Postoperative Management of the Cardiac Surgery Patient
Neurologic Care
The risk factors for neurologic injury after cardiac surgery have been well elucidated, but effective risk modification to reliably prevent neurologic complications has been elusive. In certain operations, measures to reduce risk have been implemented and have become incorporated in perioperative management.1,2 For example, patients with descending thoracic aortic operations are at significant intraoperative risk for spinal cord ischemia, as well as delayed injury (days postoperatively). These patients usually have a spinal drain placed preoperatively to reduce the risk of spinal cord injury. Spinal cord perfusion pressure (SCPP) is the difference between mean arterial pressure (MAP) and cerebrospinal fluid pressure (CSFP) (SCPP = MAP − CSFP); thus, lowering of CSFP will increase SCPP and potentially improve neurologic function as long as an adequate MAP is maintained.3
Neurologic complications are still considered one of the major risks associated with coronary artery grafting. The advanced age of bypass patients, high incidence of associated carotid occlusive disease, and increased aortic atheroembolic burden place older coronary artery bypass graft (CABG) patients at increased risk of central neurologic complications in the postoperative period.4
Other strategies for preoperative and intraoperative risk modification are becoming routine. They include selective use of preoperative carotid imaging, routine use of intraoperative transesophageal echocardiography (TEE) and epiaortic echocardiography, descending aortic cannulation with TEE guidance, aortic-no-touch technique, high-flow/high-pressure cardiopulmonary bypass (CPB), retrograde cerebral perfusion during circulatory arrest, carbon dioxide insufflation, intraoperative cerebral oxygen saturation monitoring, echocardiography de-airing, and maintenance of baseline perioperative blood pressure.5,6 Early neurologic complications vary in clinical presentation from focal to global neurologic deficit such as seizures, ischemic encephalopathy, and coma. Several authors have classified these complications as type I (focal injury, stupor, and coma) and type II (seizures, neurocognitive dysfunction, and delirium).7 This classification does not emphasize pathophysiologic mechanisms that could better inform preventive measures.5,7 Regardless of the classification system used, overlap often exists.
In comparison to the other complications, focal neurologic deficits from stroke carry a mortality rate of up to 20% in the first postoperative month and prolong ICU and hospital lengths of stay.8–10 In a mixed cardiac surgery patient population, the incidence of stroke varied among those patients having CABG, combined CABG and heart valve surgery, and ascending aorta repair.11–13 The incidence of stroke depends on the surgical procedure (isolated CABG, 1.4-3.8%; combined CABG and valve, 7.4%; isolated valve, 4.8-8.8%; multiple valve, 9.7%; and aorta, 8.7%).12
Unfortunately, most of the known baseline patient variables associated with perioperative stroke are not modifiable. Despite the identification of many intraoperative variables that cause intraoperative stroke (macroembolism of debris, air embolism, hypoperfusion, hypoxemia, coagulation status), risk remains significant and continues in the early postoperative period. Although there was early enthusiasm for off-pump CABG as an operative technique to reduce stroke, in the most recent multicenter clinical trial the 30-day incidence of stroke was not significantly decreased compared with on-pump CABG.14
The majority of strokes are ischemic (62%); fewer are due to hypoperfusion (9%) or hemorrhage (<1%).12 Hemorrhagic strokes are rare after cardiac surgery and the clinical presentation is notable for delayed awakening and coma despite discontinuing sedation or progressive clinical deterioration as intracranial pressure (ICP) increases. Hemorrhagic transformation is recognized in 20% of ischemic strokes.10 Embolic strokes tend to present with acute hemiparesis and represent a compromise of specific cerebral artery area. Thrombotic or ischemic strokes are related to diminished blood flow with the most vulnerable areas being those between major cerebral artery perfusion territories (watershed areas).
Cardiac transplantation has a higher associated occurrence of neurologic complications than CABG.15,16 Symptom onset for focal motor deficits in these patients usually occur after the second postoperative day, especially in patients receiving mechanical circulatory support or experiencing major postsurgical complications such as tamponade, severe cardiac dysfunction, prolonged CPB, or postoperative hepatic failure.
Predictive models may enable implementation of earlier interventions.14 More than half of strokes are identified within the first day after CABG.10 Because of associated morbidity and death, an objective neurologic evaluation by minimizing sedation in the first 2 postoperative hours is desirable. Weaning sedation should be initiated only when the risk of doing so is minimal (i.e., a normothermic, nonacidemic, nonagitated patient with minimal chest tube output).
Once a new focal neurologic deficit, seizure, or delayed awakening is documented, a noncontrast brain CT scan should be obtained immediately. The most important tomographic findings include loss of insular ribbon, loss of gray-white interface, loss of sulci, acute hypodensity, mass effect, and dense mean cerebral artery sign. Owing to the relative insensitivity of CT scans for diagnosing ischemia, early CT scans may be normal, despite areas of brain infarction.17 Magnetic resonance imaging (MRI) may help identify these lesions.17 MRI usually cannot be obtained safely in the early postoperative period because of the need to move the patient off the unit to a usually remote location and because non-MRI compatible pericardial pacing wires are routinely placed in cardiac surgery patients. MRI will document a new lesion in 26% to 50% of cases, but there is often lack of correlation with the neurocognitive deficit.18,19 Brain CT scans are more sensitive in diagnosing large air embolism, which is often the cause of abnormal postoperative neurologic findings in these patients. In the patient with a focal deficit or persistent embolic events, transcranial Doppler, transthoracic echocardiography (TTE), or TEE can be used to help identify sources of recurrent cardiac or aortic embolization.
Intra-arterial administration of tPA is an alternative for acute treatment of ischemic stroke within 6 hours of clinical presentation. The safety and efficacy of intra-arterial urokinase and tPA in selected patients who presented ischemic stroke within 12 days after cardiac surgery have been demonstrated.20 The mean time from operation to stroke was 4.3 days. Thrombolytic therapy was commenced within 3.6 hours. No operative intervention for bleeding was necessary and 38% of the patients had neurologic improvement. Mechanical clot retrieval is another acute stroke therapy in the first 8 hours since neurologic deficit. Patients who will likely benefit from this therapy include those with significant neurologic deficit and large vessel occlusion with core ischemia approximately less than 30% of the middle cerebral artery territory.
In the cardiac surgery patient, coma is defined as prolonged unconsciousness after discontinuation of anesthetics, sedatives, and opioids and inability to show response to motor/verbal commands. A recent retrospective investigation found an incidence of delayed awakening in approximately 0.5% of all cardiac surgery patients.21 However, the period of time in the immediate postoperative phase can be challenging as the necessity of adequate neurologic examination must be weighed against the patient’s hemodynamic stability. If a specific diagnosis is not found, and there is concomitant severe heart failure, it portends a worse outcome.21 Moreover, up to 20% of patients with postcardiotomy stroke present with delayed awakening and coma-like symptoms.21
Evaluation with brain CT is mandatory in these cases and it should occur as soon as the coma state has been recognized and reversible metabolic causes have been treated. The identified risk factors for delayed awakening after cardiac surgery in the aforementioned investigation included urgent cardiac surgery, elevated serum creatinine (Cr), and lower postoperative hemoglobin level.21 Most of these patients had normal brain CT scan imaging and ultimately recovered consciousness after being comatose during the first 24 hours.21 Although some investigators have proposed a role for altered renal excretion of sedatives in patients with postoperative renal failure or decreased oxygen delivery to the brain with anemia, the specific underlying mechanisms in these cases remain unclear.
Postoperative seizure is an independent predictor of permanent neurologic deficit and increased mortality risk.22,23 A recent study showed seizures were a strong predictor of permanent neurologic deficit and increased mortality risk. It is important to remember that any neurologic injury can present initially as a seizure. The independent risk factors for postoperative seizures included deep hypothermic circulatory arrest, aortic calcification or atheroma, and critical preoperative state. Other specific risk factors for early seizures include perioperative administration of the antifibrinolytic tranexamic acid administration and preexisting renal insufficiency.
Tranexamic acid has proconvulsant properties and it is reasonable to hold the infusion of this agent in the early postoperative period if the patient presents with seizures. The most common proposed mechanism of seizures after cardiac surgery is focal or global ischemia from hypoperfusion (air or atheroembolism) or metabolic disorders. Early brain CT scan is essential because it may detect potential reversible causes of neurologic injury such as cerebral edema, intracranial bleeding, or emboli in a major cerebral artery.10
Cardiovascular Care
Hypertension
Cardiac surgical patients presenting in the postoperative period with hypertension should be evaluated for routine causes of acute postoperative hypertension and treated accordingly. Pain, residual neuromuscular blockade, hypoxemia, hypercarbia, hypothermia, or bladder distention may provoke hypertension. If these precipitants are ruled out, pharmacologic lowering of blood pressure with an infusion of a vasodilator, sodium nitroprusside, or nitroglycerin can be initiated. The rapid titratability and short duration of action of these medications is desirable as hemodynamic lability is commonplace. Alternatively, short-acting or ultra-short-acting dihydropyridine calcium channel blockers (e.g., nicardipine or clevidipine) have been used as they exert maximal effect in the peripheral arterial system with minimal impact on cardiac function.24 While MAP is lowered, serial measurements of cardiac output and urine output should be recorded to assess the patient’s tolerance of a lower blood pressure.
Hypotension
With a reported incidence of 9% to 44%, hypotension is more common than hypertension following cardiac surgery.25 Studies of off-pump CABG surgery compared to on-pump CABG surgery have demonstrated a significant increase in the incidence of hypotension in those patients exposed to CPB.26 Some clinicians also associate the relatively common hypotensive response to more critically ill patients with prolonged medical management prior to surgery. Additionally, widespread use of angiotensin-converting enzyme inhibitors has been implicated as a potential contributor.27 Regardless of the predisposing factors, postoperative hypotension demands prompt investigation and treatment.
The most readily correctable cause of hypotension is hypovolemia. Commonly boluses of crystalloid (500 mL to 1 L of 0.9% normal saline or lactated Ringer’s solution) are administered and the patient’s hemodynamic responses (MAP, CVP, PAP, and cardiac index [CI]) are evaluated. Colloid solutions can be used, but some concern exists when administering hetastarch solutions because of its effects on the coagulation cascade and potential contribution to a bleeding diathesis.28
A low preoperative ejection fraction (<35%), advanced age (>70 years old), and prolonged CPB times (>120 minutes) correlate with an increased risk of decreased cardiac output in the postbypass period.29 Postoperatively, a depressed CI often manifests early, during separation from CPB in the operating room. The pattern observed on hemodynamic monitoring is a depressed CI (<2.2 L/minute/m2), hypotension, normal or elevated SVR, and elevated filling pressures. Echocardiography demonstrates global ventricular hypokinesia. Patients will frequently require exogenous pharmacologic support to maintain a CI of greater than 2.2 L/minute/m2. Epinephrine, dopamine, dobutamine, or milrinone may be initiated for inotropic support.30 It is not uncommon for cardiac insufficiency to persist for several days following CPB.
Even after successful coronary revascularization, the astute practitioner must be vigilant for postoperative myocardial ischemia. During weaning from CPB, air may become entrained within the heart that is not effectively evacuated by the atrioventricular (AV) bypass vents. Some of this air may enter the coronaries and lead to cardiac arrest or ischemia. In addition, during chest closure, bypass grafts may become kinked, obstructing flow. During aortic or valvular surgery, graft material or sutures may inadvertently obstruct coronary ostia or may occlude native coronary flow. Although multiple patient monitors—electrocardiogram (ECG), pulmonary artery catheter, and echocardiography—can identify ischemia, studies have demonstrated new-onset wall motion abnormalities on echocardiography as the most sensitive and earliest indicator of malperfusion.31,32 If ischemia is present, surgical revascularization or percutaneous coronary revascularization may be necessary for reestablishing flow.
For approximately 5% to 15% of patients, exposure to CPB will lead to a prolonged vasodilatory state or vasoplegia.33 The classical pattern is normal biventricular function, normovolemia, and decreased SVR. In these patients, infusing additional volume will not increase MAP and will frequently decrease systemic pressure secondary to activation of the atrial stretch receptors. In patients who are adequately volume resuscitated, the most effective treatment is infusion of a vasopressor, commonly norepinephrine, phenylephrine, or vasopressin.10 Patients requiring progressively increasing doses of vasopressors should be continually assessed for hypovolemia or anemia, as these problems might happen concurrently in the vasoplegic patient. Experimental evidence suggests that these patients may suffer from a relative vasopressin deficiency. As such, exogenous vasopressin added to one of the other aforementioned vasopressors has been used successfully to maintain an adequate MAP.34
Tamponade
Acute tamponade is a surgical emergency that demands immediate treatment. In the early postoperative period following cardiac surgery, tamponade may occur as a consequence of uncorrected coagulopathy or from chest tube obstruction. Classic signs of tamponade on physical examination include hypotension, pulsus paradoxus, diminished peripheral pulses, and oliguria. Invasive hemodynamic monitoring may demonstrate equalization of CVP, pulmonary artery (PA) diastolic, and PA systolic pressures as the pericardium fills and pericardial pressure exceeds intracardiac pressures. Echocardiographic signs include right atrial systolic collapse and right ventricular diastolic collapse.35
Arrhythmias
Postoperative arrhythmias are the most common complication after cardiac surgery with an overall reported incidence of 5% to 63%.36 The most frequent arrhythmia is atrial fibrillation, which occurs in up to 40% of patients following bypass graft surgery and 60% of patients following combined CABG valve surgery.36 Inflammation of the myocardium and mechanical disruption of the cardiac conduction system during surgery predispose cardiac surgical patients to bradyarrhythmias and heart block during the postoperative period. Accordingly, placement of temporary epicardial pacing wires in the ventricles and atria is routine.37 Atrial wires will allow for AV synchrony during contraction, but removal of these wires from the more fragile atrial tissue may place the patient at an increased risk of atrial damage and tamponade.
Given the association of supraventricular arrhythmias with increased morbidity and length of stay, considerable research effort has focused on prevention and treatment.38 In the postoperative period, 60% of arrhythmias manifest within the first 3 postoperative days.38 For the patient with new-onset atrial fibrillation or flutter, the first step is to decide whether any hemodynamic instability is present. In the unstable patient, preparations should be made for immediate cardioversion according to Advanced Cardiac Life Support (ACLS) guidelines. In the hemodynamically stable patient, examination of the serum metabolic panel should be done for evidence of hypokalemia or hypomagnesemia with repletion of potassium or magnesium as necessary. If atrial fibrillation persists, the clinician must decide whether rate or rhythm control is preferred. Although rate control will limit tachycardia and myocardial oxygen consumption, it will not provide the benefit of atrial contraction and requires long-term anticoagulation.
If rate control is deemed appropriate for the patient, the most common agents used include beta blockers (e.g., atenolol or metoprolol), calcium channel blockers (e.g., diltiazem or verapamil), and digoxin.38 Care should be avoided when administering beta blockers to patients with severe chronic obstructive pulmonary disease (COPD) or reduced ejection fraction.
Bleeding
If left untreated, hemorrhage and uncorrected coagulopathy can lead to devastating complications in the cardiac surgery patient. Excess bleeding occurs in 20% of cardiac surgical patients with approximately 5% requiring surgical reexploration.39,40 Many factors contribute to this increased risk of hemorrhage. Preoperatively, patients are routinely on antiplatelet therapy and anticoagulant medications. Intraoperative exposure to CPB initiates contact activation of platelets with resultant thrombocytopathy and thrombocyopenia. In addition, the extracorporeal circuit exacerbates inflammation and amplifies fibrinolysis.41
Thromboelastography (TEG) and point-of-care (POC) testing are useful to guide blood product administration as the rapid rate of bleeding in the cardiac surgery patient outpaces the results obtained with standard laboratory testing.42,43 In contrast to management of anemia in medical patients, transfusion of blood products in cardiac surgical patients is often determined by immediate clinical findings (e.g., intraoperative surgical field oozing, increased postoperative chest tube output, or hypotension). Increased rate of bleeding, greater than 500 mL over 2 hours, usually correlates with a source of bleeding that is amenable to surgical repair. Many institutions have established criteria upon which the surgical team will reexplore the patient for evacuation—chest tube output of greater than 500 mL within the first 2 postoperative hours requires surgical exploration. Initial evaluation of the bleeding cardiac surgery patient should focus on any abnormal physiologic parameters that may contribute to ongoing coagulopathy—severe acidemia, alkalemia, or hypothermia will impair hemostasis and should be corrected.
With the advent of cell-salvage technologies and increasing recognition of the adverse effects of red blood cell transfusion, there has been a decrease in the amount of blood given in the perioperative period.44,45 Decisions regarding administration of red blood cells are based on patient-specific factors, including comorbid conditions and the adequacy of revascularization. Current American Society of Anesthesiology Practice Guidelines recommends transfusion of red blood cells when hemoglobin levels are less than 6 g/dL. Above this level, evidence of organ malperfusion and ischemia should be demonstrated prior to red blood cell transfusion.46
Given the deleterious effects of CPB circuitry on platelet quantity and function, hemorrhage that does not appear to have an overt surgical source often requires platelet transfusion. In a cardiac surgical patient with microvascular bleeding and normal physiologic parameters (normothermia, normal acid-base balance, etc.), platelet transfusion should be strongly considered. A platelet count greater than 100 × 109/L usually excludes thrombocytopenia as a contributing factor in the hemorrhagic cardiac surgical patient.46
Although intraoperative cell salvage has reduced the need for packed red blood cell transfusion, the washing of the blood during this process eliminates essential clotting factors.47 Per American Society of Anesthesiology Guidelines, elevation of the prothrombin time (PT) greater than 1.5 times normal or elevation of the activated partial thromboplastin time (aPTT) greater than 2 times normal is an indication for fresh frozen plasma (FFP) transfusion. Although no specific algorithms exist for cardiac surgery, FFP administration is reasonable in the patient with normal platelet count and evidence of persistent bleeding.46
Pharmacologic options for reducing bleeding in the perioperative period include antifibrinolytic agents, desmopressin, and activated factor VII.48–50 For maximal efficacy, antifibrinolytics should be started in the operating room prior to incision. Studies of desmopressin have demonstrated a reduction in blood loss, but no overall decrease in transfusion requirements. The newest hemostatic agent used for excess hemorrhage in the cardiac surgical arena is activated factor VII. Although approved for hemostatic control in hemophilia A or B patients, it has been successfully used off-label in cardiac surgery patients. Studies thus far have been encouraging, demonstrating decreased bleeding and transfusion requirements.50,51
Pulmonary Care
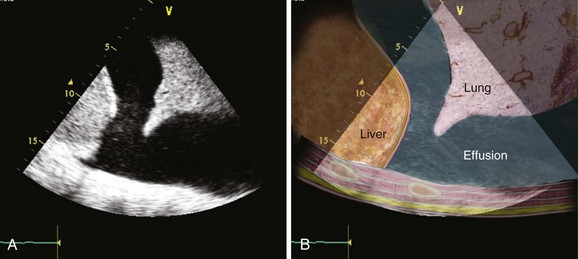
Pleural effusions present after chest tubes have been removed are common but usually do not require removal by thoracentesis or chest tube thoracostomy unless they cause respiratory symptoms (Fig. 36.1). Hemothorax not due to ongoing postoperative bleeding is typically from bleeding that was not adequately drained by pleural or mediastinal chest tubes and does not usually require specific treatment.
Unanticipated, severe pulmonary dysfunction may be due to acute lung injury and is usually associated with inflammation and hemodilution related to CPB, particularly after long operations. After CPB, the lungs are especially susceptible to cytokine-mediated systemic reperfusion-inflammation injury due to the high blood flow they receive, or they may be injured by their own ischemia-reperfusion when there is no ventilation or pulmonary blood flow during CPB and oxygen delivery is by nonpulsatile bronchial flow only. Regardless of the mechanism, some pulmonary dysfunction is nearly universal.52,53 Manifestations include decreased PaO2/FIO2 ratio, decreased pulmonary compliance, large alveolar-arterial gradients, and bronchoconstriction.
An increasing number of cardiac surgery patients are being successfully extubated in the operating room. The majority of patients who are not weaned and extubated in the operating room as part of an accelerated recovery protocol are candidates for extubation within 2 to 4 hours after ICU admission. Early postoperative conditions that mitigate against the initiation of ventilator weaning on arrival to the ICU after surgery are listed in Box 36.1.
A protocol-driven and respiratory therapist–managed reduction in initial postoperative intubation time has been achieved in many ICUs.54 Some institutions use inclusion/exclusion criteria for selecting patients to be weaned under an accelerated recovery protocol.55,56 Others employ a protocol wherein all patients are initially included, and a patient is excluded only when the patient does not meet the parameters for advancement. A practice that has been successful is to preoperatively select patients for accelerated recovery and make a final extubation decision in the operating room at the end of the case with input from both surgeon and anesthesiologist.
Although tracheostomy has conventionally been considered in patients who are ventilator dependent 2 weeks after cardiac surgery, there is little evidence to support this approach. Tracheostomy timing can be based on the determination at the end of the first ICU week of a patient’s likelihood of successful weaning over the second week. If at that time successful weaning is considered unlikely, tracheostomy should be considered. Earlier tracheostomy (before the tenth postoperative day) in patients who require prolonged mechanical ventilation after cardiac surgery is associated with lower morbidity and mortality rates as well as decreased ICU and total hospital lengths of stay, and is not associated with an increased occurrence of mediastinitis.57
Renal Care
Despite significant research efforts and considerable focus on prevention and treatment, acute kidney injury (AKI) following cardiac surgery is associated with a significantly increased risk of morbidity and mortality. Although only 1% of patients will experience the most severe form of kidney injury and require some form of renal replacement therapy, this patient population has a 60% in-hospital mortality rate.58 Risk stratification has identified several preoperative and postoperative risk factors for postoperative AKI—these include female gender, left ventricular ejection fraction (LVEF) less than 35%, exposure to angiographic contrast dye, duration of CPB, and low MAP during CPB.59
In the fluid-overloaded oliguric patient diuretics can be given to assess the patient’s responsiveness and to remove excess volume. Although conversion from oliguric to nonoliguric renal failure through diuretics does not improve overall prognosis, it can improve oxygenation and hemodynamics.60 If the patient is diuretic nonresponsive or has worsening electrolyte or acid-base imbalance, fluid overload with impairment of oxygenation or worsening heart failure, or has severe uremia, initiation of dialytic support should be considered. Several different dialysis modalities exist. In brief, continuous therapies offer more stable hemodynamics but do not clear as much solute or fluid per unit of time as intermittent dialysis.61
Applications of Echocardiography after Cardiac Surgery
The use of ultrasonography in the perioperative period has increased significantly over the past decade. In contrast to TEE usually performed by cardiologists and cardiothoracic anesthesiologists in the operating room, focused TTE is well suited for the postoperative period especially for unexplained hypotension and assessment of response to therapeutic interventions. Postoperative use of TTE can provide rapid and accurate diagnostic information in patients developing potentially life-threatening conditions. Although the image quality obtained in mechanically ventilated patients is usually better with TEE, TTE usually provides adequate views in postcardiotomy patients; and it is noninvasive, fast, performed at the bedside, reproducible, and focused on major cardiac and pleural space abnormalities.62,63
The use of TTE in periresuscitation (life support) care has two goals: (1) to assess the heart function, and (2) to identify treatable conditions. The focused echocardiographic evaluation in life support (FEEL) examination is briefly performed during cardiopulmonary resuscitation (CPR) with the primary objective of identifying potentially reversible causes of cardiopulmonary deterioration. The identification and appropriate management by TTE in the perioperative period of severe left ventricular dysfunction, pulmonary embolism, hypovolemia, or cardiac tamponade may be lifesaving.64,65
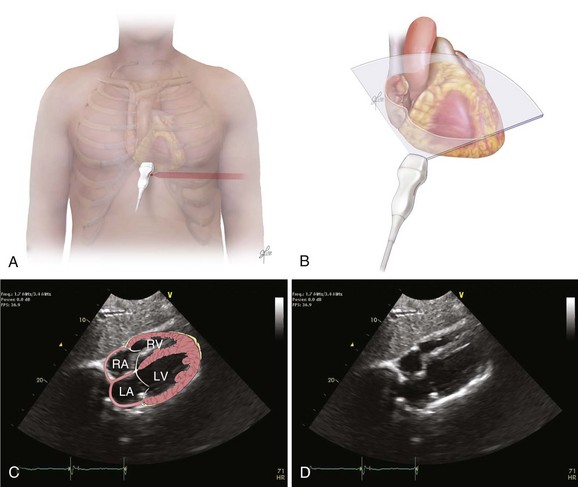
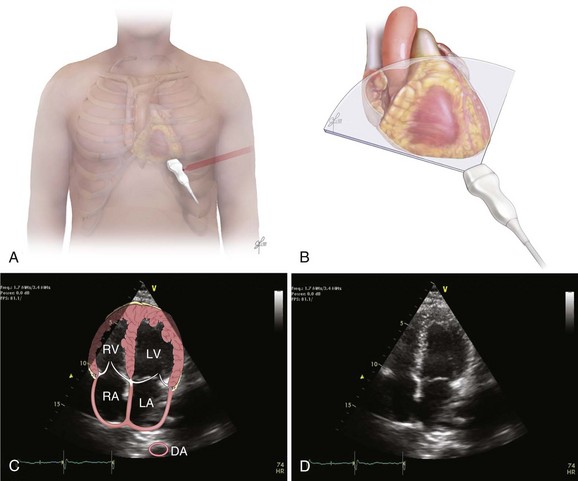
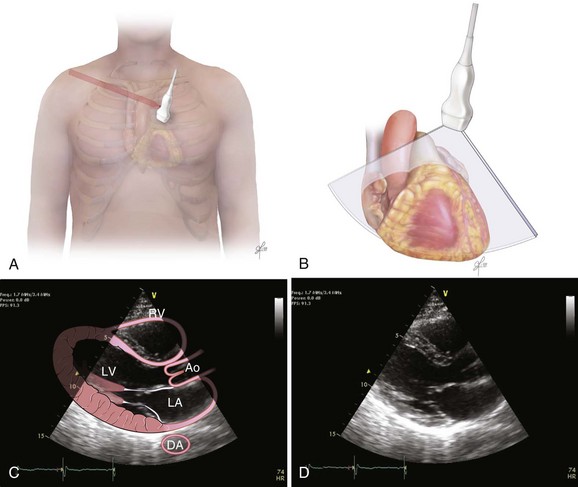
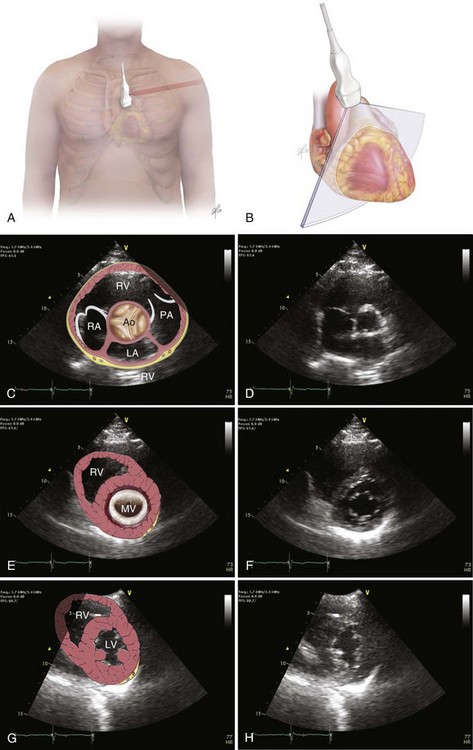
The recommended echocardiographic window to perform the FEEL examination is the subcostal view (Fig. 36.2). The FEEL examination may distinguish “true” pulseless electrical activity (PEA) from pseudo-PEA.66 A recent publication demonstrated that in 35% of patients with an ECG diagnosis of asystole, 58% of those with PEA, coordinated cardiac motion was detected (pseudo-PEA) and associated with increased survival. Echocardiographic findings altered management in 78% of cases.64
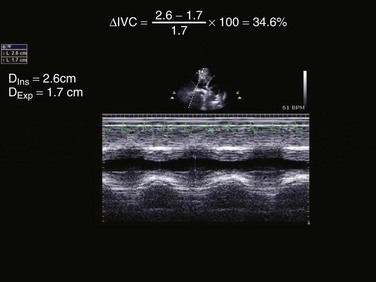
In addition, FEEL protocol can facilitate the early detection of return of spontaneous circulation. One of the most common questions in the critically ill is the fluid responsiveness. Barbier and associates described the inferior vena cava (IVC) distensibility index in mechanically ventilated patients.67 This index is based on change in size of IVC with respiration as it decreases with size with inspiration and increases with expiration:
In this equation distensibility index is expressed as a percentage. A distensibility index greater than 18% predicts fluid responsiveness.
The FATE Examination
The focused assessed transthoracic echocardiography (FATE) examination is a qualitative POC ultrasonography, which is ideal within the ICU. The FATE examination should include qualitative assessment of left and right ventricular function and intravascular volume status as minimum required information (Fig. 36.3). It supplements the critical care evaluation in the perioperative setting.68,69 However, focused echocardiography does not replace a comprehensive echocardiogram performed per cardiology specialist whenever quantitative analysis or specific diagnoses such as endocarditis or valvular dysfunction are needed. Moreover, the correct application of this tool in postcardiotomy patients depends on the appropriate utilization of ultrasonography findings in the clinical context.70
Hypovolemia (measurement of IVC diameter and distensibility index)
Myocardial dysfunction (qualitative evaluation of the right and left ventricles, measurement of cardiac output and pulmonary pressures) including patients with acute respiratory failure
Pericardial effusion/tamponade (evaluation of tamponade physiology)
Pulmonary embolism (changes in acute right ventricular dysfunction)
Acquisition of Images
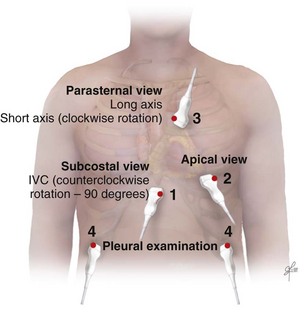
Bedside ultrasonography and acquisition of cardiac images are not exclusive of the comprehensive transthoracic examination. Moreover, the FATE examination must be considered an extension of the physical examination. Thus, before obtaining images with echocardiography it is important to keep in mind the position of the heart within the chest and the direction of the ultrasound beam (Fig. 36.4). In addition, thoracic ultrasonography offers better sensitivity and specificity than plain chest radiography. The evaluation of the pleurae mandate the knowledge of anatomic structures above and below both diaphragms. The acquisition of images can be very challenging in the postcardiotomy patient. Most of the time at least one view can be obtained and the goal of exclusion of important disease is achieved. Up to 40% of patients in the ICU have limited acquisition of echocardiography views. Although the TEE is an invasive technique, the unparalleled quality of ultrasonography examination is well known (Tables 36.1 and 36.2).
Table 36.1
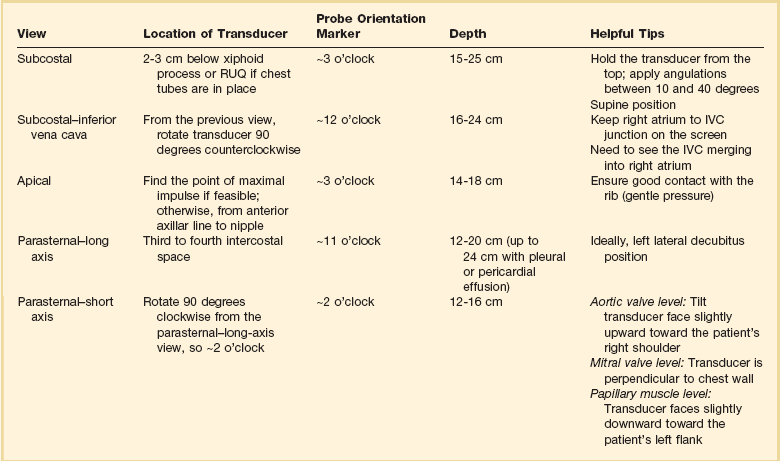
Table 36.2
Assessment of Structures with FATE Protocol
| Assessment Focus | View(s) | Classification |
| Left ventricular (LV) function (qualitative)—“eyeballing”: thickness of the myocardium | Parasternal–long-/short-axis view Apical view, subcostal view |
Normal function Mild to moderate LV dysfunction Severe LV dysfunction Hyperdynamic |
| Right ventricular function | Parasternal–long-/short-axis view Apical view, subcostal view |
|
| Pericardial space | Parasternal–long-/short-axis view Apical view, subcostal view |
Pericardial effusion Tamponade physiology |
| Pleural effusion and pericardial effusion | Parasternal–long-axis view | |
| Inferior vena cava (IVC) | Subcostal view of the IVC | Diameter measurement: 2-3 cm from right atrium Normal diameter: 2.1 cm Respiratory phase variations, ideally on spontaneously breathing patients |
Role of Ultrasonography in Postcardiotomy Tamponade
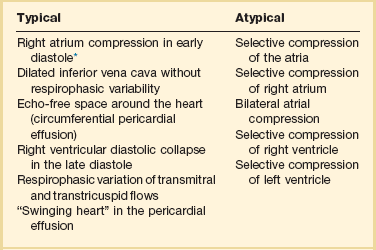
Postcardiotomy surgery tamponade (PCST) is perhaps the most important diagnosis in the patient recovering from cardiac surgery. Its early recognition and intervention have a direct impact in patients’ outcome. The process of diagnosing PCST begins with the identification of clinical signs and confirmation of the diagnosis with appropriate tools subsequently. A high index of suspicion should be maintained throughout the early postoperative cardiovascular ICU (CVICU) hours. Usually a progressive decrease of cardiac output after increased chest tube drainage and increased filling pressures occurs with PCST.71
Clinical signs such us Beck’s triad (hypotension, distended neck veins, and diminished heart sounds) and pulsus paradoxus are neither sensitive nor specific of PCST.72 Patients with other disorders such as constrictive pericarditis, severe COPD, morbid obesity, and right ventricular infarction might present with pulsus paradoxus. In contrast, this sign can be absent in patients with atrial septal defect, regional tamponade, pulmonary hypertension, COPD with cor pulmonale, aortic insufficiency, and even positive-pressure mechanical ventilation.72
The various clinical presentations of PCST impose limitations to the FATE examination, especially posterior compartmented effusion. In addition, clotted and loculated blood is echo-dense, somewhat more challenging to diagnose, and could be mistaken for the myocardium itself. As a rapid screening tool it can very helpful if collapse of the right ventricle is noticed in the long-axis parasternal view or collapse of the right atrium/right ventricle, or left ventricle in the apical or subcostal views (Figs. 36.5 to 36.7). In addition, documentation of abnormally increased diameter of the IVC (>2.5 cm) without variability with respiratory phases may help to distinguish from hemorrhagic shock in the setting of severe hypotension in the postoperative period.73
The presence of acute bleeding into the pericardial space makes it appear as echolucent space. The presence of blood in the pericardium can be graded as small (0.5 cm), moderate (0.5-2 cm), or large (>2 cm). Right atrial systolic collapse that has duration greater than 30% of the entire systole is the most sensitive and specific sign of PCST. The best views to recognize this finding are subcostal or apical view on TTE and midesophageal four-chamber view by TEE. Doppler ultrasound can reveal a 35% increase respirophasic variation of the tricuspid/mitral E-wave velocity during spontaneous inspiration and a decrease of 25% in the mitral or left ventricle outflow track flow. Another important and helpful finding is the paradoxical movement of the interventricular septum (shift of the septum to the left ventricle in diastole and toward the right ventricle in systole) during spontaneous expiration and normalization in controlled ventilation (Table 36.3). Absence of right atrial collapse in cardiac tamponade is seen in severe pulmonary hypertension, RV dysfunction, COPD with cor pulmonale, and regional posterior tamponade.
Rescue Applications of Echocardiography
• The use of focused echocardiography in periresuscitation following cardiac surgery intends to identify the four treatable causes of cardiac arrest (severe ventricular dysfunction, pulmonary embolism, hypovolemia, and cardiac tamponade).
• The most useful echocardiography view in the setting of advanced cardiac life support is the subcostal. It can be performed after 5 cycles of CPR maneuvers.
• The identification of true PEA arrest has important prognostic implications as this subgroup of patients has worse prognosis in comparison to patients with pseudo-PEA (detection of coordinated cardiac motion).
• Post–cardiac surgery tamponade has various clinical presentations and may impose limitations to focused echocardiography, especially in those cases of posterior compartmented effusion.
Miscellaneous Considerations
Intensive Care Unit Length of Stay
Initial intubation time is associated with ICU length of stay. Most cardiac surgery patients can be extubated early and transferred out of the ICU within 24 hours. Rapid patient turnover is achieved in ICUs that use care pathways based on patient parameters and not the length of stay.49 Patient-based parameters for a diuresis protocol would be based on a patient’s weight relative to the preoperative weight, the BUN/Cr, and the CI/preload, not on time (“postoperative day 1”). When time-based parameters are used, they should be based on hours rather than days. Pacing wires might be removed routinely after any 12-hour period with no need for pacing or antidysrhythmic therapy, rather than “on postoperative day 2.” Timing of chest tube removal may be based on volume and character of the drainage, rather than on an experientially derived time or postoperative day.
References
1. D’Agostino, RS, Svensson, LG, Neumann, DJ, et al. Screening carotid ultrasonography and risk factors for stroke in coronary artery surgery patients. Ann Thorac Surg. 1996; 62(6):1714–1723.
2. Bucerius, J, Gummert, JF, Borger, MA, et al. Stroke after cardiac surgery: A risk factor analysis of 16,184 consecutive adult patients. Ann Thorac Surg. 2003; 75(2):472–478.
3. Hnath, JC, Mehta, M, Taggert, JB, et al. Strategies to improve spinal cord ischemia in endovascular thoracic aortic repair: Outcomes of a prospective cerebrospinal fluid drainage protocol. J Vasc Surg. 2008; 48(4):836–840.
4. Roach, GW, Kanchuger, M, Mangano, CM, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996; 335(25):1857–1863.
5. Senay, S, Toraman, F, Akgun, Y, et al. Stroke after coronary bypass surgery is mainly related to diffuse atherosclerotic disease. Heart Surg Forum. 2011; 14(6):E366–372.
6. Gerriets, T, Schwarz, N, Sammer, G, et al. Protecting the brain from gaseous and solid micro-emboli during coronary artery bypass grafting: A randomized controlled trial. Eur Heart J. 2010; 31(3):360–368.
7. Wolman, RL, Nussmeier, NA, Aggarwal, A, et al. Cerebral injury after cardiac surgery: Identification of a group at extraordinary risk. Multicenter Study of Perioperative Ischemia Research Group (McSPI) and the Ischemia Research Education Foundation (IREF) Investigators. Stroke. 1999; 30(3):514–522.
8. Anyanwu, AC, Filsoufi, F, Salzberg, SP, et al. Epidemiology of stroke after cardiac surgery in the current era. J Thorac Cardiovasc Surg. 2007; 134(5):1121–1127.
9. Filsoufi, F, Rahmanian, PB, Castillo, JG, et al. Incidence, topography, predictors and long-term survival after stroke in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2008; 85(3):862–870.
10. Filsoufi, F, Rahmanian, PB, Castillo, JG, et al. Incidence, imaging analysis, and early and late outcomes of stroke after cardiac valve operation. Am J Cardiol. 2008; 101(10):1472–1478.
11. McKhann, GM, Grega, MA, Borowicz, LM, Jr., et al. Stroke and encephalopathy after cardiac surgery: An update. Stroke. 2006; 37(2):562–571.
12. Selim, M. Perioperative stroke. N Engl J Med. 2007; 356(7):706–713.
13. Selnes, OA, Gottesman, RF, Grega, MA, et al. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med. 2012; 366(3):250–257.
14. Lamy, A, Devereaux, PJ, Prabhakaran, D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med. 2012; 366(16):1489–1497.
15. van de Beek, D, Kremers, W, Daly, RC, et al. Effect of neurologic complications on outcome after heart transplant. Arch Neurol. 2008; 65(2):226–231.
16. Zierer, A, Melby, SJ, Voeller, RK, et al. Significance of neurologic complications in the modern era of cardiac transplantation. Ann Thorac Surg. 2007; 83(5):1684–1690.
17. Perez-Vela, JL, Ramos-Gonzalez, A, Lopez-Almodovar, LF, et al. Neurologic complications in the immediate postoperative period after cardiac surgery. Role of brain magnetic resonance imaging. Rev Esp Cardiol. 2005; 58(9):1014–1021.
18. Carrascal, Y, Guerrero, AL. Neurological damage related to cardiac surgery: Pathophysiology, diagnostic tools and prevention strategies. Using actual knowledge for planning the future. Neurologist. 2010; 16(3):152–164.
19. Restrepo, L, Wityk, RJ, Grega, MA, et al. Diffusion- and perfusion-weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting surgery. Stroke. 2002; 33(12):2909–2915.
20. Moazami, N, Smedira, NG, McCarthy, PM, et al. Safety and efficacy of intraarterial thrombolysis for perioperative stroke after cardiac operation. Ann Thorac Surg. 2001; 72(6):1933–1937.
21. Rodriguez, RA, Bussiere, M, Bourke, M, et al. Predictors of duration of unconsciousness in patients with coma after cardiac surgery. J Cardiothorac Vasc Anesth. 2011; 25(6):961–967.
22. Goldstone, AB, Bronster, DJ, Anyanwu, AC, et al. Predictors and outcomes of seizures after cardiac surgery: A multivariable analysis of 2,578 patients. Ann Thorac Surg. 2011; 91(2):514–518.
23. Manji, RA, Grocott, HP, Menkis, AH, et al. Recurrent seizures following cardiac surgery—Risk factors and outcomes. Circulation. 2011; 124(21):A13710.
24. Aronson, S, Dyke, CM, Stierer, KA, et al. The ECLIPSE trials: Comparative studies of clevidipine to nitroglycerin, sodium nitroprusside, and nicardipine for acute hypertension treatment in cardiac surgery patients. Anesth Analg. 2008; 107(4):1110–1121.
25. Levin, MA, Lin, HM, Castillo, JG, et al. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation. 2009; 120(17):1664–1671.
26. Sun, X, Zhang, L, Hill, PC, et al. Is incidence of postoperative vasoplegic syndrome different between off-pump and on-pump coronary artery bypass grafting surgery? Eur J Cardiothorac Surg. 2008; 34(4):820–825.
27. Tuman, KJ, McCarthy, RJ, O’Connor, CJ, et al. Angiotensin-converting enzyme inhibitors increase vasoconstrictor requirements after cardiopulmonary bypass. Anesth Analg. 1995; 80(3):473–479.
28. Knutson, JE, Deering, JA, Hall, FW, et al. Does intraoperative hetastarch administration increase blood loss and transfusion requirements after cardiac surgery? Anesth Analg. 2000; 90(4):801–807.
29. St Andre, AC, DelRossi, A. Hemodynamic management of patients in the first 24 hours after cardiac surgery. Crit Care Med. 2005; 33(9):2082–2093.
30. Gillies, M, Bellomo, R, Doolan, L, et al. Bench-to-bedside review: Inotropic drug therapy after adult cardiac surgery—A systematic literature review. Crit Care. 2005; 9(3):266–279.
31. Smith, JS, Cahalan, MK, Benefiel, DJ, et al. Intraoperative detection of myocardial ischemia in high-risk patients: Electrocardiography versus two-dimensional transesophageal echocardiography. Circulation. 1985; 72(5):1015–1021.
32. van Daele, ME, Sutherland, GR, Mitchell, MM, et al. Do changes in pulmonary capillary wedge pressure adequately reflect myocardial ischemia during anesthesia? A correlative preoperative hemodynamic, electrocardiographic, and transesophageal echocardiographic study. Circulation. 1990; 81(3):865–871.
33. Carrel, T, Englberger, L, Mohacsi, P, et al. Low systemic vascular resistance after cardiopulmonary bypass: Incidence, etiology, and clinical importance. J Card Surg. 2000; 15(5):347–353.
34. Argenziano, M, Chen, JM, Choudhri, AF, et al. Management of vasodilatory shock after cardiac surgery: Identification of predisposing factors and use of a novel pressor agent. J Thorac Cardiovasc Surg. 1998; 116(6):973–980.
35. Reddy, PS, Curtiss, EI, Uretsky, BF. Spectrum of hemodynamic changes in cardiac tamponade. Am J Cardiol. 1990; 66(20):1487–1491.
36. Passannante, AN. Prevention of atrial fibrillation after cardiac surgery. Curr Opin Anaesthesiol. 2011; 24(1):58–63.
37. Bethea, BT, Salazar, JD, Grega, MA, et al. Determining the utility of temporary pacing wires after coronary artery bypass surgery. Ann Thorac Surg. 2005; 79(1):104–107.
38. Echahidi, N, Pibarot, P, O’Hara, G, et al. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008; 51(8):793–801.
39. Moulton, MJ, Creswell, LL, Mackey, ME, et al. Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. J Thorac Cardiovasc Surg. 1996; 111(5):1037–1046.
40. Parr, KG, Patel, MA, Dekker, R, et al. Multivariate predictors of blood product use in cardiac surgery. J Cardiothorac Vasc Anesth. 2003; 17(2):176–181.
41. Paparella, D, Brister, SJ, Buchanan, MR. Coagulation disorders of cardiopulmonary bypass: A review. Intensive Care Med. 2004; 30(10):1873–1881.
42. Prisco, D, Paniccia, R. Point-of-care testing of hemostasis in cardiac surgery. Thromb J. 2003; 1(1):1.
43. Shore-Lesserson, L, Manspeizer, HE, DePerio, M, et al. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999; 88(2):312–319.
44. Murphy, GJ, Reeves, BC, Rogers, CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007; 116(22):2544–2552.
45. Nydegger, U. Transfusion dependency of cardiac surgery—Update 2006. Swiss Med Wkly. 2006; 136(49-50):781–788.
46. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006; 105(1):198–208.
47. Huet, C, Salmi, LR, Fergusson, D, et al. A meta-analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. International Study of Perioperative Transfusion (ISPOT) Investigators. Anesth Analg. 1999; 89(4):861–869.
48. Brown, JR, Birkmeyer, NJ, O’Connor, GT. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation. 2007; 115(22):2801–2813.
49. Cattaneo, M, Harris, AS, Stromberg, U, et al. The effect of desmopressin on reducing blood loss in cardiac surgery—A meta-analysis of double-blind, placebo-controlled trials. Thromb Haemost. 1995; 74(4):1064–1070.
50. Diprose, P, Herbertson, MJ, O’Shaughnessy, D, et al. Activated recombinant factor VII after cardiopulmonary bypass reduces allogeneic transfusion in complex non-coronary cardiac surgery: Randomized double-blind placebo-controlled pilot study. Br J Anaesth. 2005; 95(5):596–602.
51. Warren, O, Mandal, K, Hadjianastassiou, V, et al. Recombinant activated factor VII in cardiac surgery: A systematic review. Ann Thorac Surg. 2007; 83(2):707–714.
52. Clark, SC. Lung injury after cardiopulmonary bypass. Perfusion. 2006; 21(4):225–228.
53. Taggart, DP, el-Fiky, M, Carter, R, et al. Respiratory dysfunction after uncomplicated cardiopulmonary bypass. Ann Thorac Surg. 1993; 56(5):1123–1128.
54. Konstantakos, AK, Lee, JH. Optimizing timing of early extubation in coronary artery bypass surgery patients. Ann Thorac Surg. 2000; 69(6):1842–1845.
55. Ely, EW, Meade, MO, Haponik, EF, et al. Mechanical ventilator weaning protocols driven by nonphysician health-care professionals: Evidence-based clinical practice guidelines. Chest. 2001; 120(6 Suppl):454S–463S.
56. van Mastrigt, GA, Maessen, JG, Heijmans, J, et al. Does fast-track treatment lead to a decrease of intensive care unit and hospital length of stay in coronary artery bypass patients? A meta-regression of randomized clinical trials. Crit Care Med. 2006; 34(6):1624–1634.
57. Devarajan, J, Vydyanathan, A, Xu, M, et al. Early tracheostomy is associated with improved outcomes in patients who require prolonged mechanical ventilation after cardiac surgery. J Am Coll Surg. 2012; 214(6):1008–1016.
58. Stafford-Smith, M. Preservation of renal function. In: Newman MF, Fleisher LA, Fink MP, eds. Perioperative Medicine: Managing for Outcome. Philadelphia: Saunders Elsevier; 2008:723.
59. Coleman, MD, Shaefi, S, Sladen, RN. Preventing acute kidney injury after cardiac surgery. Curr Opin Anaesthesiol. 2011; 24(1):70–76.
60. Shilliday, IR, Quinn, KJ, Allison, ME. Loop diuretics in the management of acute renal failure: A prospective, double-blind, placebo-controlled, randomized study. Nephrol Dial Transplant. 1997; 12(12):2592–2596.
61. John, S, Eckardt, KU. Renal replacement strategies in the ICU. Chest. 2007; 132(4):1379–1388.
62. Breitkreutz, R, Uddin, S, Steiger, H, et al. Focused echocardiography entry level: New concept of a 1-day training course. Minerva Anestesiol. 2009; 75(5):285–292.
63. Price, S, Via, G, Sloth, E, et al. Echocardiography practice, training and accreditation in the intensive care: Document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasound. 2008; 6:49.
64. Breitkreutz, R, Price, S, Steiger, HV, et al. Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: A prospective trial. Resuscitation. 2010; 81(11):1527–1533.
65. Price, S, Uddin, S, Quinn, T. Echocardiography in cardiac arrest. Curr Opin Crit Care. 2010; 16(3):211–215.
66. Prosen, G, Krizmaric, M, Zavrsnik, J, et al. Impact of modified treatment in echocardiographically confirmed pseudo-pulseless electrical activity in out-of-hospital cardiac arrest patients with constant end-tidal carbon dioxide pressure during compression pauses. J Int Med Res. 2010; 38(4):1458–1467.
67. Barbier, C, Loubieres, Y, Schmit, C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004; 30(9):1740–1746.
68. Cowie, BS. Focused transthoracic echocardiography in the perioperative period. Anaesth Intensive Care. 2010; 38(5):823–836.
69. Faris, JG, Veltman, MG, Royse, C. Focused transthoracic echocardiography in the perioperative period. Anaesth Intensive Care. 2011; 39(2):306–307.
70. Labovitz, AJ, Noble, VE, Bierig, M, et al. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010; 23(12):1225–1230.
71. Carmona, P, Mateo, E, Casanovas, I, et al. Management of cardiac tamponade after cardiac surgery. J Cardiothorac Vasc Anesth. 2012; 26(2):302–311.
72. Spodick, DH. Acute cardiac tamponade. N Engl J Med. 2003; 349(7):684–690.
73. Bodson, L, Bouferrache, K, Vieillard-Baron, A. Cardiac tamponade. Curr Opin Crit Care. 2011; 17(5):416–424.