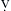Chapter 27 Postoperative Cardiovascular Management
OXYGEN TRANSPORT
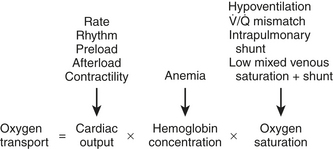
Maintaining oxygen transport (i.e., oxygen delivery [DO2]) satisfactory to meet the tissue metabolic requirements is the goal of postoperative circulatory control. Oxygen transport is the product of cardiac output (CO) times arterial content of oxygen (CaO2) (i.e., hemoglobin concentration × 1.34 mL of oxygen per 1 g of hemoglobin × oxygen saturation), and it can be affected in many ways by the cardiovascular and respiratory systems, as shown in Figure 27-1. Low CO, anemia from blood loss, and pulmonary disease can decrease DO2. Before altering the determinants of CO, including the inotropic state of the ventricles, an acceptable hemoglobin concentration (9-10 g/dL) and adequate oxygen saturation (SaO2) should be provided, enabling increases in CO to provide the maximum available DO2.1
TEMPERATURE
Patients are often admitted to the intensive care unit (ICU) after cardiac surgery with core temperatures below 35°C (95°F), especially after off-pump cardiac surgery. The typical pattern of temperature change during and after cardiac surgery and the hemodynamic outcomes are illustrated in Figure 27-2. Decreases in temperature after CPB occur in part because of redistribution of heat within the body and because of heat loss.
ASSESSMENT OF THE CIRCULATION
Echocardiography
There can be little doubt that echocardiography is the technique of choice for acute assessment of cardiac function. Just as transesophageal echocardiography (TEE) has become essential for intraoperative management in a variety of conditions, several studies document its utility in the postoperative period in the presence and absence of the PA catheter.2 It provides information that may lead to urgent surgery or prevent unnecessary surgery, gives important information about cardiac preload, and can detect acute structural and functional abnormalities. Although transthoracic echocardiography (TTE) can be performed more rapidly in this setting, adequate images can be obtained only in about 50% of patients in the ICU.
POSTOPERATIVE MYOCARDIAL DYSFUNCTION
Proposed factors that contribute to postoperative ventricular dysfunction include myocardial ischemia, residual hypothermia, preoperative medications such as β-adrenergic antagonists, and ischemia-reperfusion injury (Box 27-1).
THERAPEUTIC INTERVENTIONS
Postoperative Arrhythmias
Patients with preoperative or newly acquired noncompliant ventricles need a correctly timed atrial contraction to provide satisfactory ventricular filling, especially when they are in sinus rhythm preoperatively. Although atrial contraction provides 15% to 20% of ventricular filling, this may be more important in postoperative patients, when ventricular dysfunction and reduced compliance may be present. Rate and rhythm disorders need to be corrected when possible, using epicardial pacing wires. Approaches to postoperative rate and rhythm disturbances are shown in Table 27-1.
| Disturbance | Usual Causes | Treatments |
|---|---|---|
| Sinus bradycardia | Pre/intraoperative β-blockade | Atrial pacing |
| β-Agonist | ||
| Anticholinergic | ||
| Heart block (first, second, and third degree) | Ischemia | Atrioventricular sequential pacing |
| Surgical trauma | Catecholamines | |
| Sinus tachycardia | Agitation/pain | Sedation/analgesia |
| Hypovolemia | Volume administration | |
| Catecholamines | Change or stop drug | |
| Atrial tachyarrhythmias | Catecholamines | Change or stop drug |
| Chamber distention | Treat underlying cause (e.g., vasodilator, diuresis, give K+/Mg2+) | |
| Electrolyte disorder (hypokalemia, hypomagnesemia) | May require synchronized cardioversion or pharmacotherapy | |
| Ventricular tachycardia or fibrillation | Ischemia | Cardioversion |
| Catecholamines | Treat ischemia, may require pharmacotherapy |
Later in the postoperative period (days 1 through 3), supraventricular tachyarrhythmias become a major problem, with atrial fibrillation (AF) predominating. The overall incidence is between 30% and 40%, but with increasing age and valvular surgery the incidence may be in excess of 60%. There are probably many reasons for this, including genetic factors, inadequate atrial protection during surgery, electrolyte abnormalities, change in atrial size with fluid shifts, epicardial inflammation, stress, and irritation. Randomized trials of off-pump coronary artery bypass grafting (OPCAB) have found a similar incidence of postoperative AF compared with on-pump CABG.3
When AF or other supraventricular arrhythmias develop, treatment is often urgently required for symptomatic relief or hemodynamic benefit. The longer a patient remains in AF, the more difficult it may be to convert, and the greater is the risk for thrombus formation and embolization.4 Treatable underlying conditions such as electrolyte disturbances or pain should be corrected while specific pharmacologic therapy is being instituted. Paroxysmal supraventricular tachycardia (uncommon in this setting) can be abolished or converted by intravenous adenosine, and atrial flutter can sometimes be converted by overdrive atrial pacing by temporary wires placed at the time of surgery. Electrical cardioversion may be required if hypotension is caused by the rapid rate; however, atrial arrhythmias tend to recur in this setting. Rate control for AF or flutter can be achieved with a variety of atrioventricular nodal blocking drugs, and conversion is facilitated by many of these drugs as well. Table 27-2 summarizes the various treatment modalities for supraventricular arrhythmias. If conversion to sinus rhythm does not occur, electrical cardioversion in the presence of antiarrhythmic drug therapy should be attempted or anticoagulation with warfarin (Coumadin) instituted.
Table 27-2 Treatment Modalities for Supraventricular Arrhythmias
| Treatment | Specifics* | Indications |
|---|---|---|
| Overdrive pacing by atrial wires† | Requires rapid pacer (up to 800/min); start above arrhythmia rate and slowly decrease | PAT, atrial flutter |
| Adenosine | Bolus dose of 6-12 mg; may cause 10 seconds of complete heart block | AV nodal tachycardia |
| Bypass-tract arrhythmia | ||
| Atrial arrhythmia diagnosis | ||
| Amiodarone | 150 mg IV over 10 min, followed by infusion | Rate control/conversion to NSR in atrial fibrillation/flutter |
| β-Blockade | Esmolol, up to 0.5 mg/kg load over 1 min, followed by infusion if tolerated | Rate control/conversion to NSR in atrial fibrillation/flutter |
| Metoprolol, 0.5-5 mg; repeat effective dose q4-6h | Rate control/conversion to NSR in atrial fibrillation/flutter | |
| Propranolol, 0.25-1 mg; repeat effective dose q4h‡ | Rate control/conversion to NSR in atrial fibrillation/flutter | |
| Labetolol, 2.5-10 mg; repeat effective dose q4h‡ | Conversion of atrial fibrillation/flutter to NSR | |
| Sotalol, 40-80 mg PO q12h | Conversion of PAT to NSR | |
| Ibutilide | 1 mg over 10 min; may repeat after 10 min | Rate control/conversion to NSR in atrial fibrillation/flutter |
| Verapamil | 2.5-5 mg IV, repeated PRN‡ | Rate control/conversion to NSR in atrial fibrillation/flutter |
| Diltiazem | 0.2 mg/kg over 2 min, followed by 10-15 mg/hr¶ | Rate control/conversion to NSR in atrial fibrillation/flutter |
| Procainamide | 50 mg/min up to 1 g, followed by 1-4 mg/min | Rate control/conversion to NSR in atrial fibrillation/flutter |
| Prevention of recurrence of arrhythmias | ||
| Treatment of wide-complex tachycardias | ||
| **Digoxin | Load of 1 mg in divided doses over 4-24 hr§; may give additional 0.125-mg doses 2 hr apart (3-4 doses) | Rate control/conversion to NSR in atrial fibrillation/flutter |
| Synchronized cardioversion | 50-300 J (external); most effective with anterior-posterior patches | Acute tachyarrhythmia with hemodynamic compromise (usually atrial fibrillation or flutter) |
¶When diagnosis is unclear (ventricular vs. supraventricular) and there is no acute hemodynamic compromise (i.e., cardioversion not indicated).
AV = atrioventricular; NSR = normal sinus rhythm; PAT = paroxysmal atrial tachycardia; SVT = supraventricular tachycardia.
* See specific drug monographs for full description of indications, contraindications, and dosage. Doses are for intravenous administration; use lowest dose and administer slowly in patients with hemodynamic compromise.
† Verify pacer is not capturing ventricle.
‡ Infusion may provide better control. This drug is less useful than diltiazem owing to myocardial depression.
§ Rate of administration depending on urgency of rate control.
¶ Limited experience; may cause less hypotension than verapamil.
** Less useful than other drugs owing to slow onset and modest effect.
Afterload
Calculated SVR continues to be widely used in guiding therapy or drawing conclusions about the state of the circulation. This should only be done cautiously because SVR is not a complete indicator of afterload. Even if SVR were an accurate measure of impedance, the response to vasoactive agents depends on the coupling of ventricular-vascular function, not on impedance alone. Hemodynamic therapy should be guided based on the primary variables: BP and CO. If preload is appropriate, low BP and low CO are treated with an inotropic drug. If BP is acceptable (and preload appropriate) but CO is low, a vasodilator alone or in combination with an inotropic drug is used. If the patient is hypertensive (with low CO), vasodilators are indicated; if the patient is vasodilated (low BP and high CO), vasoconstrictors are employed (Table 27-3).
| Blood Pressure | Cardiac Output | Treatment |
|---|---|---|
| Low | Low | Inotrope |
| Normal | Low | Vasodilator ± inotrope |
| High | Low | Vasodilator |
| Low | High | Vasopressor |
POSTOPERATIVE HYPERTENSION
Hypertension has been a common complication of cardiac surgery, reported to occur in 30% to 80% of patients.5 The current population of older, sicker patients appears to have fewer problems with hypertension than with low-output syndromes or vasodilation. Although hypertension most commonly occurs in patients with normal preoperative ventricular function or a prior history, any patient may develop hypertension. Multiple factors contribute to postoperative hypertension, including awakening from general anesthesia, increases in endogenous catecholamines, activation of the plasma renin-angiotensin system, neural reflexes (e.g., heart, coronary arteries, great vessels), and hypothermia. Arterial vasoconstriction with various degrees of intravascular hypovolemia is the hallmark.
There are many alternative drugs to sodium nitroprusside for the treatment of hypertension after cardiac surgery, including nitroglycerin, adrenergic-blocking agents such as phentolamine, β-adrenergic blockers, and the mixed α- and β-adrenergic blocker labetalol. Direct-acting vasodilators, dihydropyridine calcium channel blockers (e.g., nicardipine, clevidipine), ACE inhibitors, and fenoldopam (a dopamine-1 [D1] receptor agonist) have also been used. Novel therapeutic approaches are listed in Table 27-4.
| Drug | Mechanism of Action | Half-Life |
|---|---|---|
| Nicardipine | Calcium channel blocker | Intermediate |
| Clevidipine | Calcium channel blocker | Ultra-short |
| Fenoldopam | Dopamine1 agonist | Ultra-short |
| Nesiritide | β-Natriuretic agonist | Short |
| Levosimendan | K+-ATP channel modulator | Intermediate |
Dihydropyridine calcium channel blockers relax arterial resistance vessels without negative inotropic actions or effects on atrioventricular nodal conduction and are important therapeutic options. Dihydropyridines are artery-specific vasodilators of peripheral resistance arteries, resulting in a generalized vasodilation, including the renal, cerebral, intestinal, and coronary vascular beds. In doses that effectively reduce BP, the dihydropyridines have little or no direct negative effect on cardiac contractility or conduction. The pharmacokinetic profile of nicardipine suggests that effective administration requires variable rate infusions when trying to treat hypertension because of the half-life of 40 minutes. If even more rapid control is essential, a dosing strategy consisting of a loading bolus or rapid infusion dose with a constant-rate infusion may be more efficient. The effect of nicardipine may persist even though the infusion is stopped. Clevidipine, a new ultra-short-acting dihydropyridine, is in phase III studies, has a half-life of only minutes, and may represent a potential alternative to sodium nitroprusside in the future.6
POSTOPERATIVE VASODILATION
Vasodilation alone should be associated with a hyperdynamic circulatory state presenting as systemic hypotension in association with an increased CO (and a low calculated SVR). More commonly after cardiac surgery, a combination of vasodilation and myocardial dysfunction occurs, requiring vasoconstrictor and inotropic therapy. The vasoplegic syndrome requires very high doses of vasoconstrictors and occurs after off-pump and on-pump surgery.7
DECREASED CONTRACTILITY
Drugs that increase contractility all result in increased calcium mobilization from intracellular sites to and from the contractile proteins or sensitize these proteins to calcium. Catecholamines, through β1-receptor stimulation in the myocardium, increase intracellular cyclic adenosine monophosphate (cAMP). This second messenger increases intracellular calcium, causing an improvement in myocardial contraction. Inhibition of the breakdown of cAMP by PDE inhibitors increases intracellular cAMP independent of the β-receptor. The “calcium sensitizers” constitute a new class of inotropic agents (Box 27-2).8
Catecholamines
The catecholamines used postoperatively include dopamine, dobutamine, epinephrine, norepinephrine, and isoproterenol (Box 27-3). These drugs have various effects on α- and β-receptors and therefore various effects on HR, rhythm, and myocardial metabolism. Dosing recommendations for the catecholamines are provided in Table 27-5.
| Drug | Infusion Dose (μg/kg/min) |
|---|---|
| Dopamine*,† | 2-10 |
| Dobutamine† | 2-10 |
| Epinephrine‡ | 0.03-0.20 |
| Norepinephrine‡ | 0.03-0.20 |
| Isoproterenol‡ | 0.02-0.10 |
* Less than 2 μg/kg/min predominantly “dopaminergic” (renal and mesenteric artery dilatation).
† If 10 μg/kg/min is ineffective, change to epinephrine or norepinephrine.
‡ Dose to effect; may require higher dose than indicated.
Dopamine
A precursor of norepinephrine, dopamine probably achieves its therapeutic effects by releasing myocardial norepinephrine or preventing its reuptake, especially when administered in high doses. This indirect action may result in reduced effectiveness when given to patients with chronic congestive heart failure or shock states, because the myocardium becomes depleted of norepinephrine stores. In contrast to dobutamine, the α-agonist properties of dopamine cause increases in pulmonary artery pressure (PAP), peripheral vascular resistance (PVR), and LV filling pressure. At low doses (<2 μg/kg/min), dopamine stimulates renal dopaminergic receptors to increase renal perfusion more than can be explained by an increase in CO. Despite this action, a multicenter study in New Zealand and Australia clearly demonstrated that use of low-dose dopamine in critically ill patients confers no protection from renal dysfunction.9 At doses higher than 10 μg/kg/min, tachycardia and vasoconstriction become the predominant actions of this drug.
Phosphodiesterase Inhibitors
The PDE inhibitors are nonglycosidic, nonsympathomimetic drugs that have positive inotropic effects independent of the β1-adrenergic receptor and unique vasodilatory actions independent of endothelial function or nitrovasodilators.10 Patients with CHF have downregulation of the β1-receptor, with a decrease in receptor density and altered responses to catecholamine administration. Milrinone, amrinone, and enoximone bypass the β1-receptor, causing increases in intracellular cAMP by selective inhibition of PDE fraction III (i.e., fraction IV), a cAMP-specific PDE enzyme. In vascular smooth muscle, these agents cause vasodilation in the arterial and capacitance beds. PDE inhibitors increase CO, decrease PCWP, and decrease SVR and PVR in patients with biventricular dysfunction, and they are important therapeutic approaches in postoperative cardiac surgical patients.
PDE III inhibitors have a clinical effect as inodilators; they produce dilation of arterial and venous beds, decreasing the mean arterial pressure and central filling pressures. Increases in CO are induced by multiple mechanisms, including afterload reduction and positive inotropy, but not by increasing HR. The net effect is a decrease in myocardial wall tension, representing an important contrast to most sympathomimetic agents. Catecholamine administration often needs the simultaneous administration of vasodilators to reduce ventricular wall tension. Milrinone and other PDE inhibitors also have unique mechanisms of vasodilation that may be favorable for coronary artery and IMA flow (Box 27-4).
Levosimendan
Levosimendan is a calcium-sensitizing drug that exerts positive inotropic effects through sensitization of myofilaments to calcium and vasodilation through opening of ATP-dependent potassium channels on vascular smooth muscle. These effects occur without increasing intracellular cAMP or calcium and without an increase in M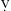 O2 at therapeutic doses. As would be expected with an inodilator, the hemodynamic effects include a reduction in pulmonary artery occlusion pressure (PAOP) in association with an increase in CO. β-Blockade does not block the hemodynamic effects of this drug. Levosimendan itself has a short elimination half-life, but it has active metabolites with elimination half-lives up to 80 hours. A study in patients with decompensated congestive heart failure found hemodynamic improvements at 48 hours were similar whether patients received the drug for 24 hours or 48 hours. Increasing plasma levels of the active metabolite were found for 24 hours after the drug infusion was stopped.11 Levosimendan is in phase III clinical studies in the United States and Europe, and it has been granted fast-track status by the U.S. Food and Drug Administration (FDA).
O2 at therapeutic doses. As would be expected with an inodilator, the hemodynamic effects include a reduction in pulmonary artery occlusion pressure (PAOP) in association with an increase in CO. β-Blockade does not block the hemodynamic effects of this drug. Levosimendan itself has a short elimination half-life, but it has active metabolites with elimination half-lives up to 80 hours. A study in patients with decompensated congestive heart failure found hemodynamic improvements at 48 hours were similar whether patients received the drug for 24 hours or 48 hours. Increasing plasma levels of the active metabolite were found for 24 hours after the drug infusion was stopped.11 Levosimendan is in phase III clinical studies in the United States and Europe, and it has been granted fast-track status by the U.S. Food and Drug Administration (FDA).
In a study after cardiac surgery, patients were given levosimendan; of 11 patients with severely impaired CO and hemodynamic compromise, 8 patients (73%) showed evidence of hemodynamic improvement within 3 hours after the start of levosimendan infusion. Specifically, cardiac index and stroke volume were significantly increased, whereas the mean arterial pressure, indexed SVR, mean PAP, right atrial pressure, and PAOP were significantly lowered.12 Clinical studies continue to evaluate the potential role for this new positive inotropic agent in patients with heart failure.
RIGHT-SIDED HEART FAILURE
Heart failure after cardiac surgery usually results from LV impairment. Although an isolated right-sided MI can occur perioperatively, most perioperative inferior MIs show variable involvement of the right ventricle.13 The myocardial preservation techniques that are best for the left ventricle may not offer ideal RV protection because the right ventricle is thin walled and more exposed to body and atmospheric temperature. Cardioplegic solution given through the coronary sinus (retrograde) may not reach parts of the right ventricle because of positioning of the cardioplegia cannula in relation to the venous outflow from this chamber and because the thebesian veins do not drain into the coronary sinus. Impairment of RV function postoperatively is more severe and persistent when preoperative right coronary artery (RCA) stenosis is present. Although depression of the ejection fraction is compensated by preload augmentation, right ventricular ejection fraction (RVEF) cannot be preserved if CPP is reduced or impedance to ejection is increased.
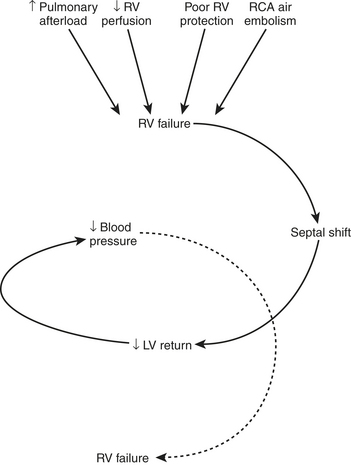
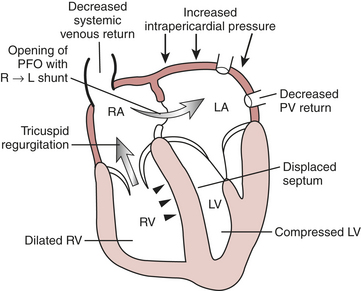
Certain aspects of the physiology of the right ventricle make it different from the left. Normally, the RV free wall receives its blood flow during systole and diastole; however, systemic hypotension or increased RV systolic and diastolic pressures may cause supply-dependent depression of contractility when M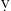 O2 is increased while CPP is decreased. The normal thin-walled right ventricle is at least twice as sensitive to increases in afterload as is the left ventricle. Relatively modest increases in outflow impedance from multiple causes in the postoperative period can exhaust preload reserve, causing a decrease in RVEF with ventricular dilation. RV pressure overload may be complicated by volume overload caused by functional tricuspid regurgitation. Decreases in RV stroke volume will diminish LV filling, and dilation of the right ventricle can cause a leftward shift of the interventricular septum, interfering with diastolic filling of the left ventricle (i.e., ventricular interaction) (Fig. 27-3). A distended right ventricle limited by the pericardial cavity further decreases LV filling. RV failure has the potential to affect LV performance by decreasing pulmonary venous blood flow, decreasing diastolic distending pressure, and decreasing LV diastolic compliance. The resulting decrease in LV output will further impair RV pump function. The mechanical outcomes of RV failure in postoperative cardiac surgical patients are depicted in Figure 27-4. It can therefore be appreciated how, once established, RV failure is self-propagating; and aggressive treatment interventions may be needed to interrupt the vicious cycle.
O2 is increased while CPP is decreased. The normal thin-walled right ventricle is at least twice as sensitive to increases in afterload as is the left ventricle. Relatively modest increases in outflow impedance from multiple causes in the postoperative period can exhaust preload reserve, causing a decrease in RVEF with ventricular dilation. RV pressure overload may be complicated by volume overload caused by functional tricuspid regurgitation. Decreases in RV stroke volume will diminish LV filling, and dilation of the right ventricle can cause a leftward shift of the interventricular septum, interfering with diastolic filling of the left ventricle (i.e., ventricular interaction) (Fig. 27-3). A distended right ventricle limited by the pericardial cavity further decreases LV filling. RV failure has the potential to affect LV performance by decreasing pulmonary venous blood flow, decreasing diastolic distending pressure, and decreasing LV diastolic compliance. The resulting decrease in LV output will further impair RV pump function. The mechanical outcomes of RV failure in postoperative cardiac surgical patients are depicted in Figure 27-4. It can therefore be appreciated how, once established, RV failure is self-propagating; and aggressive treatment interventions may be needed to interrupt the vicious cycle.
Treatment
Treatment approaches in postoperative RV failure may differ from those used in LV failure, and they are affected by the presence of pulmonary hypertension (Table 27-6). In all cases, preload should be increased to the upper range of normal; however, the Frank-Starling relationship is flat in RV failure; and to avoid ventricular dilation, the CO response to an increasing CVP should be determined. Volume loading should be stopped when the CVP exceeds 10 mmHg and CO does not increase despite increases in this pressure. The CVP should not be permitted to exceed the PAOP, because if these pressures equalize, any increase obtained in pulmonary blood flow will be offset by decreased diastolic filling of the left ventricle by means of ventricular interdependence. The atrial contribution to RV filling is important when the ventricle is dilated and noncompliant. Maintenance of sinus rhythm and use of atrial pacing are important components of treating postoperative RV failure.
Table 27-6 Treatment Approaches in Postoperative Right-Sided Heart Failure
| Preload Augmentation |
| Volume, vasopressors, or leg elevation (CVP/PCWP < 1) |
| Decrease juxtacardiac pressures (pericardium and/or chest open) |
| Establish atrial kick (sinus rhythm, atrial pacing) |
| Afterload Reduction (Pulmonary Vasodilation) |
| Nitroglycerin, isosorbide dinitrate nesiritide |
| cAMP-specific phosphodiesterase inhibitors, β2-adrenergic agonists |
| Inhaled nitric oxide |
| Nebulized PGI2 |
| Intravenous PGE1 (+ left atrial norepinephrine) |
| Inotropic Support |
| cAMP-specific phosphodiesterase inhibitors, isoproterenol, dobutamine |
| Norepinephrine |
| Levosimendan |
| Ventilatory Management |
| Lower intrathoracic pressures (tidal volume < 7 mL/kg, low PEEP) |
| Attenuation of hypoxic vasoconstriction (high Fio2) |
| Avoidance of respiratory acidosis (Paco2 30-35 mm Hg, metabolic control with meperidine or relaxants) |
| Mechanical Support |
| Intra-aortic counterpulsation |
| Pulmonary artery counterpulsation |
| Right ventricular assist devices |
CVP/PCWP = central venous pressure/pulmonary capillary wedge pressure; cAMP = cyclic adenosine monophosphate; PGI2 = prostaglandin I2; PGE1 = prostaglandin E1; PEEPs = positive end-expiratory pressures.
Although vasodilators may lead to cardiovascular collapse in RV infarction (as a result of decreases in RV filling and coronary perfusion), postoperative RV failure is often associated with increased PVR and pulmonary hypertension. In this context, attempts to decrease RV outflow impedance may be worthwhile. Intravenous vasodilators invariably reduce systemic BP, mandating the simultaneous administration of a vasoconstrictor. One way to reduce the pulmonary effects of the required vasoconstrictor is to administer the vasoconstrictor through a left atrial catheter. The PDE inhibitors are commonly used for their effect on the pulmonary vasculature and RV function, but this also usually requires systemic norepinephrine. In recent years, there has been an increased interest in and availability of aerosolized pulmonary vasodilators. This route of administration reduces or even abolishes the undesirable systemic vasodilation. Delivery of the drug directly to the alveoli improves pulmonary blood flow to these alveoli, potentially improving oxygenation by better matching blood flow to ventilation. Three drugs have been used: nitric oxide, prostaglandin I2 (i.e., epoprostenol or prostacyclin), and milrinone.14
CARDIAC TAMPONADE
The mechanism of hemodynamic deterioration during tamponade is primarily the result of impaired filling of one or more of the cardiac chambers. As the external pressure on the heart increases, the distending or transmural pressure (external-intracavitary pressure) is decreased. The intracavitary pressure increases in compensation lead to impaired venous return and elevation of the venous pressure. If the external pressure is high enough to exceed the ventricular pressure during diastole, diastolic ventricular collapse occurs. These changes have been documented in the right and the left sides of the heart after cardiac surgery.15 As the end-diastolic volume and end-systolic volume decrease there is a concomitant decrease in stroke volume. In the most severe form of cardiac tamponade, ventricular filling occurs only during atrial systole. Intense sympathoadrenergic activation increases venous return by constricting venous capacitance vessels. Tachycardia helps to maintain CO in the presence of a reduced stroke volume. Adrenergic mechanisms may also explain decreased urinary output and sodium excretion, but these phenomena may also be caused by reduced CO or a reduction in atrial natriuretic factor from decreased distending pressure of the atria.
The diagnosis of cardiac tamponade depends on a high degree of suspicion. Tamponade after heart surgery is a clinical entity distinct from the tamponade typically seen in medical patients in whom the pericardium is intact and the heart is surrounded by a compressing fluid. In the setting of cardiac surgery, the pericardial space is often left open and in communication with one or both of the pleural spaces, and the compressing blood is at least in part in a clotted, nonfluid state and able to cause localized compression of the heart. Serious consideration should be given to the possibility of tamponade after cardiac surgery in any patient with inadequate or deteriorating hemodynamics, as evidenced by hypotension, tachycardia, increased filling pressures, or low CO, especially when there has been excessive chest tube drainage. A more subtle presentation of postoperative tamponade is gradually increasing requirements for inotropic and pressor support. Many of the classic signs of tamponade may not be present in these patients, partly because they are usually sedated and ventilated but also because the pericardium is usually left open, resulting in a more gradual increase in the restraining effects of blood accumulation. There may be localized accumulations that affect one chamber more than another.16 The classic findings of elevated CVP or equalization of CVP, PAd, and PAOP may not occur.17,18 It may therefore be difficult in the presence of a declining CO and elevated filling pressures to distinguish tamponade from biventricular failure. A useful clue may be pronounced respiratory variation in BP with mechanical ventilation in association with high filling pressures and low CO, because the additional external pressure applied to the heart by positive-pressure ventilation may further impair the already compromised ventricular filling in the presence of tamponade.
SUMMARY
1. Wu W.C., Rathore S.S., Wang Y., et al. Blood transfusions in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230.
2. Costachescu T., Denault A., Guimond J.G., et al. The hemodynamically unstable patient in the intensive care unit: Hemodynamic vs TEE monitoring. Crit Care Med. 2002;30:1214.
3. Puskas J.D., Williams W.H., Mahoney E.M., et al. Off-pump versus conventional coronary artery bypass grafting: Early and 1-year graft patency, cost, and quality of life outcomes. JAMA. 1841;291:2004.
4. Hill L.L., De Wet C., Hogue C.W. Management of atrial fibrillation after cardiac surgery: II. Prevention and treatment. J Cardiothorac Vasc Anesth. 2002;16:626.
5. Vuylsteke A., Feneck R.O., Jolin-Mellgard A., et al. Perioperative blood pressure control: A prospective survey of patient management in cardiac surgery. J Cardiothorac Vasc Anesth. 2000;14:269.
6. Bailey J.M., Lu W., Levy J.H., et al. Clevidipine in adult cardiac surgical patients: A dose-finding study. Anesthesiology. 2002;96:1086.
7. Gomez W.J., Erlichman M.R., Batista-Filho M.L., et al. Vasoplegic syndrome after off-pump coronary artery bypass surgery. Eur J Cardiothorac Surg. 2003;23:165.
8. Lehmann A., Boldt J. New pharmacologic approaches for the perioperative treatment of ischemic cardiogenic shock. J Cardiothorac Vasc Anesth. 2005;19:97.
9. Bellomo R., Chapman M., Finfer S., et al. Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomized trial: Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139.
10. Levy J.H., Bailey J.M., Deeb M. Intravenous milrinone in cardiac surgery. Ann Thorac Surg. 2002;73:325.
11. Kivikko M., Lehtonen L., Colucci W.S., et al. Sustained hemodynamic effects of intravenous levosimendan. Circulation. 2003;107:81.
12. Labriola C., Siro-Brigiani M., Carrata F., et al. Hemodynamic effects of levosimendan in patients with low-output heart failure after cardiac surgery. Int J Clin Pharmacol Ther. 2004;42:204.
13. Jacobs A., Leopold J., Bates E., et al. Cardiogenic shock caused by right ventricular infarction: A report from the SHOCK registry. J Am Coll Cardiol. 2003;41:1273.
14. Meade M.O., Granton J.T., Matte-Martyn A., et al. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am J Respir Crit Care Med. 2003;167:1483.
15. Chuttani K., Pandian N.G., Mohanty P.K., et al. Left ventricular diastolic collapse: An echocardiographic sign of regional cardiac tamponade. Circulation. 1999;83:1991.
16. Kochar G.S., Jacobs L.E., Kotler M.N. Right atrial compression in postoperative cardiac patients: Detection by transesophageal echocardiography. J Am Coll Cardiol. 1990;16:511.
17. Bommer W.J., Follette D., Pollock M., et al. Tamponade in patients undergoing cardiac surgery: A clinical-echocardiographic diagnosis. Am Heart J. 1995;130:1216.
18. Chuttani K., Tischler M.D., Pandian N.G., et al. Diagnosis of cardiac tamponade after cardiac surgery: Relative value of clinical, echocardiographic, and hemodynamic signs. Am Heart J. 1994;127:913.