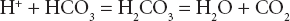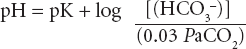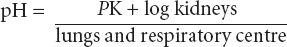Chapter 11 Postoperative problems
11.1 Introduction
Common postoperative problems occur despite the best of surgical care but can be minimised with adequate preoperative planning. Patient-related concurrent medical problems are identified prior to surgery and these conditions are optimised. This has been discussed in detail in chapter 10.
The most common postoperative problems are listed in Table 11.1.
| Wound | Infection |
| Haematoma | |
| Dehiscence | |
| Incisional hernia | |
| Fever | Atelectasis |
| Sepsis | |
| Thromboembolic disease | |
| Vomiting | Anaesthesia |
| Ileus | |
| Obstruction | |
| Shock | Haemorrhage |
| Myocardial infarction | |
| Sepsis | |
| Pulmonary embolus | |
| Haemorrhage | Wound |
| Concealed | |
| Gastrointestinal | |
| Secondary | |
| Jaundice | Septicaemia |
| Drug cholestasis | |
| Hepatitis | |
| Respiratory | Atelectasis |
| Aspiration | |
| Adult respiratory distress syndrome | |
| Pneumothorax | |
| Cardiovascular | Arrhythmias |
| Myocardial infarction | |
| Congestive cardiac failure | |
| Urinary | Retention |
| Infection | |
| Renal injury | |
| Psychiatric | Delirium |
| Vascular access | Phlebitis |
| Pneumothorax |
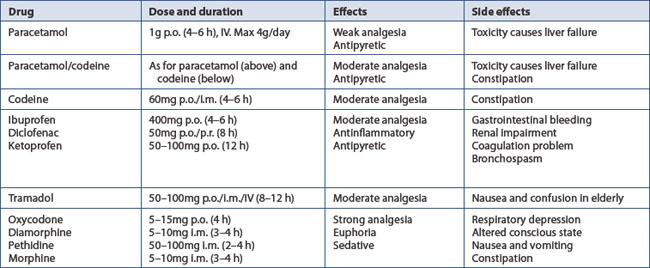
11.2 Pain
Pre-emptive analgesia is started at operation. This includes:
11.3 Nausea and vomiting
Nausea and vomiting are common in the immediate postoperative recovery. These are often due to anaesthetic-related agents and postoperative analgesia, especially opiates. The common causes are listed in Box 11.1. These include: early, postanaesthetic sickness;
acute gastric dilatation within 48 hours; paralytic ileus from two to three days; and mechanical intestinal obstruction thereafter.
A later onset of vomiting and distension, with colicky pain, in a patient who otherwise appears to be recovering satisfactorily suggests that mechanical adhesive obstruction has developed. Ileus and mechanical obstruction, however, may be very difficult to differentiate in the postoperative period. Patients with suspected adhesive obstruction are also treated conservatively initially. This consists of intermittent nasogastric aspiration and drainage, correction of fluid and electrolytes, and analgesia. But, if tenderness or a mass develop, if pain is severe or becomes continuous or if symptoms and signs persist for more than two to three days, laparotomy is indicated because of the danger of strangulation.
11.4 Tachycardia
Hypovolaemia either from excessive loss, as in haemorrhage or gastrointestinal loss, or dehydration from inadequate fluid replacement is manifested by tachycardia unless the cardiac response is suppressed by beta-blockers. Treatment is rapid correction of coagulopathy, seeking the source of haemorrhage and intravascular volume replacement. A central venous line along with a urinary catheter are helpful in monitoring the progress of treatment (Ch 11.9).
11.5 Fever
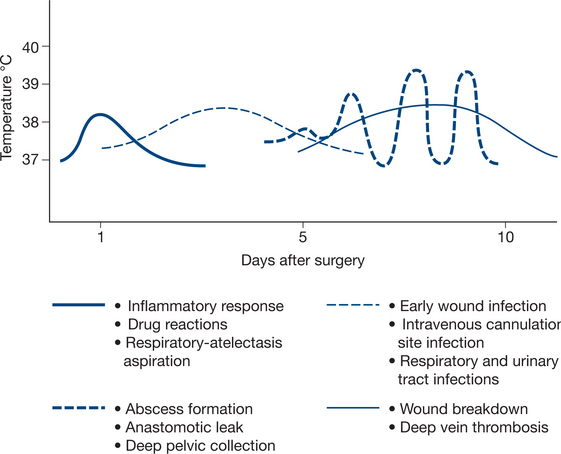
A number of factors are helpful in determining the possible causes of fever. These include the timing of fever in relation to surgery, the pattern of temperature changes and the type of surgery with its specific complications. Table 11.3 provides a summary of the common causes of postoperative fever and Figure 11.1 provides a visual summary of the timing and pattern of fevers.
| Diagnosis | Days after operation |
|---|---|
| Reaction to blood products | 0–1 |
| Atelectasis | 1–2 |
| Pneumonia | 2–4 |
| Infusion thrombophlebitis | 2–5 |
| Wound infection | 5–30 |
| Thromboembolism | 5–15 |
| Less common causes | |
| Tissue necrosis — myocardial infarction | 0–5 |
| Malignant hyperpyrexia | 0–5 |
| Neoplasm | – |
| Acute gout | 5–10 |
| Fat embolism syndrome | 2–5 |
| Drug allergy | – |
| Endocrine — thyroid or adrenal crisis | 2–5 |
| Iatrogenic-overheating patient | – |
| Faking of fever by patient | – |
The management of postoperative fever demands a careful history and examination of the patient. Particular attention is paid to the various potential septic sites and if necessary, specimens are taken for microbiological analysis including any wound discharge, urine, sputum, blood and central venous catheter tip culture. Appropriate imaging is requested, such as duplex ultrasound for deep vein thrombosis, chest X-rays for chest complications, abdominal ultrasound or CT scanning for intra-abdominal collections
All septic collections are treated by either percutaneous drainage with or without imaging guidance or by open surgery if this is unsuccessful.
11.6 Shortness of breath and tachypnoea
Common causes
1 Basal lung atelectasis, bronchitis and bronchopneumonia: ‘the postoperative chest’
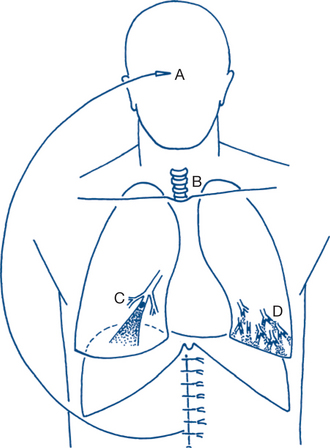
Atelectasis implies collapsed airless lung tissue and is unfortunately a very common complication of surgery — particularly after upper abdominal surgery. It usually presents within 24–48 hours of surgery (Fig 11.2) and, if untreated, will progress to bronchitis, bronchopneumonia and hypoxaemic respiratory failure.
Massive collapse of a whole lung is possible but more often the collapse is segmental (lobular or lobar atelectasis). Collapsed segments with stagnant secretions predispose to pulmonary infection. Intrapulmonary blood flow is shunted through the collapsed airless segments and results in hypoxia of moderate or profound degree; PaO2 falls from the normal 100 mmHg breathing air to 70 mmHg or less. Diffusion of CO2 is more efficient than that of O2 and the PaCO2 is usually normal or slightly subnormal because hypoxia causes overbreathing. Clinically the patient develops shortness of breath, rapid breathing, a rapid pulse and fever — usually within 24–48 hours of operation. The respiratory symptoms are generally proportional to the degree of atelectasis, as are the lung signs on examination. Minor degrees of lobular atelectasis without clinically apparent chest signs are very common and are the most usual cause of fever and tachycardia within the first day or two of surgery. Chest X-rays in the early postoperative period often reveal areas of basal lung atelectasis as streaky linear areas that were unsuspected clinically.
2 Adult respiratory distress syndrome — acute respiratory failure
A variety of factors, including complications of surgery or trauma, can cause severe generalised acute respiratory insufficiency. A plethora of names for the syndrome (‘shock lung’, ‘acute posttraumatic or postoperative pulmonary insufficiency’, ‘adult respiratory distress syndrome — ARDS’) reflects the confusion surrounding its aetiology. The syndrome may follow any one of several widely different clinical situations (Box 11.2). Clinical features are impaired arterial oxygenation (diminished PaO2), diffuse lung opacification on chest X-ray and an increasing ‘stiffness’ of the lungs (decreased compliance).
4 Pulmonary embolism
The classical presentation of pulmonary embolism is a sudden onset of dyspnoea, shock followed by pleuritic chest pain, haemoptysis and pleural rub. This generally appears a few days after surgery. This must be diagnosed and treated rapidly to prevent fatal outcome (Ch 11.9 and Ch 4).
5 Acute pulmonary oedema
Acute pulmonary oedema manifests as shortness of breath and tachypnoea with or without pink frothy sputum. It is usually the result of fluid overload and left ventricular failure, often in a patient with pre-existing cardiac disease. Acute myocardial infarction should be considered. Basal crepitations are present and the jugular venous pressure is elevated. Treatment consists of oxygen administration, morphine, diuretics and ventilatory support if necessary.
11.8 Low urine output
Common causes
Clinical features and management plan
1 Prerenal: acute vascular insufficiency
Poor urine output is often due to hypotension during surgery or inadequate fluid replacement in a fasting patient. The clinical circumstances are suggestive, with gradually diminishing urine output in association with clinical signs of intravascular volume depletion. A patent urethral catheter must be in place to ensure that urinary retention has been excluded. Urine analysis may show an elevated specific gravity (>1015) or osmolality, a high urea (>20 g/L) or creatinine content, a low sodium content (<25 mmol/L) and high urinary/plasma ratios of creatinine, urea or osmalolity (Table 11.4).
| Prerenal acute vascular insufficiency | Renal acute intrinsic renal injury | |
|---|---|---|
| Specific gravity | High (>1015) | Low |
| Osmolality | High | Low |
| Urea | High (>20 g/L) | Low |
| Creatinine | High | Low |
| Sodium | Low (<25 mmol/L) | High |
| Urinary/plasma ratio of creatinine, urea and osmolality | High | Low |
11.9 Sudden collapse or rapid deterioration
The four most common causes in the postoperative period are:
Less common causes include anaphylaxis and incompatible transfusion, tension pneumothorax, stroke, hypoxia and drug-induced respiratory depression, metabolic derangements (hypokalaemia, hyponatraemia, hypo- or hyperglycaemia) and acalculous cholecystitis (Table 11.5).
| Time after operation | |
|---|---|
| Hypovolaemia: concealed haemorrhage | 0–48 hours |
| Myocardial infarction | 1–3 days |
| Cardiac arrhythmia | |
| Septic shock: local surgical complication | 4–5 days |
| Pulmonary embolus | 5–10 days |
| Less common causes | |
| Anaphylaxis | |
| Drug reaction | Variable |
| Incompatible transfusion | Early |
| Tension pneumothorax | Variable |
| Acalculous cholecystitis | First week |
Perioperative haemorrhage is of three main types: primary during operation, reactionary within 24 hours and secondary about a week later (Box 11.3). Most cases of reactionary (and primary) haemorrhage are from an unligated vessel or a slipped ligature or clip and are not secondary to a coagulation disorder. The bleeding in secondary haemorrhage is due to erosion of an artery from spreading infection. Secondary haemorrhage is seen mainly when a heavily contaminated wound is closed primarily and can often be prevented by delayed wound closure.
Box 11.3
Characteristics of postoperative haemorrhage
Reactionary — <24 hours, due to unligated vessel
Most instances of concealed intraperitoneal haemorrhage are reactionary, occurring within 24 hours. Shock develops and the patient is noted to have a falling haemoglobin. The fall in haemoglobin may offer an important clue to the diagnosis. Physical examination and girth measurement are unreliable in the postoperative patient and drainage tubes often block with clot and so may prove to be of little diagnostic assistance. Secondary intraperitoneal haemorrhage is most commonly seen as a delayed complication of acute pancreatitis. In such inaccessible sites temporary control of bleeding can sometimes be achieved by arterial embolisation under angiographic control, before infection is definitively controlled by thorough debridement and drainage.
11.10 Wound care problems
In brief, wound healing starts immediately with surgery.
In the early phase, there is establishment of haemostasis and onset of inflammatory changes from days one to four. The intermediate phase is characterised by mesenchymal cell migration and proliferation, angiogenesis and epithelialisation over two to five days. In the late phase, collagen and other matrix proteins are laid down and the wound contracts over one to four weeks. The final phase of wound healing involves scar formation and remodelling over many months.
The wound complications encountered in the early postoperative period are: haematoma, seroma, ischaemia, infection and dehiscence. Later complications are wound sinus; painful, hypertrophic or keloid scar; and incisional hernia (Box 11.4).
Wound dehiscence usually occurs about a week after surgery, especially in the cachectic patient with increased postoperative intra-abdominal pressure from cough and ileus (Box 11.5). Sutures too widely spaced, of the wrong material, with too small bites and/or under too much tension are the faulty technical factors of most importance in the pathogenesis of burst abdomen. The usual scenario is of a sudden ‘pop’ in the wound after coughing, followed by pain, a serosanguinous discharge or evisceration. Other factors such as wound infection or erosion by a fistula can contribute to dehiscence but more often cause gradual separation of the wound depths and a subsequent hernia. The wound and eviscerated bowel should be covered with moist occlusive dressing to prevent excessive fluid loss from the bowel. Surgical repair of acute dehiscence under general anaesthesia is carried out as soon as possible. Interrupted nonabsorbable sutures with wide bites of all layers, including the skin (‘tension’ sutures), are usually employed for such secondary wound closure.
Box 11.5
Causes of wound dehiscence
Inappropriate suture (absorbable, calibre too small)
Inappropriate suturing: too far apart, bites too close, bites too small, too much tension
Postoperative distension and increased intra-abdominal pressure: ileus, coughing, straining
Wound problems can also arise after discharge from hospital. A wound sinus can persist after a preceding wound infection. Wound healing will not occur until the infected deep non-absorbable suture is removed. In patients with underlying prosthetic materials, such as artificial joints or vascular grafts, a sinus may indicate prosthetic infection. The inflammatory markers, such as C-reactive proteins (CRP) and erythrocyte sedimentation rate (ESR), are often raised. Some patients develop a painful or sensitive scar after surgery. In most instances resolution of pain occurs within six months. In the meantime pain may need to be controlled with nonspecific local measures such as massage, local steroid and anaesthetic injections and local ultrasound. Hypertrophic and keloid scars are caused by tension, infection or ischaemia, but can be idiosyncratic. Dark-skinned patients are more prone to keloid scars than those with fairer skins. The majority of patients who develop an incisional hernia have had a previous wound infection. Most appear within three months of surgery, but hernias can appear years later after initially sound primary healing. Unless a definite contraindication to surgery exists, the incisional hernia should be repaired soon after diagnosis, before the hernia becomes so large that a sound repair is difficult to achieve. A mesh is often necessary to reduce any tension on the repair.
11.11 Abnormal investigations
Hyperkalaemia
The common causes are renal failure and shock.
Emergency treatment of hyperkalaemia (>7 mmol/L) involves the following two procedures.
The combined effects of these two measures last for several hours.
Haemofiltration is an alternative technique using pressure ultrafiltration causing transfer of large volumes of fluid and electrolyte across a membrane. It is often used as an alternative to haemodialysis and requires careful monitoring of water and ECF balance and intravenous replacement of the large volumes of crystalloid fluid removed. This is done in the HDU or intensive care setting (Ch 12).
Hydrogen ion (acid–base) disorders
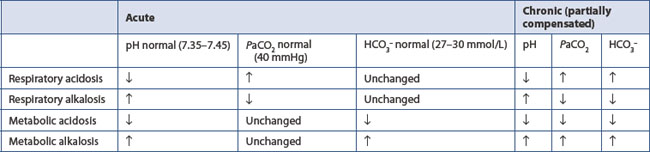
This forms the basis of renal and respiratory control of acid–base disorders. The left side of the equation is governed by the balance between production of hydrogen ion by tissue perfusion and metabolism and its excretion by the kidney; the right side by balanced excretion of CO2 via the lungs under the influence of the respiratory centre. Thus CO2 elimination is equivalent to the elimination of acid. In the renal tubules, filtered NaHCO3 is converted to carbonic acid. Sodium ions are replaced by hydrogen ions excreted by the tubular cell. Dissociation of carbonic acid to CO2 and H2O allows CO2 to diffuse back into the tubular cells to there form fresh bicarbonate equivalent to the hydrogen ions excreted. Normally 50–100 mmol of hydrogen ion is excreted daily by the kidney and 15 000 mmol (approximately 300 L) of CO2 by the lungs. Metabolic and respiratory disturbances primarily affect the left and right sides respectively of the equation (Table 11.6).
If the ratio of bicarbonate to CO2 remains unchanged, pH remains constant.
Metabolic alkalosis
The common cause is loss of acid because of obstruction of the gastric outlet (‘pyloric’ stenosis). Deficiencies of water, sodium, chloride and potassium in addition to alkalosis result from combined gastrointestinal and urinary losses of water and electrolytes. The renal response is initially to secrete an alkaline urine containing Na, K and HCO3; however, the urine subsequently becomes acid as hydrogen ion is secreted by the tubular cells in the face of severe depletion of the cations sodium and potassium.