Chapter 28 Postoperative Respiratory Care
Patients undergoing cardiac surgery experience physiologic stresses from anesthesia, thoracotomy, surgical manipulation, and cardiopulmonary bypass (CPB). Each of these interventions can create transient deleterious effects on pulmonary function even with normal lungs; the effects may be exaggerated in the presence of preexisting pulmonary pathologic processes. Important pulmonary changes after cardiac surgery include diminished functional residual capacity (FRC) after general anesthesia and muscle relaxants, transient 50% to 75% reduction in vital capacity (VC) after median sternotomy and intrathoracic manipulation, atelectasis, and increased intravascular lung water. Acute FRC reduction results in arterial hypoxemia due to mismatch between ventilation and perfusion and in diminished lung compliance with increased work of breathing. This additional work of breathing, which increases oxygen consumption by up to 20% in spontaneously breathing patients, also increases myocardial work at a time when myocardial reserves may be limited. Changes in spirometric measurements and respiratory muscle strength can last up to 8 weeks postoperatively.1
RISK FACTORS FOR RESPIRATORY INSUFFICIENCY
Assessing Risk Based on Preoperative Status
The Society of Thoracic Surgeons (STS) National Adult Cardiac Surgery Database is widely used and offers, in addition to a mortality model, a model customized to predict prolonged ventilation.2 Chronic obstructive pulmonary disease (COPD) might be expected to be a major risk for postoperative morbidity and mortality. However, hospital mortality with mild-to-moderate COPD is not especially high; it is the minority of patients with severe COPD, especially those older than the age of 75 years or receiving corticosteroids, who are at highest risk. Patients with preexisting COPD have higher rates of pulmonary complications (12%), atrial fibrillation (27%), and death (7%).3 Obesity, defined by increased body mass index, does not appear to increase the risk of postoperative respiratory failure.
Studies have used multivariate regression techniques to elucidate factors specifically associated with postoperative respiratory failure (Table 28-1).4–7 They differ in their endpoints for outcome and in their choice of preoperative versus operative versus postoperative variables. The STS model was found to be the single best predictor of mechanical ventilation support for longer than 72 hours but also identified mitral valvular disease, age, vasopressor and inotrope use, renal failure, operative urgency, type of operation, preoperative ventilation, prior cardiac surgery, female gender, myocardial infarction within 30 days, and previous stroke as contributors.5 None of these models, general or specific for respiratory complications, is sufficiently sensitive or specific to prohibit consideration of surgery for an individual patient, but all provide the clinician with early warning for patients at high risk.
Table 28-1 Factors Predicting Postoperative Respiratory Outcome
| Study | Endpoint | Risk Factors |
|---|---|---|
| Spivack et al, 19964 | Mechanical ventilation > 48 hr |
LVEF = left ventricular ejection fraction; CHF = congestive heart failure; STS = Society of Thoracic Surgeons; CABG = coronary artery bypass grafting; COPD = chronic obstructive pulmonary disease; BUN = blood urea nitrogen; DO2 = systemic oxygen delivery; CPB = cardiopulmonary bypass; Hct = hematocrit.
Postoperative Events
The expected postoperative course is a short period of ventilation support while the patient is warmed, allowed to awaken, and observed for bleeding or hemodynamic instability. Preoperative risks, issues with difficult intubation, and operating room events should be communicated from the operating room team to the ICU team at the time of ICU admission. Box 28-1 outlines criteria to be met before routine extubation.
BOX 28-1 Criteria to Be Met before Early Postoperative Extubation
Postoperative care of low-risk cardiac surgical patients has come to resemble a recovery room model, but high-risk patients benefit from postoperative involvement of anesthesiologists, cardiologists, and critical care specialists. The presence of full-time ICU staff physicians improves outcome and is now recommended by the Leapfrog Group as a patient safety standard.8
DIAGNOSIS OF ACUTE LUNG INJURY AND ACUTE RESPIRATORY DISTRESS SYNDROME
The proliferative phase of ARDS occurs on days 3 to 7 as inflammatory cells accumulate as a result of chemoattractants released by the neutrophils. At this stage, the normal repair process would remove debris and begin repair, but a disordered repair process may result in exuberant fibrosis, stiff lungs, and inefficient gas exchange. Evidence suggests that careful medical and ventilator management may affect this process. Any precipitating factors should be addressed (e.g., draining closed-space infections). Conventional ventilator support following cardiac surgery is to maintain large tidal volumes (typically 10 to 12 mL/kg) to reopen atelectatic but potentially functional alveoli. The problem is that the compromised lung is no longer homogeneous and high pressures can further damage the remaining normal lung over time. Direct mechanical injury may occur as a result of overdistention (volutrauma), high pressures (barotrauma), or shear injury from repetitive opening and closing. “Biotrauma” may also occur as a result of inflammatory mediator release and impaired antibacterial barriers. Current clinical practice with known or suspected lung injury is to limit inflation pressures. The maximal “safe” inflation pressure is not known, but evidence favors keeping peak inspiratory pressures at less than 35 cm H2O and restricting tidal volumes to about 6 mL/kg of ideal body weight. The landmark ARDSNet trial randomized patients to 6 mL/kg versus 12 mL/kg of ideal body weight and demonstrated a significant difference in 28-day survival with the low-tidal-volume group.9
Cardiopulmonary Interactions
Understanding cardiopulmonary interactions associated with mechanical ventilation is essential. Hemodynamic changes may occur secondary to changes in lung volume and intrathoracic pressure even when tidal volume remains constant.10 Pulmonary vascular resistance and mechanical heart-lung interactions play prominent roles in determining the hemodynamic response to mechanical ventilation. Because lung inflation alters pulmonary vascular resistance and right ventricular wall tension, there are limits to intrathoracic pressure that a damaged heart will tolerate. High lung volumes may also mechanically limit cardiac volumes. In patients with airflow obstruction, occult PEEP (auto-PEEP) may also contribute to hypotension and low CO. Auto-PEEP can be detected by respiratory waveform monitoring or by pressure monitoring with the ventilator’s expiratory port held closed at end-exhalation in patients who are not spontaneously breathing. Auto-PEEP often responds to bronchodilators and/or increased expiratory time to permit more complete exhalation.
GENERAL SUPPORT ISSUES
The Agency for Healthcare Research and Quality has identified a number of patient safety practices applicable to the ICU patient; at the top of the list are appropriate venothromboembolism (VTE) prophylaxis, use of perioperative β-blockers, use of maximum sterile barriers during catheter insertion, and use of antibiotic prophylaxis.11 Standard practice in icu patients includes prophylaxis against gastrointestinal bleeding with histamine blockers or proton pump inhibitors, unless the patient is receiving continuous gastric feedings; head of bed elevation to 30 degrees or more in hemodynamically stable patients; a brief daily wake-up from sedation; use of in-line suction catheters; tight glucose control; and appropriate VTE prophylaxis. Box 28-2 summarizes these risk-reduction efforts.
Impediments to Weaning and Extubation
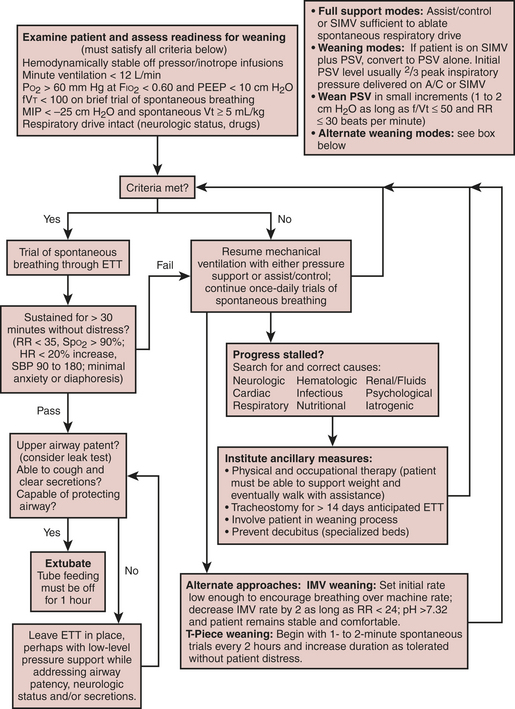
Factors limiting the removal of mechanical ventilatory support include delirium, neurologic dysfunction, unstable hemodynamics, respiratory muscle dysfunction, renal failure with fluid overload, and sepsis. Figure 28-1 outlines one approach to identifying readiness to wean and possible alternative approaches to weaning.
Neurologic Complications
Delirium is common in long-stay ICU patients and is associated with higher costs in the ICU unit and for the entire hospital stay.12 Transient postoperative delirium occurs in about 7% of patients, resolving spontaneously or with pharmacologic intervention in almost all patients by postoperative day 6. Alcohol or benzodiazepine withdrawal should be considered in the differential diagnosis of delirium. Initial management of agitation consists of reassurance and orientation of the patient and control of pain with opioids.
Cardiac Complications
Patients maintained on chronic amiodarone therapy are prone to postoperative respiratory failure, longer intubation times, and longer ICU stays, even with only subclinical evidence of pulmonary amiodarone toxicity.13 Rarely, patients taking amiodarone develop life-threatening pulmonary complications, including ARDS. Histologic lung examination of these patients demonstrates marked interstitial fibrosis with enlarged air spaces (“honeycomb” appearance) and hyperplasia of type II pneumocytes.
LIBERATION FROM MECHANICAL SUPPORT (WEANING)
Inability to Wean
The experience has been that it is rarely a single problem, but the interaction among multiple morbidities that creates a situation in which the patient may not separate from the ventilator. At this point, a frank discussion with the patient (if he or she has decisional capacity) or the health care proxy can be helpful in defining the benefits and burdens of further therapy and the patient’s desires. Consultation from the hospital’s ethics team may be very helpful. Patients who remain in a low cardiac output state and who have sustained multiorgan failure rarely if ever end their dependence on high-technology support, including ventilation and hemodialysis. On the other hand, malnutrition and deconditioning in the absence of ongoing sepsis and organ system failure sometimes respond to prolonged rehabilitation, which may be better handled by a long-term ventilation facility than an acute-care hospital. Recommendations for the difficult-to-wean cardiac surgical patient are summarized in Boxes 28-3 and 28-4.
BOX 28-4 The Difficult-to-Wean Patient
SUMMARY
1. Johnson D., Hurst T., Thompson D., et al. Respiratory function after cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:571.
2. The Society of Thoracic Surgeons. 30-Day operative mortality and morbidity risk models. Ann Thorac Surg. 2003;75:1856.
3. Samuels L.E., Kaufman M.S., Morris R.J., et al. Coronary artery bypass grafting in patients with COPD. Chest. 1998;113:878.
4. Spivack S.D., Shinozaki T., Albertini J.J., Deane R. Preoperative prediction of postoperative respiratory outcome. Coronary artery bypass grafting. Chest. 1996;109:1222-1230.
5. Branca P., McGaw P., Light R.W., et al. Factors associated with prolonged mechanical ventilation following coronary artery bypass surgery. Chest. 2001;119:537.
6. Rady M.Y., Ryan T. Perioperative predictors of extubation failure and the effect on clinical outcome after cardiac surgery. Crit Care Med. 1996;27:340.
7. Canver C.C., Chandra J. Intraoperative and postoperative risk factors for respiratory failure after coronary bypass. Ann Thorac Surg. 2003;75:853.
8. www.leapfroggroup.org/factsheets/ICU_factsheet.pdf accessed on April 28, 2004
9. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301.
10. Steingrub J.S., Tidswell M.A., Higgins T.L. Hemodynamic consequences of heart-lung interactions. J Intens Care Med. 2003;18:92.
11. http://www.AHRQ.gov/clinic/ptsafety/addend.htm. Accessed May 20, 2004
12. Milbrandt E.B., Deppen S., Harrison P.L., et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;31:955.
13. Nalos P.C., Kass R.M., Gang E.S., et al. Life-threatening postoperative pulmonary complications in patients with previous amiodarone pulmonary toxicity undergoing cardiothoracic operations. J Thorac Cardiovasc Surg. 1987;93:904.