Chapter 33
Postoperative Management
Giuseppe Papia
Vascular surgery patients remain at high risk for the development of complications in the postoperative period because of the complexity of their surgical procedures and their preoperative comorbidities. This chapter addresses management issues in the postoperative period with an emphasis on issues in the intensive care unit (ICU) and specially monitored high-dependency step-down units.
Postoperative Triage
An important aspect of postoperative medical care involves identifying patients at highest risk for postoperative complications and triaging them to the appropriate care environment. The safest postoperative environment for patients undergoing vascular surgical procedures is determined by the preoperative medical comorbid conditions (see Chapters 28, 30, 31, 37, 39, 40, and 41), the inherent risks of the type of operation performed, and the ability to maintain intraoperative and postoperative homeostasis of the patient. Emergency operations, such as those for a ruptured abdominal aortic aneurysm (AAA), have increased risk for mortality.1
Admission to the Intensive Care Unit and High-Dependency Step-Down Unit
The need for invasive hemodynamic monitoring and observation remains the most common criterion for admission of selected patients to an ICU or step-down unit. The main distinction between these two environments in most institutions is the severity of critical illness and need for invasive positive pressure ventilation. These environments can provide patients the following: decreased myocardial oxygen demand through expeditious rewarming, effective fluid resuscitation, effective analgesia, meticulous control of hemodynamics, and careful monitoring and appropriate nursing care to aid in the early diagnosis and treatment of complications. In a cohort study of patients after elective aortic surgery, Lawlor et al2 demonstrated that care in an ICU decreased mortality in patients with preoperative severe coronary artery disease defined as an ejection fraction of less than 40%, congestive heart failure, or New York Heart Association class III or IV angina. Consideration of direct admission to the ICU should also be given to patients with significant chronic obstructive pulmonary disease (COPD, defined as forced expiratory volume in 1 second of less than 1 L) and those who are dialysis dependent or have a need for continuous renal replacement therapy. Hemodynamically unstable patients (systolic blood pressure <90 mm Hg or inotropic support at the end of the operation), intubated patients with ongoing need for ventilatory support, those with clinically significant perioperative cardiac ischemia, patients with pulmonary artery catheters, patients with spinal drains, patients with hypothermia (temperature <35° C), and those who required massive transfusion of blood products (>3 L) during the procedure should be admitted to the ICU.
Selective use of high-dependency step-down units and rationing of ICU resources can be safe and cost-effective with appropriate patient risk stratification.3,4 Each patient must be individualized according to preoperative comorbid diseases, perioperative hemodynamic stability, and surgical procedure performed. Patients undergoing less invasive vascular procedures associated with fewer fluid shifts and lower surgical morbidity can be safely managed in a step-down unit. This is the case with patients undergoing peripheral arterial bypass, elective repair of infrarenal AAAs, and carotid endarterectomy. Several scoring models predicting risk in vascular patients have been studied and validated as predictors of high-risk patients undergoing vascular surgery.5
Organizational Structure of the Intensive Care Unit
The organizational structure of the ICU, independent of a patient’s medical factors, has a direct impact on outcomes.6–11 In looking at outcomes after abdominal aortic surgery, Pronovost et al7,12 have shown a relationship between increased in-hospital mortality and the absence of a full-time ICU director, less than 50% of ICU attending physicians certified in critical care, no daily rounds by an ICU physician, and decreased ICU nurse-patient ratio in the evening (<1 : 2). In patients undergoing abdominal aortic surgery, intensivists not making routine ICU rounds is associated with an increase in hospital mortality (odds ratio [OR], 3.0; 95% confidence interval [CI], 1.9-4.9), cardiac arrest (OR, 2.9; 95% CI, 1.2-7.0), acute renal failure (OR, 2.2; 95% CI, 1.3-3.9), septicemia (OR, 1.8; 95% CI, 1.2-2.6), platelet transfusion (OR, 6.4; 95% CI, 3.2-12.4), and reintubation (OR, 2.0; 95% CI, 1.0-4.1). Dang et al6 also demonstrated that ICUs with decreased nurse staffing had increased rates of cardiac and respiratory complications after abdominal aortic surgery.
Hemodynamics and Pressure Monitoring
Monitoring is required to intervene in abnormal physiology with the goal of improving outcomes. However, even patients with normal hemodynamic parameters may have inadequate organ perfusion.13 Distributive hypoxia is a state that results from inadequate oxygen delivery to tissues, exacerbated by increased metabolic demands, with resultant multiorgan dysfunction.14 Indicators of organ perfusion to guide resuscitation15 include blood pressure, heart rate, central venous pressure (CVP), pulmonary capillary wedge pressure, cardiac output,16 urine output, blood lactate concentration, tissue carbon dioxide levels, base deficit, mixed venous oxygen levels, and mixed venous carbon dioxide levels.15,17 The key to interpreting and managing these indicators appropriately is to follow trends over time rather than individual measurements.
Central Venous Catheters and Central Venous Pressure
Central venous catheters are used primarily to infuse fluids, to administer vasoactive drugs, and to assess intravascular volume. The most common sites for placement of central venous catheters are the internal jugular veins, subclavian veins, and femoral veins. To measure CVP, internal jugular or subclavian central venous catheters must be positioned with the catheter tip in the distal segment of the superior vena cava. More distal placement in the right atrium is associated with potential risk for erosion, perforation, and cardiac tamponade. CVP measures right atrial pressure, providing an estimation of preload. This measurement, however, is inaccurate in patients with COPD or in those with valvular heart disease. Femoral lines are good for resuscitation but not for CVP measurements because of the effect of intra-abdominal pressure on measurements.
The transducer must be zeroed at the level of the midaxillary line, and normal values of CVP are between 6 and 12 mm Hg. In patients being managed with positive pressure ventilation, CVP should be measured at end-expiration, when pleural pressure in the chest is approximately zero.18 The addition of positive end-expiratory pressure (PEEP) in a ventilated patient is another important consideration in measuring pressure in the chest. In normal lungs and with low levels of PEEP (between 5 and 8 cm H2O), intrapleural pressure, which affects CVP, is only slightly affected.18 The effect on CVP of high levels of PEEP (>12 to 15 cm H2O) when the lungs are probably not compliant is unclear.19 High levels of PEEP prevent backflow of fluid outside the chest to the heart, thus decreasing venous return. If sudden changes in CVP occur, one should consider a cardiorespiratory cause, such as pneumothorax or cardiac tamponade. Further discussion of CVP monitoring can be found in Chapter 32.
Peripheral Arterial Lines
Peripheral arterial catheters are the “gold standard” for direct assessment of systolic blood pressure, and they allow direct vascular access for blood sampling. Complications include bleeding, hematoma, line-related infection, thrombosis, and, rarely, limb ischemia.20 These complications occur to varying degrees, depending on the cannulation site. The most common site for cannulation is the radial artery, followed by the femoral artery, axillary artery, brachial artery, and, less commonly, dorsalis pedis and ulnar arteries. The radial artery and the femoral artery have similar cannulation complication rates,21 and some prefer the femoral artery as the primary cannulation site because of ease of insertion. The most common complication of radial artery cannulation is temporary arterial occlusion, which occurs approximately 20% (1.5% to 35%) of the time, but the occlusion is temporary in most and rarely causes acute ischemia.20 In contrast to the radial artery, cannulation of the femoral artery is associated with a higher risk of bleeding (1.58% vs 0.53%) and pseudoaneurysm (0.3% vs 0.09%) but a lower risk of thromboembolism.20 Cannulation of the axillary artery has a complication rate similar to that at other sites, and it may be the only site available for arterial access in some patients. In the ICU, however, it is not commonly used because of lack of familiarity with the approach and the potential, albeit unproven, risk for carotid embolization.20 The level of the transducer relative to the pressure being measured will determine the measured value of the arterial pressure, thus making its position critical for obtaining an accurate measurement. The standard level is the midpoint of the right atrium, approximately 5 cm below the sternal angle in the midaxillary line. This is the area where the preload pressure of the heart is determined.22
The distance of the measuring catheter from the heart, the length and compliance of the tubing, and the presence of air bubbles affect systolic and diastolic measurements in the system. These variables affect systolic and diastolic pressure proportionately in opposite directions and have no net effect on the measurement of mean arterial pressure (MAP). For this reason, measurement of MAP is a more accurate reflection of mean aortic pressure. MAP is manually calculated with the formula MAP = 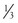 [(2 × diastolic blood pressure) + systolic blood pressure]. Electronic monitoring systems, however, measure MAP as the area under the pulse wave, often averaged over three or more cycles.
[(2 × diastolic blood pressure) + systolic blood pressure]. Electronic monitoring systems, however, measure MAP as the area under the pulse wave, often averaged over three or more cycles.
Pulmonary Artery Catheters
Pulmonary artery catheters provide central hemodynamic measurements, waveform tracings, and specific blood gas calculations such as mixed venous oxygen and carbon dioxide saturation (also see discussion of pulmonary artery catheter use in Chapter 32). Pulmonary artery catheters were initially used to assess and to treat patients with acute myocardial infarction (MI); however, the use of pulmonary artery catheters has declined significantly because several randomized trials have failed to demonstrate a reduction in mortality.23–25 Pulmonary artery catheters are typically inserted in the ICU in situations in which the clinician believes that measuring cardiac output, cardiac pressure, and mixed venous oxygen saturation will aid in guiding therapy and for direct assessment of the effect of and response to various treatments, such as fluids and vasopressors.26 Included in this category are patients in cardiogenic or refractory shock and patients in whom right ventricular dysfunction, such as right ventricular infarction, is suspected.18,27 Pulmonary artery catheters may induce arrhythmias, which are usually transient and not life-threatening.28 Left bundle branch block is a relative contraindication to the use of pulmonary artery catheterization because it can induce a temporary right bundle branch block and the potential for subsequent complete heart block.28 Other catheter-related complications include catheter knotting, pulmonary artery rupture, catheter fragmentation, and cardiac rupture secondary to forceful insertion. Long-term placement, more than 72 to 96 hours, can also lead to thrombosis and infection.
Echocardiography
Echocardiography is used to assess cardiac function by measuring ventricular contractility, chamber size, valve function, and flow.29,30 Global assessment of ventricular function with transthoracic echocardiography can be helpful in guiding hemodynamic management because one can easily differentiate between left and right ventricular dysfunction and adjust treatment appropriately.31 For instance, a patient with a poorly functioning left ventricle may benefit from an inotropic agent, whereas the presence of a hyperdynamic left ventricle and evidence of tissue hypoxia may indicate the need for fluid challenge and vasopressors.29 Echocardiography is helpful in the case of normotensive shock in a patient who has been adequately fluid resuscitated where it may be necessary to use afterload-reducing agents such as nitroglycerin.32 Calculation of ejection fraction, pulmonary artery pressure, and cardiac output is also possible, but specific expertise is required, and such measurements may not be reliable in ICU settings.31
Transesophageal Doppler ultrasound is a noninvasive modality for measuring cardiac output.33 Cardiac output is calculated by using the diameter of the descending aorta, the distribution of flow to the descending aorta, and the measured flow velocity of blood.15 The accuracy of the calculated cardiac output may be affected by the position of the ultrasound probe, which is inserted blindly in the esophagus. Poor positioning of the probe most commonly results in an underestimation of the cardiac output.15
Intra-Abdominal Pressure
Abdominal compartment syndrome is defined as clinically relevant organ dysfunction caused by intra-abdominal hypertension. Intra-abdominal pressure (IAP) is the steady-state pressure concealed within the abdominal cavity. For most critically ill patients, an IAP of 5 to 7 mm Hg is considered normal.34 Morbidly obese and pregnant individuals may have chronically elevated IAP without adverse consequences. Intra-abdominal hypertension is defined as IAP in excess of 12 to 20 mm Hg.35,36 Abdominal perfusion pressure is calculated as MAP minus IAP. Elevated IAP may reduce blood flow to the abdominal viscera. Abdominal compartment syndrome is diagnosed by the combination of intra-abdominal hypertension and evidence of organ malperfusion. Specifically, it is a constellation of symptoms consisting of new attributable organ dysfunction, such as cardiovascular (decreased cardiac output), respiratory (high peak airway pressure), and renal (oliguria) malperfusion, in the context of sustained IAP of 20 mm Hg or higher.35 Patients at increased risk for abdominal compartment syndrome are those who received large volumes of fluid for resuscitation (>10 L of crystalloid, >5 L of colloid) or transfusion of more than 10 units of packed red blood cells (PRBCs) during a 24-hour period.36
IAP is most commonly measured by the intravesicular technique.37 An indwelling bladder catheter is connected to a pressure transducer. A defined volume of fluid (25 to 50 mL of normal saline) is instilled into the catheter and used to distend the bladder and eliminate bladder wall coaptation.36 Volumes greater than 50 mL falsely elevate IAP values. IAP is then measured at end-expiration with the patient in the supine position and the manometer or other pressure measuring system at the level of the symphysis pubis. Another technique of IAP measurement uses a nasogastric or orogastric tube.38 This technique involves a lower risk for sepsis secondary to iatrogenic urinary tract infection as a result of catheter manipulation, but it is a more cumbersome method and rarely used. The timing of IAP monitoring and the frequency of monitoring are not standardized, but the highest risk for the development of abdominal compartment syndrome is during and shortly after resuscitation. The main reason for monitoring of IAP is that early recognition and treatment of abdominal compartment syndrome appear to improve survival.36
Cardiovascular Complications
Hypertension
Hypertension is common after surgery and may be caused by hypoxia, hypercapnia, hypervolemia, hypothermia, gastric or bladder distention, agitation, and pain. Furthermore, a common cause of postoperative hypertension is simply neglecting to restart preoperative antihypertensive medications. Sedation, analgesia, and rewarming often resolve most cases of mildly elevated blood pressure. However, hypertension increases myocardial oxygen consumption and can potentially cause myocardial ischemia. In addition, in the immediate postoperative period, it may contribute to increased bleeding from raw surfaces or through vascular anastomoses. After all possible causes have been evaluated and addressed, drug treatment may be considered. In general, blood pressure treatment targets should center on systolic blood pressure values 20 mm Hg above or below preoperative pressure.39 Deviations in MAP greater than 20% from preoperative values should also be treated.40,41 Several agents may be used, and the surgeon should become familiar with a few for an effective and safe approach to hypertensive patients in the postoperative period. Nitrates, beta blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, and vasodilators are the most common classes of drugs used (see Chapter 30).42
Hypertensive crisis is acute end-organ damage (heart, brain, retina, and kidney) associated with systolic blood pressure higher than 179 mm Hg or diastolic blood pressure higher than 109 mm Hg. In the absence of evidence of end-organ damage, these elevations in blood pressure constitute what are called hypertensive emergencies.43 When hypertensive emergencies are diagnosed, short-acting intravenous antihypertensive agents should be used; the reduction in blood pressure should occur within 1 hour in hypertensive crisis and within 24 hours in hypertensive emergencies.9 The goal is to reduce the patient’s diastolic blood pressure to less than 110 mm Hg during a period of 30 to 60 minutes.44 Hypertensive patients in these circumstances will manifest natriuresis, which causes intravascular fluid depletion requiring fluid administered along with antihypertensive medications. Several agents can be used in hypertensive emergencies; one of the most common and effective is labetalol because it is a combined α1-adrenergic and nonselective β-adrenergic receptor blocker.45
Hypotension
Hypotension is dangerous and is associated with an increased risk for end-organ dysfunction (MI, cerebrovascular events, and renal failure) and possibly bypass graft thrombosis. Common causes of hypotension after surgery are hypovolemia, cardiac dysfunction, and a diffuse vasodilatory state with or without sepsis. The two management strategies for correction of hypotension involve administration of fluids and vasoactive agents. A fluid challenge should be the first line of treatment of postoperative hypotension. Surgical bleeding should be excluded as a cause to prevent delays in returning the patient to the operating room.46
When administering fluids for resuscitation, one must determine whether the patient is on the ascending portion of the Frank-Starling curve and will benefit from a fluid challenge. If the patient is on the flat portion of the curve, fluid loading will only increase tissue edema and worsen tissue dysoxia with little effect on cardiac output.15 A large pulse pressure–stroke volume ratio (>10%:15%) is indicative of hypovolemia and predictive of volume responsiveness.15 A fluid challenge in these circumstances will increase preload and subsequently increase stroke volume.47 Similarly, in patients with a pulse pressure variation greater than 12%, fluid challenge is likely to have a positive effect on cardiac output.48 If the hypotension does not respond to fluid resuscitation, cardiac output must be optimized through pharmacologic means. Short-acting peripheral vasoconstrictors, such as phenylephrine and dopamine, are readily available on crash carts and can be given peripherally and titrated to effect. Once patients have adequate central intravenous access, dopamine should be switched to more definably titratable vasopressors.
In cardiogenic shock, peripheral vasoconstrictors may increase myocardial workload and dysfunction. In this setting, inotropic agents may be necessary to increase cardiac output. Patients in cardiogenic shock secondary to acute MI have mortality rates higher than 50%.49 In these situations, volume must be optimized, rhythm must be controlled, and beta agonists should be administered to enhance contractility.50 Finally, intra-aortic balloon pump counterpulsations may be necessary to aid the heart in generating cardiac output when pharmacologic agents fail. Beta blockers and calcium channel blockers have a negative inotropic effect and are relatively contraindicated in this setting.
Arrhythmias
Patients with preexisting structural heart disease are at highest risk for postoperative arrhythmias.51 Common triggers for postoperative arrhythmias are hypoxia, hypercapnia, acid-base imbalances, electrolyte abnormalities, and myocardial ischemia. Treatment must focus on predisposing factors, and the goal should be hemodynamic stabilization, control of the ventricular response, and restoration of rhythm.51
Tachyarrhythmias
The most common tachyarrhythmias are sinus tachycardia, atrial fibrillation (AF), ectopic atrial tachycardia, junctional tachycardia, multifocal atrial tachycardia, atrioventricular (AV) nodal reentry tachycardia, ventricular tachycardia (VT), and ventricular fibrillation. Tachyarrhythmias that traverse the AV node can often be controlled pharmacologically by altering conduction through the AV node.51 Tachyarrhythmias that are conducted by accessory pathways and ventricular tachyarrhythmias are more difficult to control pharmacologically than those that transverse the AV node. Supraventricular tachyarrhythmias are common after surgery, with an incidence of approximately 4% to 13%,51 and after AAA repair, with an incidence of 3.2%.52 Short bursts of atrial tachycardia do not require treatment. It is important, however, to correct any underlying causes, such as electrolyte imbalances. For sustained atrial tachyarrhythmia in a hemodynamically stable patient, the rate may be slowed with beta blockers or calcium channel blockers. Caution should be exercised with these agents, however, when they are used in patients with poor left ventricular function or congestive heart failure. Alternatively, in monitored settings, treatment can be switched to intravenous amiodarone. In stable patients with nodal reentrant rhythms, adenosine (6 to 12 mg intravenously) can also be used instead of beta blockers or calcium channel blockers. If patients are hemodynamically unstable, they should be electrically cardioverted regardless of the type of atrial tachyarrhythmia.
Ventricular tachyarrhythmias are also managed according to their duration and hemodynamic consequences. In the presence of coronary artery disease, these rhythms are ominous. Furthermore, the presence or absence of structural heart disease has prognostic significance,51 and it is important when treatment of runs of premature ventricular contractions and nonsustained VT is being considered. Prompt evaluation and treatment of predisposing factors, such as hypokalemia, hypomagnesemia, hypoxia, and myocardial ischemia, are important to prevent sudden death. In addition, unstable VT is an indication for immediate electrical defibrillation. In contrast, hemodynamically stable monomorphic VT should be treated with antiarrhythmics such as intravenous amiodarone followed by prompt cardiac evaluation.
Polymorphic VT and ventricular fibrillation occur most commonly in the setting of acute MI and can rapidly lead to hemodynamic instability. These arrhythmias are also treated with urgent defibrillation and intravenous antiarrhythmics. Polymorphic VT with prolonged QT intervals is called torsades de pointes. The most common cause of this rhythm is drug related or QT prolongation secondary to electrolyte abnormalities. Intravenous magnesium should be given empirically for suspected torsades de pointes. Positive chronotropic agents such as isoproterenol or temporary overdrive pacing to shorten the QT interval can also be used.
Atrial Fibrillation
AF is the most common arrhythmia encountered in the postoperative period; it affects 10% of patients undergoing major noncardiothoracic operations.53,54 Onset is often within the first few days after the operation, and AF is associated with increased 30-day postoperative mortality, increased ICU stay, and increased overall hospital length of stay. Common risk factors for the development of AF include advanced age, male gender, valvular heart disease, and previous history of AF.53 AF can be precipitated by hypokalemia, hypomagnesemia, volume overload, withdrawal of beta blockers or angiotensin-converting enzyme inhibitors, COPD, obesity and obstructive sleep apnea, and infections, especially pulmonary infections.53,55,56 A rapid heart rate limits ventricular filling, and loss of the atrial component of the cardiac cycle, the so-called atrial kick, decreases left ventricular stroke volume by 20% to 35%.57 This is especially important in patients with diastolic dysfunction and dilated left ventricles, whose cardiac output is volume dependent.58
Treatment focuses on controlling the heart rate, restoring normal sinus rhythm, and preventing thromboembolic complications.58 Patients who are hemodynamically unstable, who have pulmonary edema, or who have ongoing chest pain should undergo urgent electrical cardioversion. Hemodynamically stable patients should have their heart rate pharmacologically controlled with beta blockers, amiodarone, digitalis, or calcium channel blockers. Chemical cardioversion can also be used in hemodynamically stable patients, and a third of patients will convert to normal sinus rhythm with amiodarone loading alone.59
Before electrical cardioversion, one must consider the patient’s risk for thromboembolic complications. If the patient has been in AF for longer than 48 hours or for an unknown period, intracardiac thrombus must be excluded.60 This is obviously not feasible in the event of acute hemodynamic instability, and one should proceed with electrical cardioversion to stabilize the patient and subsequently monitor for thromboembolic complications. If an intracardiac thrombus cannot be excluded and the patient is hemodynamically stable, the safest course of action is anticoagulation with unfractionated heparin before cardioversion.53
Bradyarrhythmias
Bradyarrhythmias are not generally a diagnostic challenge, and treatment options are straightforward. Those associated with sinus node dysfunction are sinus bradycardia, sinus pause, sinoatrial block, and sinus arrest.61 Postoperatively, these rhythms are most often due to increased vagal tone and myocardial ischemia. If the bradyarrhythmia is transient and not associated with hemodynamic instability, it does not require treatment. Treatment of sustained bradyarrhythmias, and hemodynamic compromise, is with antimuscarinic agents such as atropine. If there is no response with continued bolus doses of atropine, the patient must be paced either externally or with a temporary transvenous pacer.
Postoperative Myocardial Infarction
Diagnosis
MI is the most common cause of cardiac complications and mortality in patients with peripheral vascular disease.46,62–65 The three modes of detecting clinically meaningful postoperative cardiac ischemia are symptoms, the electrocardiogram (ECG), and evaluation of myocardial enzymes. Symptoms elicited from the patient, often in the form of anginal chest pain, are not a frequent manifestation of postoperative myocardial ischemia but are more commonly found in those with acute coronary syndrome.66 In the postoperative period, these symptoms are also difficult to distinguish from distracting postoperative pain and are influenced by the concomitant use of analgesia and anesthetics. The presence of ST-segment changes on the ECG may indicate an MI. However, after major vascular surgery, approximately a third of patients will have ST changes on the ECG in the absence of clinically significant myocardial ischemia.67 Regardless, the presence of such changes correlates with an approximately 9- to 16-fold increased risk for MI and death.67 The third method of diagnosing postoperative MI is through the detection of myocardial proteins released into circulation as a result of myocardial cellular injury. The most common enzymes evaluated are the MB isoenzyme of creatine kinase (CK-MB) and the myocardial-specific cardiac protein troponin I.68 In vascular surgery patients, increased cardiac troponin I levels in the absence of clinical symptoms have also been demonstrated to be useful in the risk stratification of postoperative patients for cardiac morbidity.68 Elevated levels after major vascular surgery are associated with increased risk for perioperative MI and increased risk for mortality at 6 months.
ST-Segment Elevation Myocardial Infarction
ST-segment elevation MI (STEMI) is most commonly caused by acute rupture of atherosclerotic plaque and thrombosis of the involved coronary arteries. For this diagnosis to be made, the ECG must show ST-segment elevation of at least 0.1 mV (1 mm) in two consecutive leads.69 In patients with preexisting left bundle branch block, diagnosis of STEMI is difficult. Biochemically, CK-MB levels are elevated 3 to 12 hours after infarction, peak at 24 hours, and can be elevated for 3 days. Cardiac troponin levels can be detected 4 to 12 hours after infarction, peak at 12 to 48 hours, and can remain elevated for 1 week.
Treatment involves rapid resuscitation with administration of supplemental oxygen, afterload-reducing agents, antiplatelet therapy, anticoagulation with unfractionated heparin (if at low risk for bleeding), and urgent reperfusion therapy with either fibrinolysis or primary percutaneous coronary intervention (PCI). Postoperatively, fibrinolysis may be relatively contraindicated, but this decision must be individualized to the patient according to the extent of surgery and time after the operation. For clinically significant ischemia and for patients in cardiogenic shock in the postoperative period, urgent PCI and revascularization are indicated. Primary PCI is associated with primary patency rates of greater than 90%. Emergency coronary artery bypass surgery is reserved for patients who have failed to respond to PCI or fibrinolysis or who have a complication that requires surgery, such as ventricular septal rupture, cardiac rupture, or severe mitral valve insufficiency.70 Beta blockers should also be started unless patients are hypotensive or bradycardic, are in congestive heart failure, have advanced AV block, or have reactive airway disease. In addition, an angiotensin-converting enzyme inhibitor should be started in the first 24 hours after infarction. In the long term, patients also benefit from treatment with statins.
Non–ST-Segment Elevation Myocardial Infarction
Non–ST-segment elevation MI (non-STEMI) is characterized by the presence of biomarkers indicative of myocardial injury in the absence of ST elevation on the ECG. The key difference from STEMI is myocardial ischemia in the absence of significant coronary obstruction, such as occurs with acute plaque rupture. The myocardial ischemia in this setting is due to a transient reduction in coronary blood flow that causes an imbalance in myocardial oxygen supply and demand, often with shortness of breath and decompensated heart failure. The ECG can demonstrate nonspecific T-wave inversion or ST depression, and the key to diagnosis is the presence of circulating biomarkers (CK-MB, troponin I, troponin T) indicative of myocardial cellular necrosis.71 Mortality risk is directly proportional to troponin levels.72
Treatment is medical and centered on optimizing myocardial oxygen delivery and demand and preventing reinfarction and death.71 Supplemental oxygen should be administered, and pharmacologic agents to decrease preload and cardiac afterload are given. Treatment can be thought of as the ABCs (acetylsalicylic acid, angiotensin-converting enzyme inhibitor, beta blockers, and cholesterol lowering). Administration of unfractionated heparin is also associated with a mortality benefit,71 but its use must be balanced with the risk of bleeding. Statin therapy should also be instituted and treatment continued in the long term. In the setting of ongoing chest pain, the most recent American College of Cardiology/American Heart Association guidelines recommend the use of sublingual nitroglycerin if the systolic blood pressure is higher than 90 mm Hg and the heart rate is not less than 50 beats/min or more than 100 beats/min. If further analgesia is needed after nitroglycerin is given, morphine sulfate or meperidine can be used.73
Pulmonary Management
Respiratory insufficiency can be divided into either oxygenation failure or ventilation failure. Oxygenation failure is typically associated with hypoxia and ventilation failure with hypercapnia. Management of patients with acute respiratory failure and acute respiratory distress syndrome is discussed extensively in Chapter 40. The following comments focus on pulmonary support provided for the mechanically ventilated patient.
Ventilatory Support
Mechanical Ventilation
Mechanical ventilators affect oxygenation by controlling airway pressure and fraction of inspired oxygen and affect ventilation by controlling tidal volume and respiratory rate. Patients are ventilated primarily in two modes: controlled and supported. In controlled ventilation, the ventilator initiates and delivers a set amount of tidal volume; in supported mode, the breath is initiated by the patient and supported by the ventilator. Ventilators may deliver either a fixed tidal volume with variable pressure (volume control) or a variable tidal volume at a fixed pressure (pressure control). The combination of these two general modes of ventilation (controlled versus spontaneous) with either of these two settings (volume control versus pressure control) accounts for the majority of modes with which patients are ventilated. Patients with hemodynamic instability or in severe respiratory distress should be placed on a controlled mode of ventilation to limit the patient’s work of breathing. Either volume control or pressure control ventilation is adequate, and practitioners should select the mode with which they are most familiar. Pressure support mode is generally used in patients who are more stable. Pressure support mode is also used as a weaning mode (discussed in the next section).
It is important to ventilate patients with use of a lung protective strategy to reduce ventilator-induced lung injury, which can occur through two main mechanisms. The first is referred to as volutrauma, which causes overdistention of alveoli due to high tidal volume and high alveolar pressure and can lead to barotrauma (pneumothorax, pneumomediastinum). The second main mechanism is caused by shear forces on alveolar endothelium.74 These shear stresses are created in alveoli by cyclic opening and closing, and the resultant injury is called atelectatic trauma.75 PEEP applied throughout the ventilatory cycle serves to stent alveoli open, which prevents atelectatic trauma and facilitates gas exchange. The ideal level of PEEP is unknown, but an accepted range, depending on the clinical situation, is 5 to 15 cm H2O.
In ventilating patients at increased risk for volutrauma, such as those with acute respiratory distress syndrome, a lung protective strategy using pressure control ventilation to reduce peak airway pressure is most appropriate (Fig. 33-1). In this mode, inspiratory time can also be lengthened to allow increased alveolar recruitment, and alveolar distending plateau pressure and transpulmonary pressure are to be kept below 30 cm H2O by use of tidal volumes that should not exceed 6 to 8 mL/kg ideal body weight.76 However, low tidal volume ventilation causes decreased clearance of carbon dioxide with associated respiratory acidosis, but this “permissive hypercapnia” and acidosis are well tolerated if the patient’s pH is above 7.2.77 One caveat is that permissive hypercapnia is contraindicated in patients with cerebral edema.
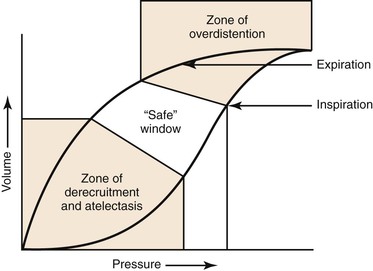
Figure 33-1 Protective ventilation. Ventilation of a patient with parameters on the left side of the pressure-volume curve places the patient at risk for atelectatic trauma, and ventilation of a patient with parameters on the right side of the curve places the patient at risk for barotrauma. (Redrawn from Froese AB: High-frequency oscillatory ventilation for adult respiratory distress syndrome: let’s get it right this time. Crit Care Med 25:906-908, 1997.)
Weaning
There are two main considerations in the weaning process. The first is weaning the support provided by the ventilator, and the second is removing the endotracheal tube (liberation from the ventilator). Patients should be assessed daily for readiness to be weaned, which can be done only after interruption of sedation.78 This method has been called “wake up and breathe.” Several randomized trials have revealed that weaning method influences ventilation duration.79,80 The traditional approach to weaning has been a progressive reduction in ventilatory support over time according to a protocol.81 A second technique is the use of spontaneous breathing trials without progressive withdrawal of ventilatory support, which may be associated with earlier extubation.82 With the traditional approach to weaning, patients are switched from a controlled to a spontaneous mode of ventilation, such as pressure support ventilation. The amount of ventilatory support and PEEP is then decreased according to oxygen saturation, respiratory rate, and tidal volume. If the patient is not ready for weaning, a decrease in tidal volume and an increase in respiratory rate will be noted.83 When a spontaneous breathing trial is used, patients are allowed to breathe spontaneously with little or no assistance. Most often, patients will be placed on minimal support (pressure support at 5 to 8 cm H2O, PEEP at 5 cm H2O) for 30 minutes to 2 hours. In a successful trial, the patient does not demonstrate respiratory distress (respiratory rate >35 for >5 minutes), desaturation (SaO2 <90% for >30 seconds), increase or decrease in heart rate (±20% for >5 minutes), systolic blood pressure higher than 180 mm Hg or lower than 90 mm Hg, or other signs of agitation and distress (paradoxical breathing, use of accessory muscles, diaphoresis).84
Indications for Extubation
Once patients are weaned from the ventilator and have successfully spent a prolonged time with minimal PEEP and on minimal pressure support, they can be assessed for the potential for extubation. If any indication for intubation exists, it is a contraindication to extubation. Contraindications to extubation are sedation, inability to protect the airway, presence of copious secretions, and lack of a cough reflex. Consideration must also be given to how difficult it would be to re-establish the airway if needed. Failure of extubation is associated with increased mortality and need for long-term ventilation.85 The harm of reintubation must be balanced by the harm created by prolonged mechanical ventilation. Reintubation rates of 10% to 15% are within the normal range in most ICUs.86
Tracheostomy
Tracheostomy relieves the patient of the portion of the work of breathing required to overcome the resistance of the endotracheal tube and the upper airway. It also allows better oral hygiene and secretion management and improves patient comfort. The major disadvantages of a tracheostomy are procedure-related complications, stomal complications, formation of a tracheoinnominate fistula, and formation of a tracheoesophageal fistula. Early tracheostomy within the first week in patients who are thought to require ventilation for more than 10 days facilitates weaning and is associated with shorter length of stay in the hospital.86 Jubran et al79 found that in patients who required prolonged mechanical ventilation, unassisted breathing through a tracheostomy resulted in shorter median weaning time compared with pressure support. However, in a retrospective observational study after abdominal and thoracoabdominal repair, Diedrich et al87 found that overall, tracheostomy correlated with worse outcomes but that patients with preexisting COPD who received a tracheostomy had improved survival.
Tracheostomy can be done either by an open surgical procedure or percutaneously. Percutaneous tracheostomy is as safe as open surgical tracheostomy, with the advantage that the patient does not require transport to the operating room, and operating room resources are thus conserved.88 Furthermore, no difference in long-term outcomes between percutaneous and open surgical tracheostomy has been shown.88 However, percutaneous tracheostomy is relatively contraindicated in patients who are hypoxic and have high PEEP requirements, in patients who are obese with short necks, in patients who are coagulopathic, and in those with recent (<10 days) anterior cervical spine fixation.89
Bleeding
Evaluation of Bleeding Patients
Patients with greater than expected fluid requirements postoperatively must be urgently evaluated for bleeding.46 The physiologic response to hypovolemia includes tachycardia, hypotension, low CVP, decreased urine output, and signs of peripheral vasoconstriction.90
Surgical Bleeding
Surgical bleeding can worsen coagulopathy and render it resistant to medical correction. A thorough patient examination is necessary to evaluate for possible sources of bleeding. Coagulopathy is generally assessed with a hemoglobin level, platelet count, prothrombin time (usually expressed as the international normalized ratio [INR]), activated partial thromboplastin time (aPTT), and fibrinogen assay. A blood sample for typing and screening should also be drawn to ensure that blood is available for transfusion if needed. Hemoglobin level may not show an initial drop when the patient is acutely bleeding. The role of imaging is dependent on the stability in an acutely bleeding postoperative patient. If the patient is stable and the bleeding source is not clear, computed tomography, ultrasonography, and angiography may be helpful.
Coagulopathy
For a summary of coagulopathy, see Table 33-1. Early in the postoperative period, coagulopathy is often dilutional or caused by incomplete reversal of the heparin administered during surgery (or both). Assessment of the coagulation system by measurement of the aPTT, prothrombin time (expressed as the INR), platelet count, and fibrinogen should be done immediately to guide therapy. The etiology, diagnosis, and management of specific coagulopathies are discussed in Chapter 37. Included here is a practical approach to the management of coagulopathic patients in the postoperative period.
Table 33-1
Postoperative Coagulopathy
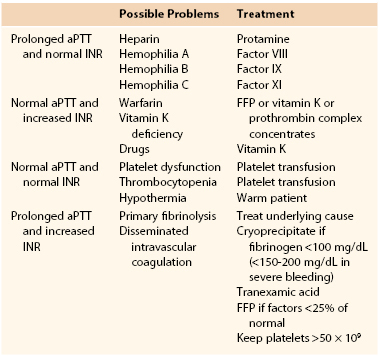
aPTT, Activated partial thromboplastin time; FFP, fresh frozen plasma; INR, international normalized ratio.
Prolonged aPTT and Normal INR.
If the patient has a prolonged aPTT and a normal INR, potential residual heparin effect may be reversed with protamine sulfate, which should be administered slowly to avoid inducing hypotension.91 However, the heparin effect may not be reduced by the administration of fresh frozen plasma (FFP) because it may increase the level of antithrombin III, and most of the effect of heparin is mediated through activation of this factor. A normal INR and a prolonged aPTT may also be found in factor VIII deficiency (hemophilia A) or von Willebrand’s disease. This may be treated with administration of the specific factor and in mild cases desmopressin (DDAVP). An increased aPTT with an often normal INR may also be found in patients with hemophilia. Diagnosis of the three types of hemophilia is made through specific factor analysis. Type A involves a deficiency of factor VIII, type B involves a deficiency of factor IX, and type C involves a deficiency of factor XI.90 In these patients, transfusion of the missing factors may be necessary. For further discussion of hemophilia, see Chapter 37.
Increased INR and Normal aPTT.
An increased INR with a normal aPTT may be caused by warfarin administration or by vitamin K deficiency secondary to chronic illness or malnutrition or as a side effect from treatment with broad-spectrum antibiotics. The coagulopathy in this setting is due to a reduction in vitamin K–dependent coagulation factors (factors II, VII, IX, and X), and FFP and vitamin K may be necessary to reverse this coagulopathy. In situations in which rapid reversal is required within 6 hours, FFP or prothrombin complex concentrates may be required.
Normal INR and aPTT.
In patients with a normal INR and aPTT, the bleeding diathesis may be caused by platelet dysfunction. This in turn may be due to lack of a sufficient number of platelets or to a defect in their function in the presence of a normal number. Other less common causes of coagulopathy associated with a normal INR and aPTT are factor XIII deficiency and hyperfibrinolysis. Causes of thrombocytopenia include bacterial infection, drugs (other than heparin), and bone marrow disease. Platelet dysfunction can also result from hypothermia. The level at which platelets should be transfused is controversial in an actively bleeding patient, but general guidelines for platelet transfusion are discussed later (and also in Chapter 37). Platelet dysfunction may be caused by platelet inhibitors or uremia. Platelet transfusion and DDAVP may be helpful in these settings. In the immediate postoperative period, patients need to be rewarmed expeditiously because platelet function is enhanced at normothermic body temperatures.46
Increased INR and Increased aPTT.
Primary fibrinolysis and disseminated intravascular coagulation (DIC) rarely occur in the postoperative period. DIC is usually associated with an increased INR and aPTT and thrombocytopenia. The hallmark of this condition is excessive stimulation of the coagulation system with an imbalance between thrombosis and fibrinolysis. The severity of the coagulopathy is dependent on the level of fibrinogen. Microthrombi are formed and induce the body’s natural mechanism of fibrinolysis. This in turn causes bleeding, and bleeding results in the release of tissue factors, including activation of factor VII, which increases coagulation. This scenario creates a vicious circle leading to depletion of stores of coagulation factors and subsequent consumptive coagulopathy. DIC can result from shock liver secondary to massive hypotensive episodes in the postoperative period, especially in the setting of chronic liver failure. Other common causes in postoperative patients are massive occult hemorrhage and sepsis. DIC has also been rarely associated with the administration of different blood products, including factor VII, in a dose-dependent manner.92
DIC is a clinical diagnosis, and laboratory tests are confirmatory.93 They show low fibrinogen levels, increased fibrin degradation products, and thrombocytopenia. The principles of management include treating the underlying cause, such as débridement of devitalized tissue and source control for sepsis or disseminated bacterial infection. Replacement of blood products is reserved for patients actively bleeding or at high risk for bleeding, not in response to laboratory abnormalities. It may be appropriate to administer cryoprecipitate when fibrinogen levels are less than 100 mg/dL (<150-200 mg/dL in severe bleeding), to transfuse FFP when other factor levels are below 25% of normal or the INR is abnormal, to transfuse platelets to increase counts to greater than 50,000/mm3, and to replace blood volume as needed.90,93
Fluids
Infusion of Fluids
Fluid administration in the postoperative period needs to be tailored to the individual patient. A balance must be reached between adequate tissue perfusion by replacing fluid loss at surgery and avoidance of fluid overloading. Hypervolemia is as detrimental to the patient as hypovolemia because it causes unwanted tissue edema and impedes tissue oxygenation. If large volumes of crystalloid solution are administered for resuscitation, it is recommended that a balanced salt solution such as lactated Ringer’s solution be given instead of normal saline to reduce the development of iatrogenic hyperchloremic metabolic acidosis. Restricted fluid regimens based on physiologic response versus standard fluid administration based on fluid algorithms and fixed rates have been associated with improved outcomes and decreased length of hospital stay after major gastrointestinal surgery and decreased pulmonary morbidity after thoracic surgery.94,95 I favor the administration of intravenous fluids in the postoperative period at low maintenance rates of 75 to 100 mL/h and small boluses of crystalloid (500 to 1000 mL) as needed, with titration to low blood pressure, increased heart rate, or low urine output (Fig. 33-2). Colloids are commonly used for fluid resuscitation because they have the theoretical benefit of expanding intravascular volume and because their osmotic activity reduces third spacing. However, there appears to be no survival benefit when albumin is used in severely ill patients as well as no difference among the different types of colloid used.96
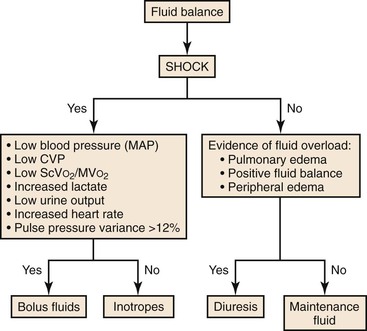
Figure 33-2 Fluid balance. CVP, Central venous pressure; MAP, mean arterial pressure; MVO2, mixed venous oxygen saturation; ScVO2, central venous oxygen saturation.
Electrolyte Management
In the immediate postoperative period, it may be dangerous to add routine electrolytes, such as potassium, to maintenance fluids because of perioperative fluid shifts. Routine testing of sodium, potassium, magnesium, calcium, and phosphate should be performed. Electrolytes should then be replaced individually as needed and can safely be done by protocol. Magnesium and potassium levels need to be maintained in the normal range to avoid cardiac arrhythmias. Maintenance of calcium and phosphate levels is also important for cardiac function as well as for several cellular functions. The serum sodium level is a reflection of total body water. Both hyponatremia and hypernatremia require assessment of the patient’s volume status and careful treatment to avoid adverse neurologic complications.
Transfusion of Blood Products
For a summary of blood product transfusion, see Table 33-2. The evidence for guiding transfusion practices is sparse with respect to the timing and volume of product administration and the ideal ratio of red blood cells (RBCs), platelets, and FFP. It is accepted that giving a disproportionate amount of one product over another can exacerbate dilutional coagulopathy. In addition, relying on laboratory tests to guide transfusion may be misleading because of the delay in obtaining results and because normal levels do not equate to normal function. Additional discussion of the following can be found in Chapters 32 and 37.
Table 33-2
Transfusion of Blood Products
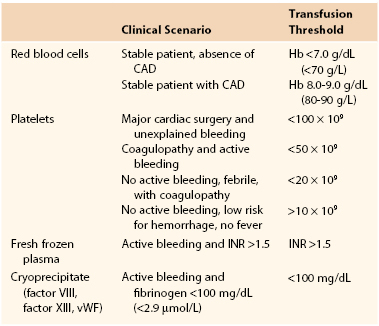
CAD, Coronary artery disease; Hb, hemoglobin; INR, international normalized ratio; vWF, von Willebrand factor.
Red Blood Cells
The transfusion threshold for RBCs has decreased for all patients on the basis of a randomized nonblinded study in critically ill euvolemic patients in the intensive care setting by Hébert et al.63 They compared 418 patients treated with a restricted transfusion strategy to maintain the hemoglobin concentration between 7.0 and 9.0 g/dL (70 and 90 g/L), in whom PRBCs were transfused when the hemoglobin level was lower than 7.0 g/dL (70 g/L), with 420 patients in whom the hemoglobin target was 10.0 to 12.0 g/dL (100 to 120 g/L), who were administered PRBCs when the hemoglobin level fell below 10.0 g/dL (100 g/L). The restrictive strategy resulted in a 54% reduction in the number of units of PRBCs transfused. Overall, 30-day mortality and organ dysfunction were similar in the two groups. Mortality at 60 days was significantly less in the restrictive group (P = .05). Furthermore, subgroup analysis suggested improved 30-day mortality in patients with an Acute Physiology and Chronic Health Evaluation (APACHE) score of less than 20 and age younger than 55 years for the restricted group (P = .02). No difference was found in patients with a primary or secondary diagnosis of cardiac disease. Thus, for clinically stable patients not at risk for coronary artery disease, transfusion of RBCs is likely to be beneficial when the hemoglobin level is less than 6.0 g/dL (60 g/L) but not when it is greater than 7.0 g/dL (70 g/L), as long as normal blood volume is maintained and the patient is carefully monitored.
In contrast to critically ill patients, guidelines state that for those at risk for coronary artery disease, transfusion may be indicated when hemoglobin is less than 8.0 to 9.0 g/dL (80 to 90 g/L). In acute blood loss, the lower limit of tolerance has not been established, but oxygen delivery will probably be adequate with a hemoglobin level of 6.0 to 8.0 g/dL (60 to 80 g/L). With ongoing blood loss, however, the measured hemoglobin concentration may be misleading because by the time that the hemoglobin value is reported, the actual oxygen-carrying capacity may have changed. In these circumstances, the decision to administer RBC transfusions should be determined by evaluating the rate of ongoing blood loss, evidence of end-organ dysfunction, and risk for or presence of coronary artery disease.90
Platelets
The definition of a threshold for platelet transfusion would be relatively easy if there were consistent, well-defined relationships among platelet count, platelet function, and bleeding manifestations. However, 10 review articles and 12 guidelines for platelet transfusion therapy were published between 1966 and 1999, thus suggesting that such a relationship is less than clear. One unit of platelets from whole blood contains 5.5 to 10 × 1010 platelets, whereas 1 unit from platelet pheresis contains 40 × 1010 platelets and is equivalent to 6 units from whole blood.
In nonsurgical patients, spontaneous bleeding is uncommon with platelet counts greater than 20 × 109/L. In contrast, the platelet count at which surgical patients are likely to experience increased bleeding is unknown. Regardless, in 1994, the College of American Pathologists recommended platelet transfusion for patients with platelet counts less than 50 × 109/L.97 For major surgery with life-threatening bleeding, this guideline also concluded that transfusions might be indicated at higher platelet counts to maintain a concentration greater than 50 × 109/L. In addition, it indicated that some experts recommend platelet transfusion after cardiopulmonary bypass in patients with normal coagulation values and platelet counts below 100 × 109/L when major unexplained bleeding occurs because platelets are dysfunctional. Similarly, the American Society of Anesthesiologists Task Force concluded that bleeding risk in surgical patients is defined by the type and extent of surgery, the ability to control the bleeding, the consequences of uncontrolled bleeding, the actual and anticipated rate of bleeding, and the presence of factors that adversely affect platelet function (e.g., extracorporeal circulation, renal failure, medications). Moreover, in the absence of contradictory evidence, the opinion of the task force is that platelet transfusion is justified at higher platelet counts in bleeding patients than in nonbleeding patients because of the increased risk for complications from bleeding in surgical patients. Similarly, massively transfused patients with microvascular bleeding suspected to be secondary to platelet deficiency may benefit from empirical platelet therapy. However, the task force recommends that surgical patients with microvascular bleeding usually require platelet transfusion if the platelet count is less than 50 × 109/L but rarely require therapy if the count is greater than 100 × 109/L. With intermediate platelet counts (50 to 100 × 109/L), the decision to transfuse platelets should be based on the patient’s risk for more significant bleeding.
In summary, for patients who are actively bleeding after major surgery, platelet counts should be maintained at a level greater than 50,000/mm3. In nonbleeding patients and those in whom the risk for hemorrhage is low, platelet counts can be maintained above 10,000/mm3. However, if patients have a fever or are coagulopathic, the target should be a level greater than 20,000/mm3.
Fresh Frozen Plasma
According to the American Society of Anesthesiologists, after major transfusion, defined as transfusion of more than 10 units of RBCs, or after major surgery with active bleeding, FFP should be given to reduce the INR to a level below 1.5 because pathologic microvascular bleeding does not tend to occur until coagulation test results are greater than 1.5 times normal. In massive resuscitation such as for trauma, it has also been suggested that FFP be given in a 1 : 1 ratio with RBCs to reduce the risk of dilutional coagulopathy, although definitive evidence supporting this practice is lacking. FFP is also indicated for warfarin overdose, but it will not correct the anticoagulant effect of heparins because it contains antithrombin, which is augmented by both unfractionated heparin and low-molecular-weight heparin.
Cryoprecipitate
Cryoprecipitate consists of fibrinogen, factor VIII, factor XIII, and von Willebrand factor. The majority of acquired hypofibrinogenemias are due to consumptive coagulopathy and fibrinolysis. With postoperative bleeding, administration of cryoprecipitate should be reserved for patients with fibrinogen levels less than 100 mg/dL (2.9 µmol/L). Each unit of cryoprecipitate contains approximately 150 mg of fibrinogen and is compatible only with normal saline. The volume needed to raise fibrinogen concentration 0.5-1.0 g/L can be estimated as one unit of cryoprecipitate per 5-10 kg of body weight.97a
Summary of the Transfusion of Blood Products
For clinically stable patients not at risk for complications of coronary artery disease, transfusion of PRBCs is likely to be beneficial when the hemoglobin level is less than 7.0 g/dL (70 g/L), as long as normal blood volume is maintained and the patient is carefully monitored. Critically ill patients and those at risk for coronary artery disease will probably benefit from maintenance of hemoglobin in the range of 8 to 9 g/dL (80 to 90 g/L). In acute blood loss, the lower limit of tolerance has not been established, but oxygen delivery will probably be adequate with a hemoglobin level between 6 and 8 g/dL (60 and 80 g/L). With ongoing blood loss, however, the measured hemoglobin concentration may be misleading because by the time that the hemoglobin value is reported, the actual oxygen-carrying capacity may have changed. In this circumstance, the decision to administer RBC transfusions should be determined by evaluating the rate of ongoing blood loss, evidence of end-organ dysfunction, and risk for or presence of coronary artery disease. FFP should be administered in the context of massive transfusions if there is microvascular bleeding associated with a significant (>1.5 times the normal value) increase in INR or aPTT. If these tests are not readily available, however, plasma may be transfused in an attempt to stop diffuse nonsurgical bleeding. Cryoprecipitate should be given when the fibrinogen level is less than 100 mg/dL (2.9 µmol/L) or possibly when time or the intravascular volume does not allow transfusion of FFP. Platelets should be transfused to patients who are bleeding from nonsurgical causes and have a platelet count of 50 × 109/L or less. Clinical circumstances may also require transfusion of platelets at a higher threshold.
Adjuncts in Hemostasis
Several adjuncts may be used with the aim of improving hemostasis. Recombinant activated factor VII (VIIa) has been used to control bleeding in patients with hemophilia type A and type B. Recombinant factor VIIa also generates thrombin at the site of vessel injury and induces coagulation.98 It has been used in the treatment of patients with hemorrhage from blunt trauma, thrombocytopenia, warfarin overdose, obstetric bleeding, and intracranial bleeding.98,99 It is no longer recommended for off-label use in patients without hemophilia because of increased thromboembolic arterial events and lack of mortality benefit.100
Antifibrinolytic agents such as tranexamic acid and aminocaproic acid have been successfully used to treat postoperative hemorrhage.101 These synthetic lysine analogues inhibit plasminogen and plasmin-mediated fibrinolysis.91 Similarly, serine proteases such as aprotinin, which neutralize trypsin, plasmin, and kallikrein, have been used when fibrinolysis contributes to bleeding.102 These agents have been shown to decrease transfusion of blood products in cardiac, hepatic, and orthopedic surgery.102 Aprotinin has also been used prophylactically in coronary artery bypass surgery, but there has been concern because of an increased incidence of renal failure and increased risk for MI in these patients that led to removal from the market for a time.98 Finally, DDAVP, a synthetic vasopressin analogue that stimulates endothelial release of factor VIII and von Willebrand factor, has been used to enhance platelet aggregation.103 It is used mainly in patients with hemophilia and those with both congenital and acquired platelet disorders, but its value in surgical hemostasis is unproven. Thus, its use in postoperative patients who do not have bleeding disorders is not recommended.98
Complications of Massive Transfusion
The definition of massive transfusion is replacement of 50% of a patient’s blood volume in 3 hours or one blood volume in 24 hours.104 The major complication of massive transfusion is the development of coagulopathy from multiple causes, including hemodilution, hypothermia, acidosis secondary to tissue hypoxia, and DIC.104 Hemodynamically unstable patients are initially resuscitated with crystalloids, which are acellular and dilute the blood components, thereby aggravating the coagulopathy. Hypothermia associated with the infusion of large volumes of cold solution also inhibits the enzymatic coagulation cascade, synthesis of coagulation factors, and platelet function.105 Administration of large volumes of fractionated blood products also causes coagulopathy by complex mechanisms, some known and some unknown: dilution of coagulation factors (particularly factors V and VIII : C, which have a limited shelf life), hypocalcemia because of the citrate used as an anticoagulant, and platelet dysfunction as a result of aging and metabolic alterations caused by storage.90 Furthermore, when RBCs are stored, their extracellular potassium levels increase over time, so patients receiving large volumes of RBCs are at risk for hyperkalemia.
One of the most common sequelae of massive transfusion is acute lung injury secondary to congestion caused by overload, alveolar inflammation, and increased permeability.106 Passive transfer of leukocyte antibodies in donated blood resulting in immune-mediated transfusion-associated lung injury can also occur.107 Finally, massive transfusion has been described as an immunosuppressant leading to sepsis and sepsis-induced lung injury.106 (See Chapter 32 for further discussion of transfusion-associated lung injury.)
Renal Failure
Detailed discussion of renal failure can be found in Chapter 41. Therefore, this discussion focuses on issues related primarily to the care of patients with postoperative renal failure. Briefly, patients with preoperative renal insufficiency are at the highest risk for postoperative renal failure. After vascular surgery, the major cause of postoperative renal failure is perioperative hypotension and ischemic injury leading to acute tubular necrosis.46 Unfortunately, postoperative renal failure is a marker for increased mortality, with rates of 60% to 80% after major vascular surgery.46 Physiologically, patients are found to have oliguria or anuria and rising creatinine concentration despite volume resuscitation.
Few strategies have proved effective in reducing the incidence of this complication in the postoperative period. Diuretics may improve diuresis but have no demonstrated effect on prevention of renal failure. Low-dose dopamine has also been used but without a reduction in morbidity or mortality.108 In small studies, the use of fenoldopam (a renal vasodilator with natriuretic effect) given intraoperatively and postoperatively has been associated with some beneficial effect in patients with preexisting renal insufficiency.109,110
Early management of postoperative renal dysfunction should be focused on reperfusion of the kidneys, which is most often achieved by fluid resuscitation. Forced diuresis has also been used in addition to fluid loading, but diuresis can be detrimental because it increases oxygen demand in the kidney and exacerbates ischemic injury.111 Loop diuretics are the drugs of choice when forced diuresis is used because they offer the theoretical benefit of decreased oxygen consumption by the kidney through inhibition of medullary thick ascending limb adenosine triphosphatase.112 In patients with renal dysfunction, doses of many drugs used in intensive care (particularly antibiotics excreted through the kidneys) should be adjusted according to creatinine clearance.
General Issues
Gastrointestinal Ischemia
Postoperative gastrointestinal ischemia is a rare but potentially lethal postoperative complication. Colonic ischemia occurs in 2% of patients after elective AAA repair and carries an overall mortality of 40% to 60% if it is transmural.113 The incidence of ischemic colitis in patients undergoing repair of a ruptured AAA is reported to be 15% to 65%.114 A high index of suspicion for colonic ischemia is necessary after aortic repair in patients with persistent acidosis and shock with no other obvious cause, greater than expected fluid requirements, new onset of gastrointestinal bleeding, or simply bloody bowel movements. Laboratory investigations often demonstrate anion gap acidosis and leukocytosis. In advanced cases, a plain abdominal radiograph may demonstrate free air in the peritoneal cavity or in the portal venous system. Abdominal computed tomography may demonstrate evidence of pneumatosis intestinalis. Bedside flexible sigmoidoscopy or colonoscopy can be diagnostic of colon ischemia. Stable patients with colonoscopic results suggesting ischemia limited to the mucosa may be treated conservatively with hydration and broad-spectrum intravenous antibiotics. However, immediate exploration is warranted in such patients if the condition worsens or hemodynamic instability ensues. Unstable patients with evidence of bowel ischemia should undergo urgent surgical exploration to reduce mortality. In selected cases of colon ischemia that has not progressed to gangrene, reimplantation of the inferior mesenteric artery can be considered. In the setting of frank gangrene, the colon should be resected and a colostomy created.
Nutrition
The nutritional state of critically ill patients has a direct effect on outcome. This is due to the catabolic stress state that critical illness creates. Malnutrition in this patient population is associated with increased morbidity and mortality as well as increased length of stay. In the general medical and surgical ICU population, it has been shown that commencing enteral nutrition within 24 to 48 hours of ICU admission is associated with improved outcomes.115 It has been associated with reduced infections, increased protection against stress ulceration as well as intestinal ischemia, maintenance of gut flora, decreased catabolism, and decreased mortality.116 Nutritional support in the ICU has had the goals of maintaining lean body mass to preserve immune function and to avert metabolic complications.117 This includes delivery of both micronutrients and macronutrients and meticulous glycemic control.117 Early enteral nutrition has been shown to be safe in patients admitted to the ICU after major abdominal surgery.118
Several studies have also shown that patients undergoing major vascular surgery have a significant incidence of preoperative and postoperative malnutrition.119 Patients undergoing surgery for AAA have higher nutritional depletion and increased rates of postoperative infection compared with patients having carotid or peripheral vascular surgery.120 Interestingly, after AAA surgery, it has also been demonstrated that small bowel function returns to normal by 18 hours and starting enteral nutrition within 3 hours of surgery can promote small intestinal activity compared with fasting. This suggests that enteral nutrition may in fact aid the return of normal bowel function.121 As suggested by others, it is my practice to initiate oral or enteral feeding within the first 48 hours after AAA surgery, even in the absence of traditional clinical markers for readiness to feed (presence of bowel sounds, flatus, bowel movement, or volume of gastric aspirate).122 Most ICUs use enteral nutrition protocols and guidelines for critically ill patients for this reason.116 Although there is a paucity of studies of the applicability of these protocols specifically to vascular patients, both our vascular surgeons and intensivists think that these guidelines are safe and beneficial in this patient population.123
There are several newly updated North American and European guidelines available to help guide assessment of nutrition and support therapy in critically ill adult patients.116,117 These patients represent a heterogeneous population, and the nutritional needs of patients should be individualized. These guidelines are intended for patients who are expected to require more than 2 or 3 days of ICU care and have had significant metabolic or surgical stress. They are not intended for those patients who require only temporary monitoring or who have had minimal surgical stress. Some of the general highlights of the guidelines are reviewed here.
Nutrition in the ICU can be subdivided between dosing and route of administration. Several predictive equations exist that are used to estimate calorie requirements for patients in the ICU. However, these predictive equations are often less accurate than indirect calorimetry. These predictive equations are also more problematic in obese patients in making accurate assessments of nutritional goals. Once goal calories are determined, patients should be given 50% to 65% of goal calories in their first week of ICU admission, thereafter increasing to goal rate. In obese patients (body mass index >30) with critical illness, permissive underfeeding or hypocalorie feeding with enteral nutrition is recommended. The target in obese patients should remain at 60% to 70% of ideal weight.
The two main routes of administering nutrition in critically ill patients are the enteral route and the parenteral route. As a general rule, the enteral route has the most benefit for the patient. In patients who are unable to eat, enteral nutrition is preferred to parenteral. As mentioned before, feeding should be started within the first 24 to 48 hours of admission to the ICU and advanced toward goal during the next 48 to 72 hours. Enteral feeding can be started through either a gastric tube or small bowel tube in the absence of traditional markers of bowel function (bowel sounds, flatus, or passage of stool). Patients are often challenged with trickle feeding at 10 mL/h to ensure tolerance before increasing rapidly toward goal.
It is recommended that either parenteral or enteral nutrition be provided to critically ill patients who are unable to voluntarily maintain their nutritional needs. In general, enteral feeding is preferred to parenteral feeding because of superior nutritional efficacy and better safety profile. Enteral feeding through a nasogastric tube or small bowel feeding tube should be started within the first 24 to 48 hours after admission to the ICU and continued until adequate oral intake is firmly established. Placement of a feeding tube in the proximal small bowel should be considered in patients with inadequate gastric emptying as evidenced by residual gastric volumes in excess of 500 mL. If early enteral feeding is not feasible, parenteral nutrition should be considered if the anticipated duration of nutritional support will exceed 7 days. Shorter periods of parenteral nutrition (e.g., <5-7 days) expose the patient to a variety of vascular access, metabolic, and infectious risks without proven benefit. Even in patients stabilized on parenteral nutrition, periodic efforts should be made to start enteral feeding. Parenteral nutrition should not be stopped until enteral nutrition is providing at least 60% of target requirements. Recommended nutrition doses based on guidelines are up to 20 to 25 kcal/kg actual body weight per day in the acute phase of critical illness (72-96 hours) and 25 to 30 kcal/kg body weight per day in the recovery phase.116
Pain Management
Effective postoperative analgesia is important and may be associated with a reduction in the incidence of myocardial ischemia after surgery.62 This beneficial effect does not appear to be related to changes in heart rate, blood pressure, or requirement for postoperative vasoactive agents compared with patients with inadequate pain control.62 In nonintubated patients who are able, patient-controlled analgesia is preferred to intermittent on-demand narcotic dosing because patients can be in a significant amount of pain while waiting for the next narcotic dose. Epidural analgesia can also be effective in the postoperative period, and it offers the potential benefit of reducing the dose of opiates, which improves gastric emptying and ileus and increases patient mobility.124 Patients who are mobilized earlier can benefit from reduced thrombotic events and pulmonary complications. However, care must be taken to prevent significant hypotension, which can be caused by the administration of epidural anesthesia. The balance between analgesia and sedation is discussed in the section on sedation and delirium in the ICU.
Deep Venous Thrombosis Prophylaxis
The etiology, treatment, and prophylaxis of deep venous thrombosis (DVT) are reviewed in depth in Chapter 51. Thus, only issues relevant to the prophylaxis and treatment of DVT after vascular surgical procedures are discussed here. Because most vascular surgery involves the intraoperative administration of heparin, it has been suggested that these patients do not carry the same postoperative risk for DVT as other surgical patients do. However, the incidence of DVT after aortic surgery has been reported to be between 2% and 18%,125 and therefore all patients undergoing vascular surgical procedures should receive postoperative DVT prophylaxis.
General measures in postoperative care, such as elevation of the lower extremities, early ambulation, and leg exercises, may reduce the incidence of DVT.126 Graduated compression stockings, intermittent pneumatic compression, and venous foot pumps have also been shown to decrease the incidence of DVT, but they have not been proved to prevent fatal pulmonary embolism when used as monotherapy.126 It is also generally accepted that aspirin is not effective in the prevention of DVT.127 In contrast, treatment with either low doses of subcutaneous unfractionated heparin (5000 units subcutaneously two or three times per day) or subcutaneous low-molecular-weight heparin at prophylactic doses is effective and safe in the prevention of postoperative DVT.126 The potential benefit to prophylaxis with low-molecular-weight heparin is a reduced rate of heparin-induced thrombocytopenia. Prophylaxis is generally given until patients become ambulatory and mobile. This topic is extensively reviewed in Chapter 51.
Bridging Postoperative Oral Anticoagulation
Patients taking oral anticoagulants in the preoperative period require specific considerations. The most common strategy is to interrupt the oral anticoagulation and to use either subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin.128
The American College of Chest Physicians has published guidelines with regard to patient risk for thromboembolic events.129 Patients are categorized as being at low, moderate, or high risk. Bridging with full-dose anticoagulation is recommended only for patients at high risk. Such patients include those with mechanical mitral valves or old-model mechanical aortic valves. However, in patients with low stroke and thromboembolic risk, such as those with AF and no other risk factors for stroke such as rheumatic valve disease, bridging may be unnecessary and potentially harmful.130 In these patients, oral anticoagulation can be stopped several days before the procedure and restarted several days after it. Although limited data regarding benefit are available, prophylaxis-dose heparin may be used for these patients at low risk.
In patients taking oral anticoagulants who are at high risk for perioperative stroke, such as those with mechanical mitral valves or old-model mechanical aortic valves, oral anticoagulants should be stopped 4 days before and heparin started 2 days before surgery, when the oral agent is assumed to become subtherapeutic.128 In the absence of postoperative bleeding, bridging doses of heparin can be restarted 12 to 24 hours after the surgical procedure. If unfractionated heparin is used, it should be restarted without a bolus dose. However, the risk of bleeding caused by restarting anticoagulation after major vascular surgery must be carefully considered. If anticoagulation is delayed because the risk of bleeding is deemed too high, prophylactic doses of heparin may be given.128 After minor surgical procedures, oral anticoagulation can be restarted the evening of surgery. Regardless, clinicians must decide on a strategy that balances stroke risk with bleeding risk in each individual patient.
Alcohol Withdrawal Syndrome
Alcohol is the most abused drug worldwide.131 Autonomic hyperactivity, including tremulousness, sweating, nausea, vomiting, anxiety, and agitation, appears within the first 24 to 48 hours after withdrawal in patients who abuse alcohol.131 Symptoms can peak between the third and fifth postoperative days. Serious complications that may develop are delirium tremens and grand mal epileptiform seizures. Delirium tremens, which occurs in less than 5% of patients abusing alcohol, is associated with an increase in postoperative mortality132 and is characterized by fever, tachycardia, hypertension, tremors, diaphoresis, hallucinations, disorientation, agitation, and urinary incontinence. Physicians must exclude other life-threatening causes of confusion, such as myocardial ischemia, congestive heart failure, hypotension, pulmonary embolism, bleeding, stroke, metabolic and electrolyte disorders, and sepsis. Before sedation is initiated for presumed alcohol withdrawal, hypoxia must be ruled out because hypoxia is the most common cause of confusion and agitation in the postoperative period.
Patients can be identified preoperatively to be at risk by the quantity and frequency of their alcohol consumption. Preoperative detoxification or abstinence before elective surgery may prevent adverse postoperative complications, but data in this regard are lacking. Patients known to be at risk should be administered prophylactic doses of benzodiazepines throughout the entire perioperative period to prevent the development of alcohol withdrawal syndrome.132 Patients who regularly consume alcohol at dependency levels (determined by how alcohol consumption affects relationships, jobs, educational goals, and family life and not by the specific quantity consumed) have been shown to have increased postoperative morbidity and mortality.133
First-line treatment of patients with alcohol withdrawal syndrome is benzodiazepines.134 They mimic the central nervous system effects of alcohol on γ-aminobutyric acid receptors and suppress the withdrawal response.135 Alcohol replacement therapy with oral or intravenous alcohol is still used but is controversial and has not been shown to be superior to benzodiazepine treatment.136 Treatment of severe acute alcohol withdrawal may require intubation and admission to the ICU. Beta blockers may also be used to reduce sympathetic overactivity and cardiac complications. Seizures should be treated with intravenous benzodiazepines. All patients should also be given daily thiamine and multivitamins to prevent Wernicke-Korsakoff syndrome.
Sedation and Delirium in the Intensive Care Unit
Emphasis on sedation and analgesia in critically ill patients has recently come to the forefront of patient care in the ICU. The primary goal is achieving adequate pain control and anxiolysis with appropriate sedation and analgesia. Unfortunately, in critically ill patients on mechanical ventilation, this is very challenging and can lead to oversedation, delirium, and harm to the patient.137 It is very important to titrate the medications used for analgesia and sedation in a goal-directed manner to safely provide comfort without impeding recovery, affecting weaning from the ventilator, and prolonging length of stay. Several sedation-monitoring systems have been developed in the ICU, such as the Sedation Agitation Scale, the Motor Activity Assessment Scale, and the Richmond Agitation-Sedation Scale (RASS).137 A multidisciplinary team at Virginia Commonwealth University in Richmond has developed the RASS, and it is a validated method used to avoid oversedation in the ICU. It is unique from other methods of evaluation in that it separates verbal from physical stimulation in generating the score, thereby assessing the patient’s arousal with varying potency of stimulus.138 The RASS assesses consciousness by use of a 10-point scale from −5 to +4 (−5, unarousable, to 0, alert and calm, to +4, combative) with three defined steps that incorporate the duration of eye contact to determine arousal and content of thought.139 The entire assessment takes approximately 20 seconds.137 The first step is simply to observe the patients; if they are alert, restless, or agitated, they receive a score of 0 to +4 accordingly. If they are not spontaneously alert, they are called to look at the rater, with duration of eye contact rated (more or less than 10 seconds) and scored from −1 to −3 accordingly. If patients do not respond to verbal stimulation, they are stimulated physically and scored −4 or −5.139 However, if patients are calm but not alert before verbal and physical stimulation, they are rated from −1 to −5 even if they become agitated on stimulation.
Ely et al137 compared the RASS with the Glasgow Coma Scale, which is a trauma-specific consciousness assessment tool, and demonstrated excellent correlation and discrimination between the scores in assessing levels of consciousness. They also compared RASS with cumulative lorazepam, propofol, fentanyl, and morphine doses of the prior 8-hour and 24-hour periods before RASS assessment. Interestingly, they found significantly different correlations with RASS between opiates (analgesics) and benzodiazepines (sedatives), with higher likelihood of reintubation with deeper sedation measured by RASS. There was further agreement in this study that RASS provided a consensus target for goal-directed sedation and improved team communication regarding sedation management.
Consciousness is considered a combination of level of arousal plus the content of that consciousness, such as delirium.140 Unaddressed pain and anxiety in critically ill patients has been shown to lead to increased morbidity and mortality as well as posttraumatic stress disorder long term.141 RASS is used to assess the level of arousal, but another key to patient outcome is delirium, a key feature of which is the presence or absence of inattention. Because the RASS incorporates eye contact as part of its assessment, it also correlates with the onset of inattention and delirium. The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) is a delirium-monitoring tool that complements the RASS and is used to assess acute brain dysfunction.142 Whereas RASS measures the level of consciousness, CAM-ICU determines the content of that consciousness and the presence or absence of delirium. Delirium is a common but underdiagnosed form of organ dysfunction in the ICU. It was once thought to be a normal part of the process as patients arose from coma to consciousness, but it also occurs in the absence of this process or preexisting coma. Critically ill patients at risk for the development of delirium are those with preexisting cognitive impairment, advanced age, use of psychoactive drugs, mechanical ventilation, untreated pain, and sleep deprivation.142
Three subtypes of delirium are described: hyperactive, hypoactive, and mixed. Hyperactive delirium is represented by the patient who is agitated and attempting to pull out lines and tubes; patients with hypoactive delirium typically are characterized by a flat or withdrawn affect; and those who fluctuate between the two have mixed delirium. ICU physicians have concentrated on treating multiorgan dysfunction, often dismissing delirium as an unavoidable, often iatrogenic consequence of critical illness with little long-standing ill effect. However, the development of delirium has been shown to be an independent predictor of higher 6-month mortality and longer hospital stay in mechanically ventilated patients.143 In this study, the investigators demonstrated that the development of delirium, and the number of days spent in a delirious state, was associated with a threefold increase in mortality in mechanically ventilated patients compared with those who did not develop delirium. Current guidelines suggest in fact that critically ill patients be monitored daily for delirium.144
The assessment for delirium combines the RASS score with inattention and either disorganized thinking or an altered level of consciousness.142 If patients respond to verbal stimulation (RASS score of −3 to +4) and have a positive CAM-ICU assessment, they are found to be delirious. Monitoring delirium with the CAM-ICU daily takes approximately 1 to 2 minutes, and nurses can easily be trained to perform it with their routine assessments.143 One important aspect in daily assessment for delirium is identifying patients with the hypoactive delirium subtype. As mentioned before, this subtype is characterized by a flat affect present in a calm, seemingly alert patient.145 These patients have normal or nearly normal arousal compared with the agitated patients with delirium, which is likely why they are underdiagnosed and may have a worse outcome. Outcomes can be improved by reducing unnecessary use of sedatives and analgesics.
Treatment of delirium can be divided into nonpharmacologic and pharmacologic agents. Nonpharmacologic methods concentrate on minimizing risk factors with repeated reorientation of patients, early mobilization and activity, nonpharmacologic sleep protocol, and timely removal of catheters and lines; unnecessary noise and stimuli are minimized, and patients are given their normal aids, such as glasses and hearing aids. Pharmacologic strategies first involve reduction or removal of unnecessary sedatives and analgesics. Many drugs, such as benzodiazepines, used to treat confusion often exacerbate it; lorazepam is a well-recognized offender. Pharmacologic agents with procognitive advantage, such as haloperidol, have also been recommended for the treatment of delirium, although this is based on sparse data in the ICU population.144 Other antipsychotic and neuroleptic agents, such as risperidone, quetiapine, and olanzapine, are often used in both hyperactive and hypoactive delirium, but patients receiving these agents must be closely monitored for adverse cardiac and extrapyramidal events. An important resource for physicians is available at www.icudelirium.org. Figure 33-3 gives an overall approach in critically ill patients to balance both pharmacologic and nonpharmacologic approaches. It attempts to improve patient outcomes by addressing oversedation, immobilization, and delirium at the same time in high-risk critically ill patients.
Figure 33-3 Sedation, mobility, sleep, delirium harm reduction model. This diagram demonstrates the inter-relationship between unrecognized delirium, over-sedation, immobility and lack of sleep. The red and green denote pathways that make this problem worse where the blue and yellow pathways make this problem better. For example, the overuse of sedation decreases the likelihood of mobility, increases delirium risk, and decreases restorative sleep. Alternatively mobilizing patients can make apparent the decreased need for sedation, can improve sleep, and can help delirium clear. (From Clemmer T: Rethinking critical care: reducing patient harm from sedation, immobility, and delirium, 2001, Institute for Healthcare Improvement.)
Outside the ICU, postoperative delirium requires a hypoxic, metabolic, and septic workup. Medications and medication interactions have to be ruled out, and older patients such as our vascular patients suffer from multisensory deprivation, which makes them extremely confused at night.
Selected Key References
Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(Suppl):204S–233S.
Excellent set of evidence-based guidelines on antithrombotic and thrombolytic therapy..
Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Ornato JP. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:E1–E211.
Extensive evidence-based guidelines..
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. [National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee] JAMA. 2003;289:2560–2572.
Extensive evidence-based review..
Crawford TC, Oral H. Cardiac arrhythmias: management of atrial fibrillation in the critically ill patient. Crit Care Clin. 2007;23:855–872.
Extensive evidence-based review..
Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417.
Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373.
Excellent evidence-based guidelines on how to address nutrition in critically ill patients..
Kim LJ, Martinez EA, Faraday N, Dorman T, Fleisher LA, Perler BA, Williams GM, Chan D, Pronovost PJ. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation. 2002;106:2366–2371.
Petralia GA, Kakkar AK. Venous thromboembolism prophylaxis for the general surgical patient: where do we stand? Semin Respir Crit Care Med. 2008;29:83–89.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. [Early Goal-Directed Therapy Collaborative Group] N Engl J Med. 2001;345:1368–1377.
Wojciechowski PJ, Samol N, Walker J. Coagulopathy in massive transfusion. Int Anesthesiol Clin. 2005;43:1–20.
Extensive evidence-based review..
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Lindenauer PK, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361.
2. Lawlor DK, et al. Is intensive care necessary after elective abdominal aortic aneurysm repair? Can J Surg. 2004;47:359–363.
3. Bastounis E, et al. Selective use of the intensive care unit after elective infrarenal abdominal aortic aneurysm repair. Int Angiol. 2003;22:308–316.
4. Vandy FC, et al. Specialized vascular floors after open aortic surgery: cost containment while preserving quality outcomes. Ann Vasc Surg. 2013;27:45–52.
5. Patterson BO, et al. Predicting risk in elective abdominal aortic aneurysm repair: a systematic review of current evidence. Eur J Vasc Endovasc Surg. 2008;36:637–645.
6. Dang D, et al. Postoperative complications: does intensive care unit staff nursing make a difference? Heart Lung. 2002;31:219–228.
7. Pronovost PJ, et al. Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery. JAMA. 1999;281:1310–1317.
8. Zimmerman JE, et al. Improving intensive care: observations based on organizational case studies in nine intensive care units: a prospective, multicenter study. Crit Care Med. 1993;21:1443–1451.
9. Carson SS, et al. Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of ‘open’ and ‘closed’ formats. JAMA. 1996;276:322–328.
10. Ryan D, et al. Why routine intensive care unit admission after elective open infrarenal abdominal aortic aneurysm repair is no longer an evidence based practice. Surgeon. 2010;8:297–302.
11. Levy MM, et al. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med. 2008;148:801–809.
12. Pronovost PJ, et al. Intensive care unit nurse staffing and the risk for complications after abdominal aortic surgery. Eff Clin Pract. 2001;4:199–206.
13. Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
14. Ince C, et al. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999;27:1369–1377.
15. Marik PE, et al. Noninvasive hemodynamic monitoring in the intensive care unit. Crit Care Clin. 2007;23:383–400.
16. De Backer D, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104.
17. Lindert J, et al. OPS imaging of human microcirculation: a short technical report. J Vasc Res. 2002;39:368–372.
18. Bellemare P, et al. Variations in pulmonary artery occlusion pressure to estimate changes in pleural pressure. Intensive Care Med. 2007;33:2004–2008.
19. Carter RS, et al. LV filling pressure during PEEP measured by nadir wedge pressure after airway disconnection. Am J Physiol. 1985;249:H770–H776.
20. Scheer B, et al. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002;6:199–204.
21. Frezza EE, et al. Indications and complications of arterial catheter use in surgical or medical intensive care units: analysis of 4932 patients. Am Surg. 1998;64:127–131.
22. Courtois M, et al. Anatomically and physiologically based reference level for measurement of intracardiac pressures. Circulation. 1995;92:1994–2000.
23. Wiener RS, et al. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA. 2007;298:423–429.
24. Binanay C, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633.
25. Sandham JD, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14.
26. Polanczyk CA, et al. Right heart catheterization and cardiac complications in patients undergoing noncardiac surgery: an observational study. JAMA. 2001;286:309–314.
27. Magder S. Invasive intravascular hemodynamic monitoring: technical issues. Crit Care Clin. 2007;23:401–414.
28. Slung HB, et al. Complications of the Swan-Ganz catheter. World J Surg. 1984;8:76–81.
29. Beaulieu Y, et al. Bedside ultrasonography in the ICU: part 1. Chest. 2005;128:881–895.
30. Beaulieu Y, et al. Bedside ultrasonography in the ICU: part 2. Chest. 2005;128:1766–1781.
31. Duvall WL, et al. Can hand-carried ultrasound devices be extended for use by the noncardiology medical community? Echocardiography. 2003;20:471–476.
32. Spronk PE, et al. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360:1395–1396.
33. Lefrant JY, et al. Training is required to improve the reliability of esophageal Doppler to measure cardiac output in critically ill patients. Intensive Care Med. 1998;24:347–352.
34. Biffl WL, et al. Secondary abdominal compartment syndrome is a highly lethal event. Am J Surg. 2001;182:645–648.
35. Sugrue M. Abdominal compartment syndrome. Curr Opin Crit Care. 2005;11:333–338.
36. Lui F, et al. Abdominal compartment syndrome: clinical aspects and monitoring. Crit Care Clin. 2007;23:415–433.
37. Cheatham ML, et al. Preload assessment in patients with an open abdomen. J Trauma. 1999;46:16–22.
38. Collee GG, et al. Bedside measurement of intra-abdominal pressure (IAP) via an indwelling naso-gastric tube: clinical validation of the technique. Intensive Care Med. 1993;19:478–480.
39. Papia G, et al. Intensive care of the patient following open abdominal aortic surgery. Curr Opin Crit Care. 2006;12:340–345.
40. Gopalan PD, et al. Critical care of the vascular surgery patient. Crit Care Clin. 2003;19:109–125.
41. Haas CE, et al. Acute postoperative hypertension: a review of therapeutic options. Am J Health Syst Pharm. 2004;61:1661–1673.
42. Marik PE, et al. Hypertensive crises: challenges and management. Chest. 2007;131:1949–1962.
43. Chobanian AV, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572.
44. Khan IA, et al. Clinical, diagnostic, and management perspectives of aortic dissection. Chest. 2002;122:311–328.
45. Kanto J, et al. Pharmacokinetics of labetalol in healthy volunteers. Int J Clin Pharmacol Ther Toxicol. 1981;19:41–44.
46. McArdle PJ, et al. Postoperative care of vascular surgery patients. Anesthesiol Clin North Am. 2004;22:333–347.
47. Michard F, et al. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–2008.
48. De Backer D, et al. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31:517–523.
49. Babaev A, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294:448–454.
50. Gurm HS, et al. Cardiogenic shock complicating myocardial infarction. Crit Care Clin. 2007;23:759–777.
51. Heintz KM, et al. Perioperative cardiac issues: postoperative arrhythmias. Surg Clin North Am. 2005;85:1103–1114.
52. Johnston KW. Multicenter prospective study of nonruptured abdominal aortic aneurysm. Part II. Variables predicting morbidity and mortality. J Vasc Surg. 1989;9:437–447.
53. Valentine RJ, et al. The clinical course of new-onset atrial fibrillation after elective aortic operations. J Am Coll Surg. 2001;193:499–504.
54. Goodman S, et al. Supraventricular arrhythmias in intensive care unit patients: short and long-term consequences. Anesth Analg. 2007;104:880–886.
55. Gami AS, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367.
56. Wang TJ, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477.
57. Daoud EG, et al. Effect of an irregular ventricular rhythm on cardiac output. Am J Cardiol. 1996;78:1433–1436.
58. Crawford TC, et al. Cardiac arrhythmias: management of atrial fibrillation in the critically ill patient. Crit Care Clin. 2007;23:855–872.
59. Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;356:935–941.
60. Klein AL, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411–1420.
61. Hollenberg SM, et al. Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(Suppl):N145–N150.
62. Mangano DT, et al. Postoperative myocardial ischemia. Therapeutic trials using intensive analgesia following surgery. The Study of Perioperative Ischemia (SPI) Research Group. Anesthesiology. 1992;76:342–353.
63. Hébert PC, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417.
64. Stevens RD, et al. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg. 2003;97:623–633.
65. Boersma E, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA. 2001;285:1865–1873.
66. Raby KE, et al. Detection and significance of intraoperative and postoperative myocardial ischemia in peripheral vascular surgery. JAMA. 1992;268:222–227.
67. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72:153–184.
68. Kim LJ, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation. 2002;106:2366–2371.
69. Karve AM, et al. Acute ST-segment elevation myocardial infarction: critical care perspective. Crit Care Clin. 2007;23:685–707.
70. Antman EM, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). J Am Coll Cardiol. 2004;44:671–719.
71. Bhatheja R, et al. Acute coronary syndromes: unstable angina/non–ST elevation myocardial infarction. Crit Care Clin. 2007;23:709–735.
72. Antman EM. Decision making with cardiac troponin tests. N Engl J Med. 2002;346:2079–2082.
73. 2011 ACCF/AHA Focused update of the guidelines for the management of patients with unstable angina/non-ST elevation myocardial infarction. Circulation. 2011;123:2022–2060.
74. Whitehead T, et al. The pulmonary physician in critical care 7: ventilator induced lung injury. Thorax. 2002;57:635–642.
75. Adams AB, et al. Ventilator-induced lung injury. Respir Care Clin North Am. 2003;9:343–362.
76. Brower RG, et al. Lung-protective ventilation strategies in acute lung injury. Crit Care Med. 2003;31(Suppl):S312–S316.
77. Hickling KG. Permissive hypercapnia. Respir Care Clin North Am. 2002;8:155–169.
78. Kress JP, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477.
79. Jubran A, et al. Effect of pressure support vs unassisted breathing through a tracheostomy collar on weaning duration in patients requiring prolonged mechanical ventilation: a randomized trial. JAMA. 2013;309:671–677.
80. McConville JF, et al. Weaning patients from the ventilator. N Engl J Med. 2012;367:2233–2239.
81. Epstein SK, et al. Independent effects of etiology of failure and time to reintubation on outcome for patients failing extubation. Am J Respir Crit Care Med. 1998;158:489–493.
82. Esteban A, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000;161:1450–1458.
83. Yang KL, et al. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445–1450.
84. Eskandar N, et al. Weaning from mechanical ventilation. Crit Care Clin. 2007;23:263–274.
85. Epstein SK, et al. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112:186–192.
86. Rothaar RC, et al. Extubation failure: magnitude of the problem, impact on outcomes, and prevention. Curr Opin Crit Care. 2003;9:59–66.
87. Diedrich DA, et al. Tracheostomy after major vascular surgery. J Cardiothorac Vasc Anesth. 2006;20:14–19.
88. Delaney A, et al. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta-analysis. Crit Care. 2006;10:R55.
89. De Leyn P, et al. Tracheotomy: clinical review and guidelines. Eur J Cardiothorac Surg. 2007;32:412–421.
90. Dagi TF. The management of postoperative bleeding. Surg Clin North Am. 2005;85:1191–1213.
91. Levy JH. Hemostatic agents. Transfusion. 2004;44(Suppl):58S–62S.
92. McKenna R. Abnormal coagulation in the postoperative period contributing to excessive bleeding. Med Clin North Am. 2001;85:1277–1310.
93. Labelle CA, et al. Disseminated intravascular coagulation: treat the cause, not the lab values. Cleve Clin J Med. 2005;72:377–378.
94. Nisanevich V, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103:25–32.
95. Brandstrup B, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648.
96. Bunn F, et al. Colloid solutions for fluid resuscitation. Cochrane Database Syst Rev. 2008;(1).
97. Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. Fresh-Frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. JAMA. 1994;271:777–781.
97a. Callum J, et al. Cryoprecipitate: the current state of knowledge. Transfus Med Ref. 2009;23:177–188.
98. Goodnough LT, et al. Blood management. Arch Pathol Lab Med. 2007;131:695–701.
99. Goodnough LT, et al. Recombinant factor VIIa: safety and efficacy. Curr Opin Hematol. 2007;14:504–509.
100. Lin Y, et al. The evidence for the use of recombinant factor VIIa in massive bleeding: revision of the transfusion policy framework. [National Advisory Committee on Blood and Blood Products] Transfus Med. 2012;22:383–394.
101. Ker K, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054.
102. Ozier Y, et al. Pharmacological approaches to reducing blood loss and transfusions in the surgical patient. Can J Anaesth. 2006;53(Suppl):S21–S29.
103. Mahdy AM, et al. Perioperative systemic haemostatic agents. Br J Anaesth. 2004;93:842–858.
104. Wojciechowski PJ, et al. Coagulopathy in massive transfusion. Int Anesthesiol Clin. 2005;43:1–20.
105. Valeri CR, et al. Effect of skin temperature on platelet function in patients undergoing extracorporeal bypass. J Thorac Cardiovasc Surg. 1992;104:108–116.
106. Nathens AB. Massive transfusion as a risk factor for acute lung injury: association or causation? Crit Care Med. 2006;34(Suppl):S144–S150.
108. Friedrich JO, et al. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142:510–524.
109. Halpenny M, et al. The effects of fenoldopam on renal function in patients undergoing elective aortic surgery. Eur J Anaesthesiol. 2002;19:32–39.
110. Miller Q, et al. The effects of intraoperative fenoldopam on renal blood flow and tubular function following suprarenal aortic cross-clamping. Ann Vasc Surg. 2003;17:656–662.
111. Brezis M, et al. Hypoxia of the renal medulla—its implications for disease. N Engl J Med. 1995;332:647–655.
112. Rahman SN, et al. Effects of atrial natriuretic peptide in clinical acute renal failure. Kidney Int. 1994;45:1731–1738.
113. Champagne BJ, et al. Outcome of aggressive surveillance colonoscopy in ruptured abdominal aortic aneurysm. J Vasc Surg. 2004;39:792–796.
114. Kehlet H, et al. Prophylaxis against postoperative complications in gastroenterology. Scand J Gastroenterol Suppl. 1996;216:218–224.
115. Heyland DK, et al. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. [Canadian Critical Care Clinical Practice Guidelines Committee] J Parenter Enteral Nutr JPEN. 2003;27:355–373.
116. Critical Care Nutrition guidelines 2013. [Available at] www.criticalcarenutrition.com [Accessed November 2013] .
117. Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009;37:1757–1761.
118. Han-Geurts IJ, et al. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br J Surg. 2007;94:555–561.
119. Chaar CI, et al. Endovascular aneurysm repair is associated with less malnutrition than open abdominal aortic aneurysm repair. Am J Surg. 2009;198:623–627.
120. Westvik TS, et al. Malnutrition after vascular surgery: are patients with chronic renal failure at increased risk? Am J Surg. 2006;192:22–27.
121. Nuguyen NQ, et al. Relationship between altered small intestinal motility and absorption after abdominal aortic aneurysm repair. Intensive Care Med. 2011;37:610–618.
122. Heys SD, et al. Nutrition and the surgical patient: triumphs and challenges. Surgeon. 2005;3:139–144.
123. Abstract CCCF 2012. Presented at Canadian Society of Vascular Surgery annual meeting. September 13–14, 2013 in Edmonton, Alberta, Canada.
124. Kock M, et al. Thoracic epidural anesthesia improves global and regional left ventricular function during stress-induced myocardial ischemia in patients with coronary artery disease. Anesth Analg. 1990;71:625–630.
125. Bani-Hani MG, et al. Interventions for preventing venous thromboembolism following abdominal aortic surgery. Cochrane Database Syst Rev. 2008;(1).
126. Petralia GA, et al. Venous thromboembolism prophylaxis for the general surgical patient: where do we stand? Semin Respir Crit Care Med. 2008;29:83–89.
127. Geerts WH, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(Suppl):338S–400S.
128. Dunn A. Perioperative management of oral anticoagulation: when and how to bridge. J Thromb Thrombolysis. 2006;21:85–89.
129. Ansell J, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(Suppl):204S–233S.
130. Douketis JD, et al. Physician preferences for perioperative anticoagulation in patients with a mechanical heart valve who are undergoing elective noncardiac surgery. Chest. 1999;116:1240–1246.
131. Lieber CS. Alcohol and coronary heart disease. N Engl J Med. 2003;348:1719–1722.
132. Spies C, et al. Perioperative morbidity and mortality in chronic alcoholic patients. Alcohol Clin Exp Res. 2001;25(Suppl ISBRA):164S–170S.
133. Tonnesen H, et al. Preoperative alcoholism and postoperative morbidity. Br J Surg. 1999;86:869–874.
134. Ntais C, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2005;(3).
135. Mayo-Smith MF, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164:1405–1412.
136. Gordon AJ, et al. Identification and treatment of alcohol use disorders in the perioperative period. Postgrad Med. 2006;119:46–55.
137. Ely EW, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
138. Riker R, et al. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329.
139. Sessler CN, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
140. Dubois MJ, et al. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304.
141. Scragg P, et al. Psychological problems following ICU treatment. Anaesthesia. 2001;56:9–14.
142. Ely EW, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29:1370–1379.
143. Ely EW, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
144. Jacobi J, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. [Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American Society of Health-System Pharmacists (ASHP), American College of Chest Physicians] Crit Care Med. 2002;30:119–141.
145. Truman B, et al. Monitoring delirium in critically ill patients. Using the confusion assessment method for the intensive care unit. Crit Care Nurse. 2003;23:25–36.