and leaning forward and difficulty with horizontal gaze. Compensation can be gained with extension of the hips, increased positive tilt (retroversion) of the pelvis, and flexion of the knees, although at the expense of increased fatigue.15
Flatback deformity or syndrome is the term commonly applied to postoperative malalignment of the spine. Flatback syndrome first was recognized by Doherty in 1973 as a postural disorder of the spine caused specifically by distraction instrumentation in the correction of scoliosis. Doherty recognized the loss of normal lordosis that occurred as a result of distraction across the lumbar spine and the thoracolumbar junction with the use of Harrington instrumentation for correction of coronal deformity in adolescent idiopathic deformity.16 Kyphotic decompensation syndrome is a more general term for postoperative kyphosis that is caused by immediate malalignment of the spine.17 Kyphotic decompensation may occur at a single segment of the spine or, more commonly, in fusion of the spine over multiple segments.
Fixation of the spine in coronal plane malalignment also is an important cause of immediate postoperative malalignment. Coronal plane malalignment after surgery may result in significant truncal shift, shoulder asymmetry, apparent leg-length discrepancy, pelvic obliquity with imbalance, rib-on-pelvis deformity, and complaints regarding appearance.18 A small malalignment or coronal tilt at the lumbosacral segments may cause a significant truncal shift and imbalance. A common cause of postoperative coronal malalignment is asymmetrical correction of balanced curves in a double major deformity or in a thoracolumbar scoliosis with a fixed lumbosacral obliquity.
Avoiding Fusion of the Spine in Malalignment
Preoperative planning and recognition of global sagittal and coronal plane alignment are important for avoiding postoperative malalignment of the spine.19 On physical examination, evaluation of the patient with knees fully extended is essential to assess the sagittal plane. Recognition of pelvic tilt (version of the pelvis) is important to determine whether a patient may be compensating for sagittal deformity in the spine with hyperextension of the hips and increase in pelvic tilt. In the coronal plane, correction of pelvic obliquity during the examination is important to assess coronal alignment of the spine prior to surgery. Leg-length discrepancy or apparent pelvic obliquity may be corrected with standing blocks to evaluate the spine with a level pelvis. Standing 36-inch radiographs are important in the evaluation of preoperative deformity and for identifying goals of surgical correction. Preoperative planning is based on calculations of sagittal and coronal changes needed for spinal realignment and may include digital image manipulation or modeling of the spine.20 Preoperative planning in deformity surgery may be characterized by significant variability within and between observers.21
During the procedure, patient positioning and intraoperative radiographs are critical for obtaining optimal alignment of the spine and for avoiding immediate postoperative deformity. Patient positioning has a significant impact on spinal alignment in the coronal and sagittal planes, and use of an appropriate operative frame to optimize passive correction of deformity and to avoid generation of deformity is important.22,23 Positioning the patient in a flexed posture may be useful for decompressive procedures, but arthrodesis of the spine with the patient in a knee-chest position, or in a flexed posture, is an important cause of postoperative deformity in even short-segment fusions.24,25 Intraoperative radiology is useful in assessing alignment of the spine after placement of fixation. Full-length, 36-inch radiographs obtained in the sagittal and coronal planes are most useful for assessment of global balance.
Treatment of Postoperative Malalignment of the Spine
When postoperative malalignment is recognized immediately after surgery, the patient may be treated with early revision and may not require osteotomies to correct a fixed deformity. Early revision may include improving segmental or regional sagittal alignment in a mobile spine with facetectomies or, more significantly, with Ponte osteotomies.26 In a patient with coronal imbalance due to asymmetrical correction of double curves, revision surgery may include improving the correction of the curve contralateral to the coronal offset or reducing the correction of the curve ipsilateral to the coronal offset Early detection of immediate postoperative deformity facilitates correction in a mobile spine.
The patient in whom postoperative deformity is recognized late, or after solid arthrodesis of the spine in a malaligned position, can present a difficult challenge for realignment. Osteotomies of the spine are the most commonly used technique for correction of postoperative malalignment.27 Surgical correction of fixed sagittal deformity was first reported by Smith-Petersen et al. in 1945.28 This osteotomy pivots on the posterior longitudinal ligament, with consequent lengthening of the anterior column through the disc space.29 The technique is well suited for patients with flexible discs ventrally and dorsal pseudarthrosis at multiple levels. Combined ventral and dorsal surgery offers the advantage of a controlled manipulation of the anterior column and fusion with structural graft. LaChapelle described a combined ventral and dorsal osteotomy for the management of ankylosing spondylitis that involved cutting the anterior column directly, followed by grafting to avoid risks of a ventral opening wedge.30 The combined ventral and dorsal osteotomy for the correction of iatrogenic lumbar kyphosis has led to good restoration of lumbar lordosis, maintained at more than 2 years’ follow-up.31
A dorsal-only approach to correction of sagittal deformity offers several advantages, including single-stage surgery, reduced morbidity compared with combined surgery, addressing the deformity at the apex, creation of compressive forces at the osteotomy site, and maximal correction with a minimal number of osteotomies.32 Dorsal surgical options can be categorized by the fulcrum across which correction is achieved. Dorsal osteotomies that hinge on the posterior longitudinal ligament (e.g., Smith-Petersen) cause opening of the anterior column. Spinal shortening osteotomies gain sagittal plane correction without distraction of the anterior column. Heinig’s eggshell decancellation procedure includes a controlled compression fracture of the anterior column with differentially more closure dorsally.33 In contrast, the Thomasen osteotomy maintains the height of the anterior column and hinges on the anterior longitudinal ligament.34 Asymmetrical closure of the pedicle subtraction osteotomy may permit up to 6 cm correction of coronal plane deformity as well as sagittal plane deformity.35 Larger corrections of trunk translation or coronal deformity may require a vertebral column resection.18,36,37
Case Examples: Immediate Postoperative Deformity
Case 1
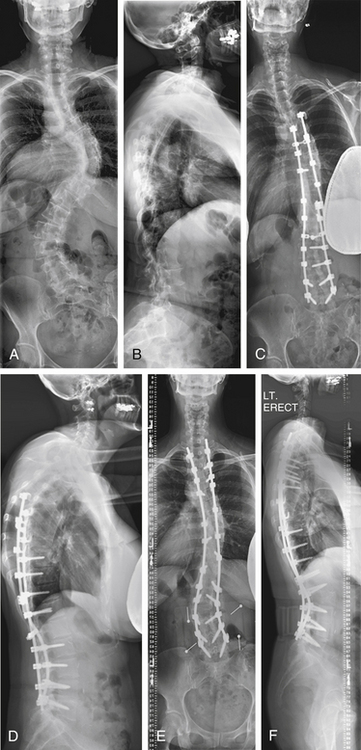
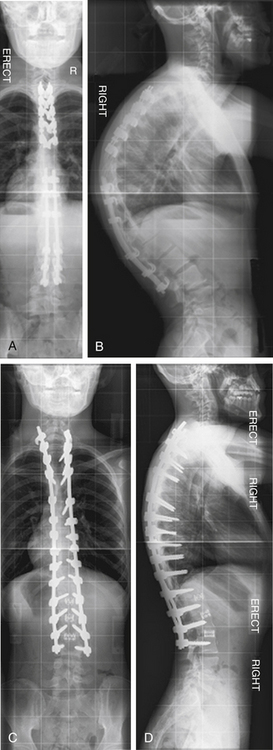
A 54-year-old woman presented with a double major scoliosis. Asymmetrical flexibility of the lumbar and thoracic curves resulted in overcorrection of the lumbar curve relative to the thoracic curve and postoperative coronal decompensation. Realignment of the spine with an asymmetrical pedicle subtraction osteotomy (PSO) at L3 resulted in improvement of sagittal and coronal plane alignment (Fig. 195-1).
Case 2
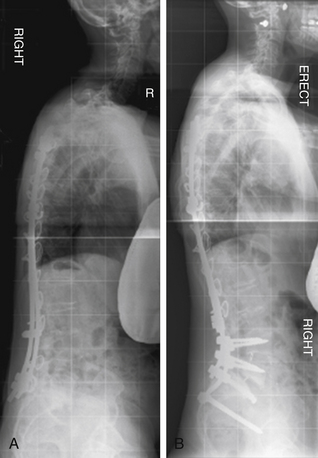
A 41-year-old woman was treated with combined ventral and dorsal surgery for lumbar adult scoliosis. The patient was fused in a fixed kyphotic and coronal plane decompensation. An asymmetrical PSO provided correction of deformity in the sagittal and coronal planes (Fig. 195-2).
Postoperative Decompensation
Late decompensation within the fused segments is most characteristic of incomplete fusion, or pseudarthrosis of the spine. Failure to achieve solid arthrodesis of the spine may lead to symptoms including progressive deformity, pain, dyspnea, and neurologic deficit. Pseudarthrosis of the spine is a contributing factor in as many as 80% of reoperations in failed spinal fusions.38–40 Symptomatic pseudarthroses are most common in the thoracolumbar and lumbosacral regions. Because revision surgery for symptomatic pseudarthrosis is characterized by high rates of recurrent pseudarthrosis and clinical failure,41,42 obtaining solid arthrodesis of the spine at the time of initial surgery is valuable because this may help avoid revision for pseudarthrosis. The role of biologics in improving arthrodesis in ventral dorsal spinal fusion surgery may reduce the burden of revision surgery for late postoperative deformity due to pseudarthrosis.43–45
Postoperative deformity due to pseudarthrosis may occur in both instrumented and noninstrumented fusions. Recognition and detection of pseudarthrosis may be challenging. The reported rates of pseudarthrosis are highly variable, and an important cause of observed variability in published rates is the difficulty of making a radiographic diagnosis of pseudarthrosis.46,47 In an instrumented fusion, pseudarthrosis may be apparent by implant loosening with osteolysis around screws, or by rod breakage. In noninstrumented fusions, identification of a pseudarthrosis may be more challenging. In a study correlating radiographic findings with intraoperative findings, Dawson et al. reported that plain anteroposterior and lateral views of the spine accurately identified a pseudarthrosis in only 48% of cases.48 The authors identified CT in the coronal plane as the most accurate study for identifying pseudarthrosis, with a 96% correlation with findings at surgery. Deckey et al. reported a finding of progressive deformity in 4 of 14 patients (28%) who underwent removal of instrumentation with an apparently solid fusion.49 This finding brings into question the effectiveness of even second-look surgery. DePalma and Rothman reported that the condition of pseudarthrosis is iatrogenic and responsibility for it lies entirely within the hands of the surgeon.50 Although surgical technique is an important consideration in the generation of a successful arthrodesis, other factors also have an important influence on the occurrence of nonunion in the spine, including the region of the spine fused, surgical approach, underlying diagnosis, implant stiffness, local conditions for bone formation, smoking, infection, and bone graft choices.7, 51–57
Crankshaft deformity of the spine is an important example of postoperative deformity within a fused region of the spine. In children with spinal deformity, progressive curvature, vertebral rotation, and rib prominence after a dorsal fusion may be due to continued anterior column growth of the spine despite a solid dorsal arthrodesis. This pattern of deformity progression was first identified by Hefti and McMaster.58 Duboisset et al. introduced the term “crankshaft phenomenon” as a descriptive term for this type of progressive deformity, making the analogy with an automotive crankshaft rotating around a center arm.59 Young patients who have significant ventral growth remaining at the time of a dorsal spine fusion are at risk of postoperative deformity progression, and incidences of 37% to 43% have been reported in patients who are Risser 0 and have open triradiate cartilages at the time of dorsal fusion.60,61 A more accurate radiographic parameter for predicting the risk of the crankshaft phenomenon after dorsal surgery may be the assessment of peak height velocity. Saunders et al. reported a high incidence (8/8) of crankshaft phenomenon in patients fused before reaching peak height velocity, and a low incidence (2/15) in patients fused after reaching peak height velocity.62 The risk of crankshaft phenomenon causing progressive deformity in children may be limited in the setting of contemporary rigid segmental instrumentation systems. Burton et al. reported only one case of progressive deformity after dorsal-only surgery in a cohort of skeletally immature patients, suggesting that ventral surgery may be unnecessary to prevent crankshaft deformity.63
Late decompensation of the unfused segments of the spine in patients undergoing selective thoracic or lumbar fusions for deformity is another important cause of postoperative deformity. It may lead to deformity in the sagittal or coronal plane. Late decompensation of the unfused segments in the sagittal plane is commonly due to proximal junctional kyphosis, a cause of postoperative deformity that is addressed in the next section of this chapter. Late decompensation in the coronal plane most commonly is due to progression of scoliosis in the unfused region of the spine. The choice of fusion levels at the time of initial surgery, and the techniques of correction used in the initial surgery are important determinants of the rate of late coronal plane decompensation in spine surgery.
In the coronal plane, decompensation after surgical correction of deformity may occur due to either deformity progression in the mobile lumbar spine or the exclusion of a structural curve from the segments chosen for arthrodesis. The development of progressive lumbar deformity after selective thoracic fusion, or fusion to the mobile lumbar spine, is an important cause of postoperative degenerative changes, pain, and decompensation in the coronal plane.64–66 Spontaneous correction of lumbar deformity may be reliably expected to occur in some cases of selective thoracic fusion with compensatory lumbar deformity.67 However, Miller et. al demonstrated an incidence of coronal decompensation after selective thoracic fusion of nearly 17% at 2 years in patients with adolescent scoliosis.68 Newton et al. demonstrated that factors determining the surgical choice to include a nonstructural lumbar curve in the fusion levels include the magnitude of the curve, the apical displacement, and the ratio of the size of the lumbar curve to the thoracic curve.69 Late decompensation of the lumbar unfused segments may occur secondary to degenerative changes. Cochran et al. reported a high incidence of retrolisthesis, facet joint changes, and disc degeneration subjacent to the fused levels in patients fused below L3 with Harrington instrumentation.70 Correction of thoracic deformity using derotation of the thoracic spine may increase rotation of the unfused lumbar segments.64,65,71 The choice of an appropriate lower-end vertebra for deformity surgery is important in limiting the occurrence of early or late coronal decompensation in scoliosis surgery. Accurate and reproducible recognition of the stable, end, and neutral vertebrae is important in choosing a lowest instrumented segment. Similarly, recognizing whether a curve within a deformity is structural or nonstructural and classifying the type of curve are critical to surgical strategies in scoliosis surgery.72
The choice of fusion levels in the upper end of a spinal deformity construct may also have a significant impact upon coronal decompensation. Recognition and inclusion of a structural upper thoracic curve is important in the preoperative planning of the cephalad extent of spinal arthrodesis for deformity. Lenke et al. demonstrated that the failure to identify and include a structural upper thoracic curve may lead to shoulder asymmetry and postoperative coronal plane decompensation.73 The authors recommend extending a dorsal fusion to the level of T2 when preoperative criteria are present, including a proximal thoracic curve measuring more than 30 degrees and correcting to more than 20 degrees on side-bending films, the presence of rotation or translation at the apex of the thoracic curve, and preoperative shoulder elevation on the concave side or T1 tilt toward the concave side. Subsequently, Kuklo et al. demonstrated that spontaneous curve correction of the unfused upper thoracic curve occurs more reliably after ventral fusion of the main thoracic curve than after dorsal fusion.74 The authors identify T1 tilt toward the convexity of more than 5 degrees, shoulder asymmetry with elevation of the concave-sided shoulder, and bending correction to more than 25 degrees as factors that may predict postoperative shoulder asymmetry and coronal decompensation after selective thoracic ventral spine fusion. Overall, careful radiographic assessment of the thoracic spine is useful in predicting postoperative decompensation in the upper thoracic region.
Avoiding Postoperative Deformity Due to Late Decompensation
Regarding pseudarthrosis, the lumbosacral junction is especially susceptible due to strain on the S1 screws, especially in fusions above L2 to the sacrum.75 Supplementing lumbosacral fixation with instrumentation to the pelvis is a useful technique for avoiding loss of fixation to the sacrum and progressive loss of sagittal or coronal balance.76–78 Circumferential arthrodesis of the spine also may be useful in avoiding late revision surgery due to nonunion or progressive deformity.79,80 The role of biologics and bone graft substitutes in reducing postoperative deformity due to pseudarthrosis remains to be demonstrated in a prospective clinical study.43
Avoiding late postoperative deformity in the coronal plane may be difficult, because progressive deformity of the unfused segments may be part of the natural history of spinal deformity rather than an avoidable complication. Accurate identification of structural and nonstructural deformity in the lumbar spine is important in determining when the lumbar curve may correct spontaneously after a selective thoracic fusion, and when the lumbar curve is likely to progress. Lenke et al. demonstrated that in King II deformity, spontaneous correction of the lumbar curve was predictable after selective thoracic fusion using either a ventral or a dorsal approach, with a ventral approach resulting in improved thoracic and lumbar curve correction.67 Behensky et. al demonstrated that fixed apical vertebral rotation in Lenke 3C curve type is predictive of progressive deformity and spinal decompensation after surgery.81 Differentiation between a King type II and type III or a Lenke 1 and Lenke 3 deformity is essential in determining the risk of decompensation through progressive deformity in the lumbar mobile segments. Overall, the maintenance of mobile segments in the lumbar spine remains an important goal of deformity correction surgery in both children and adults, and an accurate assessment of symptomatic changes of the lumbosacral spine and of the mobility of the lumbar compensatory curve is important in choosing to end a fusion in the mobile lumbar spine. Moderating surgical correction of the thoracic spine in patients with compensatory or structural lumbar deformity also may be useful for the avoidance of progressive lumbar deformity after selective thoracic fusion.82
Treatment of Postoperative Decompensation of the Spine
The treatment of symptomatic late postoperative deformity of the spine often is surgical. Operative management of postoperative deformity due to pseudarthrosis is challenging, and is characterized by high rates of failure and recurrent pseudarthrosis. In lumbar and lumbosacral pseudarthrosis, circumferential fusion with combined ventral and dorsal surgery is the most reliable technique for treating a symptomatic pseudarthrosis.83 In the thoracic spine, progressive deformity due to pseudarthrosis may be treated effectively with dorsal-based osteotomies and dorsal-only fusion (see Case 4).84 Biologics, including recombinant bone morphogenetic proteins, may have a role in improving union rates after revision surgery for pseudarthrosis, but in patients with deformity due to pseudarthrosis, realignment of the spine will still be required.
Patients with coronal decompensation of the spine due to progression of unfused regions of the spine may require revision surgery with extension of the fusion to include the region that was not fused originally (see Case 5). Bracing a compensatory curve after selection of a structural curve may be useful in preventing curve progression but will not be useful in treating symptomatic decompensation Arlet et. al described two cases in which they released the overcorrection of the main thoracic curve in patients with postoperative coronal plane decompensation and observed a spontaneous correction of the lumbar compensatory curve.85 They proposed that the derotation of the main thoracic curve directly caused decompensation of the contralateral lumbar curve. Late symptomatic degeneration below a fusion to the mobile lumbar spine may require extension of fusion to the pelvis (see Case 6).
Case Examples: Scoliosis and Kyphosis
Case 4
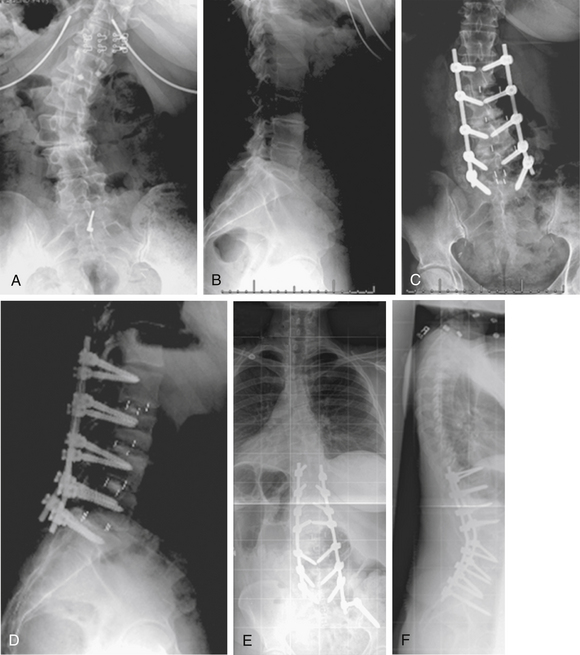
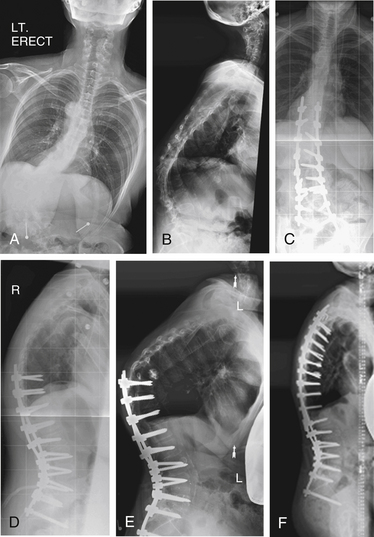
A 14-year-old girl presented with adolescent idiopathic scoliosis and a Lenke 1CN deformity. Fusion was performed at levels from T3 to L1. The patient’s lumbar deformity progressed over the 2 years following surgery, requiring extension of fusion to L4 (Fig. 195-4).
Case 5
A 26-year-old man presented with Scheuermann kyphosis. Dorsal spine fusion was attempted, with implant failure at the apex of the patient’s kyphosis followed by removal of apical implants for prominence. Progressive deformity developed due to nonunion. The patient was treated with dorsal-only deformity correction with Smith-Petersen osteotomies across the apex of the deformity (Fig. 195-5).
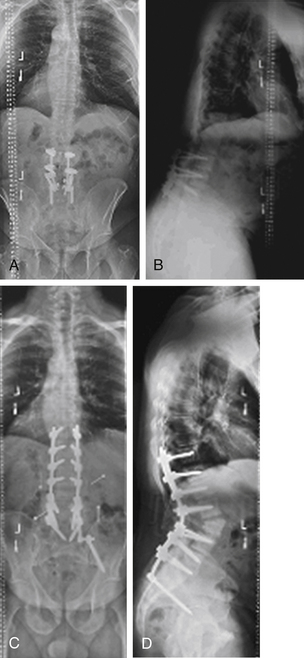
Case 6
A 62-year-old woman had undergone prior spinal fusion to L5 15 years earlier. She presented with progressive kyphotic decompensation due to advanced degenerative changes at L5-S1. Revision surgery with a combined ventral and dorsal approach with extension of fusion to the pelvis resulted in improvement of her sagittal malalignment (Fig. 195-6).
Postoperative Deformity Due to Adjacent-Segment Pathology
Postoperative deformity due to sagittal decompensation adjacent to a fused section is an important pathology in both degenerative and deformity surgery. Adjacent-segment pathology encompasses degenerative changes adjacent to a fusion, including disc degeneration and stenosis, and deformity adjacent to a fusion, including spondylolisthesis and kyphosis. Adjacent-segment degeneration is defined radiographically, and there is significant variability in the criteria used to define the pathology.86 Adjacent-segment disease is defined by the need for revision surgery due to adjacent-level pathology. There is significant variability in the indications for revision surgery among and between surgeons and patients, resulting in imprecise outcome measures. This section of the chapter focuses on adjacent-segment kyphosis, with or without spondylolisthesis.
Proximal junctional kyphosis is a postoperative deformity that is defined radiographically as segmental kyphosis of more than 5 degrees greater than the summed normal angular segments.87 Postoperative proximal junctional kyphosis is common after long spinal fusions, and the rates of proximal junctional kyphosis have been reported variably in adolescents and adults. In adolescent patients with scoliosis, proximal junctional kyphosis has been reported in up to 46% of patients postoperatively.88 In adult patients with long fusions, the postoperative prevalence of proximal junctional kyphosis was up to 39% of patients.89,90 The association between proximal junctional kyphosis and clinical symptoms has been variable, and Kim et al. reported that SRS outcome scores were not adversely affected by the presence of proximal junctional kyphosis radiographically.90 However, proximal junctional kyphosis often is a clinically significant pathology. Mok and Pichelmann, in separate studies, both recognized proximal junctional kyphosis as an important cause of reoperation after surgery for spinal deformity.5,8 Sagittal thoracic decompensation is a progressive kyphotic deformity of the thoracic spine that results in a global sagittal imbalance of more than 8 cm from C7 plumb relative to the dorsal aspect of the sacrum. Sagittal thoracic decompensation is associated with significant disability and poor outcomes.91 Proximal junctional kyphosis and sagittal decompensation may be associated with significant pain, disability, and neural compromise.
The cause of proximal junctional kyphosis is not well defined. Lee et al. described sagittal decompensation in the thoracic spine in 11 of 23 cases (48%) after osteotomies for lumbar degenerative kyphosis.92 The authors proposed that atrophy of the thoracic extensor muscles after surgery and postural habits may account for the predominance of thoracic decompensation. Yagi et al. proposed a classification of proximal junctional kyphosis into three types: ligamentous and disc failure, bone failure, and bone/implant interface failure.93 The authors identified most cases in their series as ligamentous and disc failure with little clinical consequence. However, Watanabe et al. described two mechanisms for proximal junctional kyphosis: upper instrumented vertebra fracture with subluxation of the adjacent vertebra, and fracture above the instrumented vertebra.94 Patients with fracture of the upper instrumented vertebra with subluxation of the adjacent vertebra presented early, on average within 3 months of surgery, and two of the five patients with fracture at the upper instrumented vertebra and subluxation presented with severe neural impairment. The authors identified marked correction of preoperative sagittal malalignment as a risk factor for upper instrumented vertebra fracture with adjacent vertebral subluxation. Lowe and Kasten confirm an increased risk of proximal junctional kyphosis with correction of kyphotic deformity greater than 50%.95
Avoiding Postoperative Adjacent-Segment Kyphosis
Surgical strategies to avoid proximal junctional kyphosis include preoperative planning and intraoperative techniques. The choice of an upper instrumented vertebra may be important in avoiding proximal junctional kyphosis. Lee et al. demonstrated that fusion and instrumentation ending below a segment with more than 5 degrees of kyphosis was a significant risk factor for the development of progressive adjacent-segment kyphosis.92 There is no reliable difference in the occurrence of proximal junctional kyphosis in fusion to the structural thoracic spine (T9-10) compared with the mobile thoracolumbar junction (T11-12, L1- 2).96
Implant rigidity may have a role in the development of proximal junctional kyphosis. The pathology was relatively rare when less rigid implants were used, including Harrington instrumentation of the spine. The stress gradient between rigid internal fixation and the adjacent unfused and uninstrumented spine may be a risk factor for developing proximal junctional pathology.97–100 The role of a more gradual transition in stiffness from the instrumented spine to the noninstrumented spine with dynamic implants or with implants of variable stiffness has not been defined.101,102 Augmentation of the adjacent segments of the spine with ligamentoplasty to prevent dorsal ligamentous failure, or with cement augmentation to prevent fracture of the upper instrumented vertebra, or above the upper instrumented vertebra also may be useful strategies, but their effectiveness has not been well demonstrated in a prospective study.103,104
Treatment of Postoperative Adjacent-Segment Kyphosis
Proximal junctional kyphosis may be radiographically apparent with limited clinical impact. However, proximal sagittal decompensation, or fracture of an upper instrumented vertebra with adjacent subluxation, may be associated with significant disability and possible neural deterioration. Extension of fusion and instrumentation above the junctional kyphosis is the most reliable technique for correcting postoperative kyphotic deformity and for stabilizing the spine above an unstable subluxation (see Cases 7 and 8). The choice of proximal levels in revision surgery may help to avoid recurrence of junctional pathology, and principles including avoiding cantilever forces to gain large corrections and avoiding fusion below a kyphotic segment will be useful in revision surgery as well as primary approaches. In a series of 26 patients undergoing revision surgery for thoracic proximal junctional kyphosis and postoperative sagittal decompensation, significant improvement in sagittal alignment and in SRS-30 scores was obtained with dorsal-based osteotomy and extension of fusion and instrumentation to a proximal lordotic or neutral segment.105
Case Examples: Scoliosis and Kyphosis
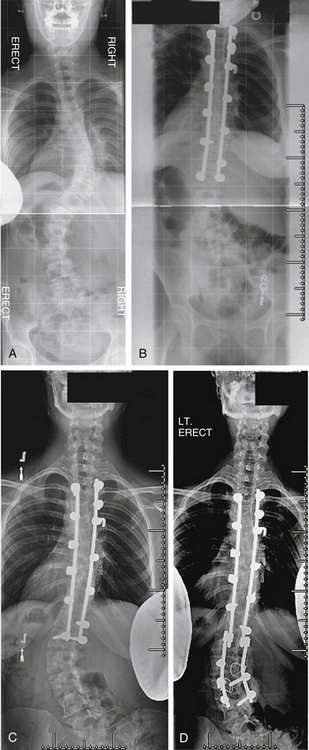
Case 7
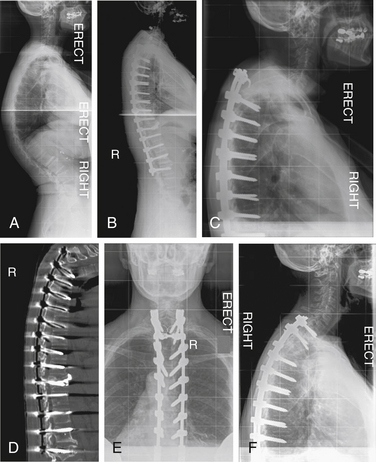
A 73-year-old woman presented with adult scoliosis. She was treated with a combined ventral and dorsal approach to the deformity. Three months after surgery she developed progressive sagittal plane decompensation due to fracture above the fusion. Revision surgery with dorsal Smith-Petersen osteotomies and extension of fixation to T3 resulted in improvement of deformity and clinical symptoms (Fig. 195-7).
Case 8
A 67-year-old woman presented with systemic lupus erythematosus and steroid-dependent osteoporosis. She complained of progressive thoracolumbar kyphosis and pain. She underwent a dorsal spine fusion from T3 to L4 with greater than 50% correction of thoracic kyphosis. Six weeks after surgery, she developed adjacent-segment kyphosis at T3-4 with fracture of the upper instrumented vertebra and subluxation of the adjacent segment. Revision surgery involved extension of fusion to T1 with hybrid fixation (Fig. 195-8).
Summary
Postoperative deformity is an important and significant sequela of surgery for spinal disorders including degenerative pathologies and deformity. Postoperative deformity includes malalignment of the spine at the time of initial surgery, and progressive deformity of the spine over time due to curve progression within the fused region of the spine or adjacent to the fused region. The occurrence and prevalence of postoperative deformity may be managed and minimized with strategies that include preoperative planning and choice of levels for instrumentation and fusion, with attention to sagittal and coronal alignment prior to surgery. Intraoperative strategies including optimizing patient position on the operating frame and modifying corrective forces to minimize adjacent-segment decompensation in the sagittal and coronal planes are useful to avoid postoperative deformity. Surgical techniques to achieve effective arthrodesis and minimize the risk of nonunion or implant migration will reduce the incidence of late deformity due to pseudarthrosis or subsidence of fixation. Prevention is the most valuable and cost-effective technique for avoiding clinical consequences of postoperative deformity, and the most effective way to avoid the need for revision surgery. However, in many cases, late decompensation and deformity progression may be unavoidable, and represent the natural history of disease progression. Ongoing and critical clinical and radiographic follow-up is most important for identifying postoperative deformity, and for guiding subsequent care.
Bridwell K.H. Decision making regarding Smith-Petersen vs. pedicle subtraction osteotomy vs. vertebral column resection for spinal deformity. Spine (Phila Pa 1976). 2006;31(Suppl 19):S171-S178.
Glassman S.D., Bridwell K., Dimar J.R., et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976). 2005;30(18):2024-2029.
Kim Y.J., Bridwell K.H., Lenke L.G., et al. Sagittal thoracic decompensation following long adult lumbar spinal instrumentation and fusion to L5 or S1: causes, prevalence, and risk factor analysis. Spine (Phila Pa 1976). 2006;31(20):2359-2366.
Luhmann S.J., Lenke L.G., Bridwell K.H., Schootman M. Revision surgery after primary spine fusion for idiopathic scoliosis. Spine (Phila Pa 1976). 2009;34(20):2191-2197.
Mok J.M., Cloyd J.M., Bradford D.S., et al. Reoperation after primary fusion for adult spinal deformity: rate, reason, and timing. Spine (Phila Pa 1976). 2009;34(8):832-839.
Park P., Garton H.J., Gala V.C., et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976). 2004;29(17):1938-1944.
1. Malter A.D., McNeney B., Loeser J.D., et al. Five-year reoperation rates after different types of lumbar spine surgery. Spine (Phila Pa 1976). 1998;23:814-820.
2. Hu R.W., Jaglal S., Axcell T., et al. A population-based study of reoperations after back surgery. Spine (Phila Pa 1976). 1997;22:2265-2270. discussion 71
3. Keskimaki I., Seitsalo S., Osterman H., et al. Reoperations after lumbar disc surgery: a population-based study of regional and interspecialty variations. Spine (Phila Pa 1976). 2000;25:1500-1508.
4. Martin B.I., Mirza S.K., Comstock B.A., et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine (Phila Pa 1976). 2007;32:382-387.
5. Mok J.M., Cloyd J.M., Bradford D.S., et al. Reoperation after primary fusion for adult spinal deformity: rate, reason, and timing. Spine (Phila Pa 1976). 2009;34(8):832-839.
6. Lapp M.A., Bridwell K.H., Lenke L.G., et al. Long-term complications in adult spinal deformity patients having combined surgery: a comparison of primary to revision patients. Spine (Phila Pa 1976). 2001;26:973-983.
7. Cook S., Asher M., Lai S.M., et al. Reoperation after primary posterior instrumentation and fusion for idiopathic scoliosis. Toward defining late operative site pain of unknown cause. Spine (Phila Pa 1976). 2000;25:463-468.
8. Pichelmann M.A., Lenke L.G., Bridwell K.H., et al. Revision rates following primary adult spinal deformity surgery: six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine (Phila Pa 1976). 2010;35(2):219-226.
9. Luhmann S.J., Lenke L.G., Bridwell K.H., Schootman M. Revision surgery after primary spine fusion for idiopathic scoliosis. Spine (Phila Pa 1976). 2009;34(20):2191-2197.
10. Bernhardt M., Bridwell K.H. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine (Phila Pa 1976). 1989;14:717-721.
11. Jackson R.P., McManus A.C. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size: a prospective controlled clinical study. Spine (Phila Pa 1976). 1994;19:1611-1618.
12. DeWald R.L. Osteotomy of the thoracic/lumbar spine. In: Bradford D.S., editor. Master techniques in orthopaedic surgery: the spine. Philadelphia: Lippincott-Raven; 1997:229-248.
13. White A.A., Panjabi M.M. Practical biomechanics of scoliosis and kyphosis. In: White A.A., Panjabi M.M., editors. Clinical biomechanics of the spine. Philadelphia: Lippincott-Raven; 1990:127-168.
14. White A.A. Biomechanical considerations in the surgical management of the spine. In: White A.A., Panjabi M.M., editors. Clinical biomechanics of the spine. Philadelphia: Lippincott-Raven; 1990:127-168.
15. Glassman S.D., Bridwell K., Dimar J.R., et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976). 2005;30(18):2024-2029.
16. Doherty J.H. Complications of fusion in lumbar scoliosis: proceedings of the Scoliosis Research Society. J Bone Joint Surg [Am]. 1973;55:438.
17. Farcy J.P., Schwab F.J. Management of flatback and related kyphotic decompensation syndromes. Spine (Phila Pa 1976). 1997;22:2452-2459.
18. Bradford D.S., Tribus C.B. Vertebral column resection for the treatment of rigid coronal decompensation. Spine. 1997;22(14):1590-1599.
19. Potter B.K., Lenke L.G., Kuklo T.R. Prevention and management of iatrogenic flatback deformity. J Bone Joint Surg [Am]. 2004;86(8):1793-1808.
20. Yang B.P., Chen L.A., Ondra S.L. Novel mathematical model of the sagittal spine: application to pedicle subtraction osteotomy for correction of fixed sagittal deformity. Spine J. 2008;8(2):359-366.
21. Robitaille M., Aubin C.E., Labelle H. Intra and interobserver variability of preoperative planning for surgical instrumentation in adolescent idiopathic scoliosis. Eur Spine J. 2007;16(10):1604-1614.
22. Harimaya K., Lenke L.G., Mishiro T., et al. Increasing lumbar lordosis of adult spinal deformity patients via intraoperative prone positioning. Spine (Phila Pa 1976). 2009;34(22):2406-2412.
23. Petit Y., Aubin C.E., Labelle H. Relation between patient positioning, trunk flexibility and surgical correction of the scoliotic spine. Stud Health Technol Inform. 2002;88:400-403.
24. Stephens G.C., Yoo J.U., Wilbur G. Comparison of lumbar sagittal alignment produced by different operative positions. Spine (Phila Pa 1976). 1996;21(15):1802-1806. discussion 1807
25. Tribus C.B., Belanger T.A., Zdeblick T.A. The effect of operative position and short-segment fusion on maintenance of sagittal alignment of the lumbar spine. Spine (Phila Pa 1976). 1999;24(1):58-61.
26. Geck M.J., Macagno A., Ponte A., Shufflebarger H.L. The Ponte procedure: posterior only treatment of Scheuermann’s kyphosis using segmental posterior shortening and pedicle screw instrumentation. J Spinal Disord Tech. 2007;20(8):586-593.
27. Bridwell K.H. Decision making regarding Smith-Petersen vs. pedicle subtraction osteotomy vs. vertebral column resection for spinal deformity. Spine (Phila Pa 1976). 2006;31(Suppl 19):S171-S178.
28. Smith-Peterson M.N., Larson C.B., Aufranc O.E. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. J Bone Joint Surg [Am]. 1945;27:1-11.
29. Van Royen B.J., DeGast A. Lumbar osteotomy for correction of thoracolumbar kyphotic deformity in ankylosing spondylitis: a structured review of three methods of treatment. Ann Rheum Dis. 1999;58:399-406.
30. LaChapelle E.H. Osteotomy of the lumbar spine for correction of kyphosis in a case of ankylosing spondylo-arthritis. J Bone Joint Surg [Am]. 1946;28:851-858.
31. Kostuik J.P., Maurais G.R., Richardson W.J., et al. Combined single stage anterior and posterior osteotomy for the correction of iatrogenic lumbar kyphosis. Spine (Phila Pa 1976). 1988;13:256-266.
32. Shufflebarger H.L., Clark C.E. Thoracolumbar osteotomy for postsurgical sagittal imbalance. Spine (Phila Pa 1976). 1992;17(Suppl):287-292.
33. Heinig C.F. Eggshell procedure. In: Luque E.R., editor. Segmental spinal instrumentation. Thorofare, NJ: Slack; 1984:221-234.
34. Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res. 1985;194:142-152.
35. Berven S.H., Deviren V., Smith J.A., et al. Management of fixed sagittal plane deformity: results of the transpedicular wedge resection osteotomy. Spine (Phila Pa 1976). 2001;26(18):2036-2043.
36. Suk S.I., Chung E.R., Lee S.M., et al. Posterior vertebral column resection in fixed lumbosacral deformity. Spine (Phila Pa 1976). 2005;30(23):E703-E710.
37. Lenke L.G., Sides B.A., Koester L.A., et al. Vertebral column resection for the treatment of severe spinal deformity. Clin Orthop Relat Res. 2010;468(3):687-699.
38. Cummine J.L., Lonstein J.E., Moe J.H., et al. Reconstructive surgery in the adult for failed scoliosis fusion. J Bone Joint Surg [Am]. 1979;61:1151-1161.
39. Lonstein J.E. Salvage and reconstructive surgery. In: Lonstein J.E., Bradford D.S., Winter R.B., Oglivie J.W., editors. Moe’s textbook of scoliosis and other spinal deformities. ed 3. Philadelphia: WB Saunders; 1995:387-398.
40. Steinmann J.C., Herkowitz H.N. Pseudarthrosis of the spine. Clin Orthop Relat Res. 1992;284:80-90.
41. Lauerman W.C., Bradford D.S., Ogilvie J.W., et al. Results of lumbar pseudarthrosis repair. J Spinal Disord. 1992;5:149-157.
42. Carpenter C.T., Dietz J.W., Leung K.K., et al. Repair of a pseudarthrosis of the lumbar spine: a functional outcome study. J Bone Joint Surg [Am]. 1996;78(5):712-719.
43. Maeda T., Buchowski J.M., Kim Y.J., et al. Long adult spinal deformity fusion to the sacrum using rhBMP-2 versus autogenous iliac crest bone graft. Spine (Phila Pa 1976). 2009;34(20):2205-2212.
44. Abdullah K.G., Steinmetz M.P., Benzel E.C., Mroz T.E. The state of lumbar fusion extenders. Spine (Phila Pa 1976). 2011;36(20):E1328-E1334.
45. Dimar J.R., Glassman S.D., Burkus K.J., Carreon L.Y. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine (Phila Pa 1976). 2006;31(22):2534-2539. discussion 2540
46. Larsen J.M., Capen D.A. Pseudarthrosis of the lumbar spine. J Am Acad Orthof Surg. 1997;5(3):153-162.
47. Heggeness M.H., Esses S.I. Classification of pseudarthrosis of the lumbar spine. Spine (Phila Pa 1976). 16(Suppl 8), 1991. S499–S454, 1991
48. Dawson E.G., Clader T.J., Bassett L.W. A comparison of different methods used to diagnose pseudarthrosis. J Bone Joint Surg [Am]. 1985;67(8):1153-1159.
49. Deckey J.E., Court C., Bradford D.S. Loss of sagittal plane correction after removal of spinal implants. Spine. 2000;25(19):2453-2460.
50. DePalma A.F., Rothman R.H. The nature of pseudarthrosis. Clin Orthop Relat Res. 1968;59:113-118.
51. Dawson E.G., Lotysch M.3rd, Urist M.R. Intertransverse process lumbar arthrodesis with autogenous bone graft. Clin Orthop Relat Res. 1981;154:90-96.
52. Kim D.H., Albert T.J. Update on use of instrumentation in lumbar spine disorders. Best Pract Res Clin Rheumatol. 2002;16(1):123-140.
53. McAfee P.C., Lee G.A., Fedder I.L., Cunningham B.W. Anterior BAK instrumentation and fusion: complete versus partial discectomy. Clin Orthop Relat Res. 2002;394:55-63.
54. Berven S., Tay B.K., Kleinstueck F.S., Bradford D.S. Clinical applications of bone graft substitutes in spine surgery: consideration of mineralized and demineralized preparations and growth factor supplementation. Eur Spine J. 2001;10(Suppl 2):S169-S177.
55. Cleveland M., Bosworth D.M., Thompson F.R. Pseudarthrosis in the lumbosacral spine. J Bone Joint Surg [Am]. 1948;30:302-312.
56. Boden S.D., Sumner D.R. Biologic factors affecting spine fusion and bone regeneration. Spine (Phila Pa 1976). 1995;20(Suppl 24):S102-S112.
57. Brown C.W., Orme T.J., Richardson H.D. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine (Phila Pa 1976). 1986;11:942-943.
58. Hefti F.L., McMaster M.J. The effect of the adolescent growth spurt on early posterior spinal fusion in infantile and juvenile idiopathic scoliosis. J Bone Joint Surg [Br]. 1983;65:247-254.
59. Dubousset J., Herring J.A., Shufflebarger H. The crankshaft phenomenon. J Pediatr Orthop. 1989;9(5):541-550.
60. Sanders J.O., Herring J.A., Browne R.H. Posterior arthrodesis and instrumentation in the immature (Risser-grade-0) spine in idiopathic scoliosis. J Bone Joint Surg [Am]. 1995;77:39-45.
61. Hamill C.L., Bridwell K.H., Lenke L.G., et al. Posterior arthrodesis in the skeletally immature patient. Assessing the risk for crankshaft: is an open triradiate cartilage the answer? Spine (Phila Pa 1976). 1997;22(12):1343-1351.
62. Saunders J.O., Little D.G., Richards S. Prediction of the crankshaft phenomenon by peak height velocity. Spine (Phila Pa 1976). 22(12), 1997. 1352–1356, 1997
63. Burton D.C., Asher M.A., Lai S.M. Scoliosis correction maintenance in skeletally immature patients with idiopathic scoliosis. Is anterior fusion really necessary? Spine (Phila Pa 1976). 2000;25(1):61-68.
64. Thompson J.P., Transfeldt E.E., Bradford D.S., et al. Decompensation after Cotrel–Dubousset instrumentation of idiopathic scoliosis. Spine (Phila Pa 1976). 1990;15:927-931.
65. Bridwell K.H., McAllister J.W., Betz R.R., et al. Coronal decompensation produced by Cotrel–Dubousset “derotation” maneuver for idiopathic right thoracic scoliosis. Spine (Phila Pa 1976). 1991;16:769-777.
66. Kalen V., Conklin M. The behavior of the unfused lumbar spine following selective thoracic fusion for idiopathic scoliosis. Spine (Phila Pa 1976). 1990;15:271-274.
67. Lenke L.G., Betz R.R., Bridwell K.H., et al. Spontaneous lumbar curve coronal correction after selective anterior or posterior thoracic fusion in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 1999;24(16):1663-1671. discussion 1672
68. Miller D.J., Jameel O., Matsumoto H., et al. Factors affecting distal end and global decompensation in coronal/sagittal planes 2 years after fusion. Stud Health Technol Inform. 2010;158:141-146.
69. Newton P.O., Faro F.D., Lenke L.G., et al. Factors involved in the decision to perform a selective versus nonselective fusion of Lenke 1B and 1C (King-Moe II) curves in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2003;28(20):S217-S223.
70. Cochran T., Irstam L., Nachemson A. Long-term anatomic and functional changes in patients with adolescent idiopathic scoliosis treated by Harrington rod fusion. Spine (Phila Pa 1976). 1983;8(6):576-584.
71. Moore M.R., Baynham G.C., Brown C.W., et al. Analysis of factors related to truncal decompensation following Cotrel-Dubousset instrumentation. J Spinal Disord. 1991;4(2):188-192.
72. Erickson M.A., Baulesh D.M. Lowest instrumented vertebra selection in AIS. J Pediatr Orthop. 2011;31(Suppl 1):S69-S76.
73. Lenke L.G., Bridwell K.H., O’Brien M.F., et al. Recognition and treatment of the proximal thoracic curve in adolescent idiopathic scoliosis treated with Cotrel-Dubousset instrumentation. Spine. 1994;19(14):1589-1597.
74. Kuklo T.R., Lenke L.G., Won D.S., et al. Spontaneous proximal thoracic curve correction after isolated fusion of the main thoracic curve in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2001;26(18):1966-1975.
75. Cunningham B.W., Sefter J.C., Hu N., et al. Biomechanical comparison of iliac screws versus interbody femoral ring allograft on lumbosacral kinematics and sacral screw strain. Spine (Phila Pa 1976). Feb 26, 2010. [Epub ahead of print]
76. Cho K.J., Suk S.I., Park S.R., et al. Risk factors of sagittal decompensation after long posterior instrumentation and fusion for degenerative lumbar scoliosis. Spine (Phila Pa 1976). 2010;35(17):1595-1601.
77. Emami A., Deviren V., Berven S., et al. Outcome and complications of long fusions to the sacrum in adult spine deformity: Luque-Galveston, combined iliac and sacral screws, and sacral fixation. Spine (Phila Pa 1976). 2002;27(7):776-786.
78. Moshirfar A., Rand F.F., Sponseller P.D., et al. Pelvic fixation in spine surgery. Historical overview, indications, biomechanical relevance, and current techniques. J Bone Joint Surg [Am]. 2005;87(Suppl 2):89-106.
79. Soegaard R., Bünger C.E., Christiansen T., et al. Circumferential fusion is dominant over posterolateral fusion in a long-term perspective: cost-utility evaluation of a randomized controlled trial in severe, chronic low back pain. Spine (Phila Pa 1976). 2007;32(22):2405-2414.
80. Bridwell K.H. Causes of sagittal spinal imbalance and assessment of the extent of needed correction. Instr Course Lect. 2006;55:567-575.
81. Behensky H., Cole A.A., Freeman B.J., et al. Fixed lumbar apical vertebral rotation predicts spinal decompensation in Lenke type 3C adolescent idiopathic scoliosis after selective posterior thoracic correction and fusion. Eur Spine J. 2007;16(10):1570-1578.
82. Goshi K., Boachie-Adjei O., Moore C., Nishiyama M. Thoracic scoliosis fusion in adolescent and adult idiopathic scoliosis using posterior translational corrective techniques (Isola): is maximum correction of the thoracic curve detrimental to the unfused lumbar curve? Spine J. 2004;4(2):192-201.
83. Albert T.J., Pinto M., Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion. Spine (Phila Pa 1976). 2000;25:123-129.
84. Berven S., Kao H., Deviren V., et al. Treatment of thoracic pseudarthrosis in the adult: is combined surgery necessary?. Clin Orthop Relat Res. 2003;411:25-31.
85. Arlet V., Marchesi D., Papin P., Aebi M. Decompensation following scoliosis surgery: treatment by decreasing the correction of the main thoracic curve or “letting the spine go. Eur Spine J. 2000;9(2):156-160.
86. Park P., Garton H.J., Gala V.C., et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976). 2004;29(17):1938-1944.
87. Lee G.A., Betz R.R., Clements D.H.3rd, Huss G.K. Proximal kyphosis after posterior spinal fusion in patients with idiopathic scoliosis. Spine (Phila Pa 1976). 1999;24(8):795-799.
88. Kim Y.J., Bridwell K.H., Lenke L.G., et al. Proximal junctional kyphosis in adolescent idiopathic scoliosis following instrumented posterior only spinal fusion: more than 5 year follow-up. Spine (Phila Pa 1976). 2005;30:2045-2050.
89. Glattes R.C., Bridwell K.H., Lenke L.G., et al. Proximal junctional kyphosis in adult scoliosis following instrumented posterior spinal fusion: incidence and risk factor analysis. Spine (Phila Pa 1976). 2005;30:1643-1649.
90 Kim Y.J., Bridwell K.H., Lenke L.G., et al. Proximal junctional kyphosis in adult spinal deformity after segmental posterior spinal instrumentation and fusion: minimum five-year follow-up. Spine (Phila Pa 1976). 2008;33:2179-2184.
91. Kim Y.J., Bridwell K.H., Lenke L.G., et al. Sagittal thoracic decompensation following long adult lumbar spinal instrumentation and fusion to L5 or S1: causes, prevalence, and risk factor analysis. Spine (Phila Pa 1976). 2006;31(20):2359-2366.
92. Lee Sang-Hun, Kim Ki-Tack, Suk Kyung-Soo D., et al. Sagittal decompensation after corrective osteotomy for lumbar degenerative kyphosis: classification and risk factors. Spine (Phila Pa 1976). 2011;36(8):E538-E544.
93. Yagi M., Akilah K.B., Boachie-Adjei O. Incidence, risk factors and classification of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Spine (Phila Pa 1976). 2011;36(1):E60-E68.
94. Watanabe K., Lenke L.G., Bridwell K.H., et al. Proximal junctional vertebral fracture in adults after spinal deformity surgery using pedicle screw constructs: analysis of morphological features. Spine (Phila Pa 1976). 2010;35(2):138-145.
95. Lowe T.G., Kasten M.D. An analysis of sagittal curves and balance after Cotrel-Dubousset instrumentation for kyphosis secondary to Scheuermann’s disease. A review of 32 patients. Spine (Phila Pa 1976). 1994;19(15):1680-1685.
96. Kim Y.J., Bridwell K.H., Lenke L.G., et al. Is the T9, T11, or L1 the more reliable proximal level after adult lumbar or lumbosacral instrumented fusion to L5 or S1? Spine (Phila Pa 1976). 2007;32(24):2653-2661.
97. Lee C.K. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976). 1988;13:375-377.
98. Weinhoffer S.L., Guyer R.D., Herbert M., et al. Intradiscal pressure measurements above an instrumented fusion: a cadaveric study. Spine (Phila Pa 1976). 1995;20:526-531.
99. Kim Y.E., Goel V.K., Weinstein J.N., et al. Effect of disc degeneration at one level on the adjacent level axial mode. Spine (Phila Pa 1976). 1991;16:331-335.
100. Phillips F.M., Reuben J., Wetzel F.T. Intervertebral disc degeneration adjacent to a lumbar fusion. An experimental rabbit model. J Bone Joint Surg [Br]. 2002;84:289-294.
101. Kanayama M., Togawa D., Hashimoto T., et al. Motion-preserving surgery can prevent early breakdown of adjacent segments: comparison of posterior dynamic stabilization with spinal fusion. J Spinal Disord Tech. 2009;22(7):463-467.
102. Panjabi M.M., Henderson G., James Y., Timm J.P. StabilimaxNZ versus simulated fusion: evaluation of adjacent-level effects. Eur Spine J. 2007;16(12):2159-2165.
103. Imagama S., Kawakami N., Matsubara Y., et al. Preventive effect of artificial ligamentous stabilization on the upper adjacent segment impairment following posterior lumbar interbody fusion. Spine (Phila Pa 1976). 2009;34(25):2775-2781.
104. Hart R.A., Prendergast M.A., Roberts W.G., et al. Proximal junctional acute collapse cranial to multi-level lumbar fusion: a cost analysis of prophylactic vertebral augmentation. Spine J. 2008;8(6):875-881.
105. Okada E, Hu SS, Deviren V, Berven S: Results of surgical treatment for proximal junctional kyphosis after adult spinal deformity surgery. Minimum 2 year follow-up study. In Preparation.