CHAPTER 304 Posterior, Transforaminal, and Anterior Lumbar Interbody Fusion
Techniques and Instrumentation
Background
In the early 1900s, Muller recognized that dorsal lumbar fusion techniques were sometimes unsuccessful and attempted to treat patients afflicted with Pott’s disease via an anterior approach.1 In 1933, Burns successfully performed an interbody fusion for a traumatic L5-S1 spondylolisthesis via the transabdominal approach.1 In 1944, Briggs and Milligan introduced the posterior interbody approach and placed laminectomy autograft in the interbody space.2 In 1946, Jaslow expanded the concept by inserting an excised spinous process into the intervertebral space.3 In 1953, Cloward popularized the technique by using impacted blocks of iliac crest to provide rigid interbody fixation.4 The advantage of supplemental instrumentation was soon realized, and in 1961 Humphreys and colleagues were the first to place an anterior lumbar compression plate with unicortical screws.5
Technical challenges and device limitations initially restricted the application of interbody procedures until the advent of new interbody fixation devices increased technical ease and efficacy.6–14 The modern concept of interbody fixation placement was introduced by Wagner and coworkers following the implantation of a bone-filled cage in horses with wobbler syndrome.15,16 The intervertebral disk space was distracted by placement of an oversized, perforated, stainless steel cylinder (the Bagby basket) filled with autogenous bone graft, ultimately yielding a reported fusion rate nearing 88%.17
Material evolution and physical modifications of the Bagby basket led to the production of the Bagby and Kuslich (BAK) implant.18 The popularity of interbody fixation continued to grow as numerous interbody graft options were developed, including autologous iliac crest graft, structural allograft, and bone chips within metallic cages.11,19–22 More recently synthetic implants including titanium mesh cages,23 carbon fiber cages, and polyetheretherketone (PEEK) implants have gained recognition as viable options.24 Augmentation of the interbody implant with pedicle screw fixation has further added to fusion success in comparison to stand-alone grafts.13,25
A circumferential fusion can be achieved via an ALIF (anterior lumbar interbody fusion, PLIF (posterior lumbar interbody fusion), or TLIF (transforaminal lumbar interbody fusion), each of which can be supplemented with posterior segmental fixation through traditional open or minimally invasive means. The choice of approach depends on multiple factors including the age, gender, and comorbidities of the patient, the levels of pathology, the presence of scar tissue from previous procedures, and anatomic considerations.26
The PLIF procedure with pedicle screw fixation can be performed through a single incision, but has been associated with a radiculopathy rate of up to 13%27 and requires violation of the structural integrity of both facet joints to achieve adequate graft placement. The ALIF can achieve the same interbody support as the PLIF, but without manipulation of the dural or posterior neural structures. However, it often requires significant retraction of the iliac vessels, hypogastric nerves, and peritoneum, exposing them to direct manipulation injury. Other complications include an increased risk of deep venous thrombosis, muscular atony, abdominal wall hernias, and retrograde ejaculation in men.26,28 When combined with posterior fixation, the additional time, expense, and morbidity of the two-stage procedure remains a limiting factor in its widespread usage.29
To circumvent the difficulties associated with the PLIF and ALIF procedures, Harms and Rolinger in 1982 suggested the placement of bone graft and titanium mesh via a transforaminal route.23 The TLIF approach minimized retraction on the thecal sac and neural structures, enabled lordosis restoration via anterior graft placement, and spared the contralateral lamina, facet, and pars, thus providing increased surface area for fusion along both the anterior and posterior elements.23 Furthermore, the TLIF can be performed safely in revision cases with significant epidural fibrosis because of its unique posterolateral trajectory.29–32
The introduction of minimally invasive and laparoscopic techniques represents the most recent modifications to enable lumbar interbody fusion. Laparoscopic and mini-open ALIF procedures aim to minimize the incidence of hernias and abdominal wall atony.33 The muscle-splitting posterior minimally invasive approaches aim to diminish iatrogenic soft tissue injury, thus reducing intraoperative blood loss, postoperative pain, and hospital stay.34,35 Recently, the minimally invasive TLIF procedure has become an increasingly popular method of lumbar arthrodesis.35,36
Indications For Lumbar Interbody Fusion
The indications for LIF have expanded during the past decade. Current indications include the following: spondylolisthesis (usually grade I or II); DDD causing diskogenic low back pain (with or without radiculopathy); recurrent lumbar disk herniation inducing significant mechanical back pain; postdiscectomy collapse with neural foraminal stenosis and secondary radiculopathy; three or more recurrent lumbar disk herniations with or without back pain; treatment of pseudarthrosis; treatment of postlaminectomy kyphosis; treatment of traumatic instability, and treatment of lumbar coronal and/or sagittal deformities (Table 304-1).26
Table 304-1 Primary Indications for Anterior, Posterior, or Transforaminal Lumbar Interbody Fusion
The relative contraindications for lumbar interbody fusion have also evolved during this period and now include the following: multilevel (three levels) disk disease (in patients without lumbar spinal deformities); single-level disk disease causing radicular pain without symptoms of mechanical low back pain or instability; severe osteoporosis (with attendant risk of graft subsidence through the end plates) (Table 304-2).6,7,11,26,37
Table 304-2 Relative Contraindications for Lumbar Interbody Fusion
Biomechanical Considerations
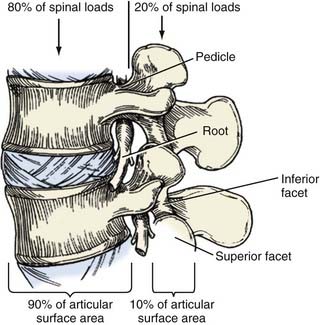
Circumferential fusion (360) techniques have distinct theoretical advantages over posterolateral fusion techniques.4,6,11 As Wolff’s law indicates, fusion potential is enhanced if grafts are placed under compression.26 Interbody fusions place the bone graft in the load-bearing position of the anterior and middle spinal columns, which support 80% of spinal loads and provide 90% of the osseous surface area, thereby maximally enhancing the potential for fusion (Fig. 304-1; Table 304-3).26
Table 304-3 Advantages of Lumbar Interbody Fusion Compared with Posterolateral Fusion
Furthermore, supplemental posterior pedicle screw-rod constructs have been shown to increase biomechanical rigidity and decrease pseudoarthrosis rates when associated with an interbody graft.26,38–41 Lastly, restoration of intervertebral height and segmental lordosis is possible with interbody fusion devices and this restoration has been found to correspond with favorable clinical outcomes.4,7,11,21
Interbody Grafts
Following a complete interbody discectomy, an interbody device must have design characteristics to not only restore disk height, but create lordosis through the segment, maintain sagittal balance, distract the neuroforaminal space, and restore anatomic weight bearing to the anterior column.26,42
Over the past 50 years, numerous interbody grafts have been used, including autograft iliac crest, cortical allograft (donor) or synthetic bone, threaded cylindrical titanium cages, impact titanium cages, carbon fiber reinforced or plain PEEK polymer, and impacted carbon fiber reinforced PEEK wedges.43–46 Osteoinductive materials have also evolved over this time period, from iliac crest bone to bone morphogenetic protein (rhBMP-2).47,48
The safety and efficacy of iliac crest have been demonstrated over the years by various authors.5,23,49,50 However, despite its ability to promote interbody fusion, iliac autograft is associated with significant harvest site morbidity in up to 25% of patients.26,51 This led to the use of tricortical bone allograft for circumferential fusions. Unfortunately, mechanical failures developed because some of these grafts were unable to provide sufficient structural support, thus leading to the open box metal and carbon fiber designs that are routinely used for circumferential fusions in the modern era.24
No interbody graft device is perfect, and metallic cages have some drawbacks. The modulus of elasticity of titanium is significantly greater than that of bone, thus facilitating subsidence through the vertebral end plates in patients with osteoporosis.26 Furthermore, metal implants have been found to incur micromotion and dislodge debris through the fusion segment, which may result in a cellular reaction with subsequent loosening of the bone and device interface.42 Metal implants additionally create imaging artifacts secondary to their opacity and scatter potential, thus limiting postoperative fusion evaluation.42
The modulus of elasticity of bone is more closely approximated by nonresorbable polymers such as carbon fiber, which has an unlimited supply and no risk of viral disease transmission or recipient rejection (unlike allograft).26 The carbon fibers are often embedded in a composite material such as PEEK to prevent their breakdown and release.42 The biomechanical drawback of a carbon fiber implant is its relative brittleness, thus allowing the potential for splintering, micromotion, and composite material failure. In several large series, interbody graft collapse, slippage, and migration were reported in 3% to 10% of cases.7 To circumvent this confound, newer carbon fiber graft designs have incorporated threaded or ridged interfaces, thus minimizing slippage and migration.42
Bone graft substitutes and extenders are alternatives to autograft and allograft and can be used to fill interbody cages. They are classified into two main categories: (1) biologic agents that induce bone formation from native tissues such as BMP analogues (e.g., rhBMP-2), and (2) bone scaffold substitutes such as calcium phosphate salts, including beta-tricalcium phosphate, hydroxyapatite, and wollanosite.52
TransForaminal Lumbar Interbody Fusion
As with the PLIF procedure, patients are placed prone on a surgical frame to facilitate lumbar lordosis. A vertical incision is performed and the muscles and soft tissues are retracted laterally to expose the spinous processes, lamina, and facet joints. At a minimum, a unilateral laminotomy and complete facetectomy are performed on the symptomatic side. If needed, further decompression may be obtained by a full laminectomy and contralateral foraminotomy.53
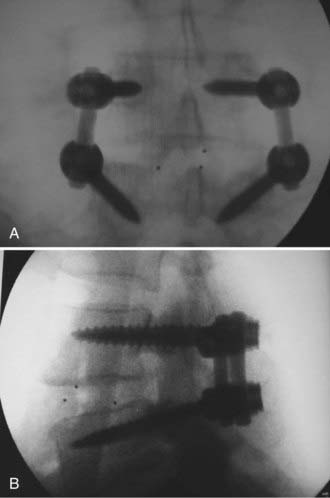
After adequate decompression of the neural elements has been performed, pedicle screws are placed in the standard fashion. A total discectomy is performed and the disk space is gradually distracted using pedicle screws and interbody dilators.53 To facilitate interbody access, the posterior bony lips of the end plates may be removed with a 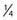 -inch osteotome while carefully protecting the thecal sac and nerve roots. The interbody graft is then placed, followed by pedicle screw-rod compression to restore lumbar lordosis while maintaining disk height (Fig. 304-2).53 The transverse processes are then decorticated for posterolateral fusion, following which the wound is closed in a standard layered fashion.
-inch osteotome while carefully protecting the thecal sac and nerve roots. The interbody graft is then placed, followed by pedicle screw-rod compression to restore lumbar lordosis while maintaining disk height (Fig. 304-2).53 The transverse processes are then decorticated for posterolateral fusion, following which the wound is closed in a standard layered fashion.
Minimal Access Approach
The minimally invasive approach enables access through a fixed tubular retractor, whereas the mini-open approach involves an expandable tubular system. The minimally invasive approach is associated with the least amount of tissue trauma but conversely offers the least exposure for decompression and instrument manipulation.26
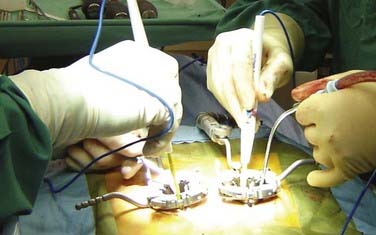
In contrast to the open approach, pedicle screws are placed on the ipsilateral side after the decompression is complete because the hardware often visually limits an already compact surgical field. In the mini-open approach, the ipsilateral pedicle screws are placed under direct visualization through the expandable tube retractor (Fig. 304-3). The contralateral pedicle screws can be inserted either through a paramedian incision through a second expandable retractor or via a percutaneous screw system. In the minimally invasive approach, percutaneous pedicle screws are placed bilaterally. The pedicle screws contralateral to the interbody exposure are connected to a rod to allow sequential distraction of the interspace. Using a combination of interspace wedges, spreaders, and serial distractors, the increased disk height is maintained by fixing the contralateral pedicle screws in the distracted position on the rod.
Anterior Lumbar Interbody Fusion
The mini-open ALIF has been found to be superior to laparoscopic approaches. Laparoscopic approaches have been reported to have higher complication rates, which are probably related to the two-dimensional view afforded by the laparoscope.26,28
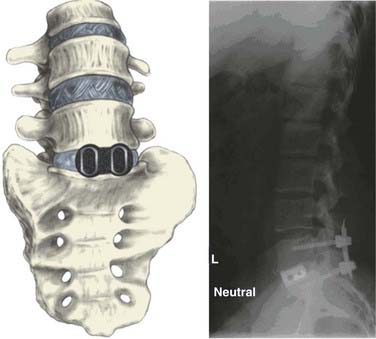
Irrespective of the invasiveness of the approach, following interbody graft placement, the peritoneum and its contents are returned to their normal anatomic position, any peritoneal openings are closed, and the abdominal wall is closed in layers (Fig. 304-4).
Proposed Benefits Of Minimal Access Approaches
Conventional lumbar fusion surgery is associated with iatrogenic soft tissue injury that can have deleterious short- and long-term effects on patient outcomes. Numerous studies have demonstrated that irreversible muscle injury occurs as a result of muscle stripping and retraction and can be associated with poor clinical results.54–58 The goal of any minimally invasive procedure is to achieve the same surgical objectives as the corresponding open procedure through a less traumatic exposure.
Schwender and coworkers initially confirmed the feasibility and safety of performing the TLIF procedure through a minimally invasive approach in a series of 49 patients using standardized outcome measures.59 Visual analogue scale (VAS) and Oswestry disability index (ODI) scores were equivalent to their open counterparts, although mean hospital stay (1.9 days) and average blood loss (140 mL) were significantly lessened. Jang and Lee also reported significant benefits in their minimally invasive TLIF patients.60 Isaacs and coworkers compared endoscopically assisted minimally invasive TLIF patients to their open PLIF counterparts and also found advantages with minimally invasive surgery.61 Mummaneni and Rodts reported the mini-open TLIF technique to enable bilateral direct visualization of both pedicles for the placement of screws through bilateral expandable retractors.62 Despite the growing literature base in these minimally invasive surgical endeavors, the learning curve remains steep, and prospective randomized trials comparing them to their open counterparts remain limited, thus providing guarded conclusions at best to date.
Bagby GW. The Bagby and Kuslich (BAK) method of lumbar interbody fusion. Spine. 1999;24:1857.
Barnes et al Barnes B, Rodts GEJr, Haid RWJr, et al. Allograft implants for posterior lumbar interbody fusion: results comparing cylindrical dowels and impacted wedges. Neurosurgery. 2002;51:1191-1198.
Branch CL, Branch CLJr. Posterior lumbar interbody fusion with the keystone graft: technique and results. Surg Neurol. 1987;27:449-454.
Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18:2106-2107.
Brodke DS, Dick JC, Kunz DN, et al. Posterior lumbar interbody fusion. A biomechanical comparison, including a new threaded cage. Spine. 1997;22:26-31.
Cloward RB. Posterior lumbar interbody fusion updated. Clin Orthop Relat Res. 1985;193:16-19.
Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10:154-168.
Foley KT, Lefkowitz MA. Advances in minimally invasive spine surgery. Clin Neurosurg. 2002;49:499-517.
Fraser RD. Interbody, posterior, and combined lumbar fusions. Spine. 1995;20:167S-177S.
Harms J, Rolinger H. A one-stage procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120:343-347.
Holly LT, Schwender JD, Rouben DP, et al. Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus. 2006;30(3):E6. 1-5
Humphreys SC, Hodges SD, Patwardhan AG, et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine. 2001;26:567-571.
Kaiser MG, Haid RWJr, Subach BR, et al. Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: a retrospective review. Neurosurgery. 2002;51:97-105.
Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine. 1995;20:356-361.
Lin PM. Posterior lumbar interbody fusion technique: complications and pitfalls. Clin Orthop Relat Res. 1985;193:90-102.
Mummaneni PV, Haid RW, Lanman T, et al. Transforaminal lumbar interbody fusion: technical advances. Contemp Neurosurg. 2003;25:1-8.
Mummaneni PV, Haid RW, Rodts GE. Lumbar interbody fusion: state-of-the-art technical advances. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:24-30.
Mummaneni PV, Haid RW. Transforaminal lumbar interbody fusion. In: Haid RW, Fessler R, editors. Lumbar Interbody Fusion: Cages, Dowels, and Grafts. St Louis: Quality Medical; 2003:227-234.
Mummaneni PV, Lin FJ, Haid RW, et al. Current indications and techniques for anterior approaches to the lumbar spine. Contemp Neurosurg. 2002;24:1-8.
Mummaneni PV, Rodts GEJr. The mini-open transforaminal lumbar interbody fusion. Neurosurgery. 2005;57:256-261.
Prolo DJ, Oklund SA, Butcher M. Toward uniformity in evaluating results of lumbar spine operations. A paradigm applied to posterior lumbar interbody fusions. Spine. 1986;11:601-606.
Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine. 2005;2:692-699.
Rodts GEJr, McLaughlin MR, Zhang J, et al. Laparoscopic anterior lumbar interbody fusion. Clin Neurosurg. 2000;47:541-556.
Rosenberg WS, Mummaneni PV. Transforaminal lumbar interbody fusion: technique, complications, and early results. Neurosurgery. 2001;48:569-574.
Wang ST, Goel VK, Fu CY, et al. Comparison of two interbody fusion cages for posterior lumbar interbody fusion in a cadaveric model. Int Orthop. 2006;30:299-304.
1 Mummaneni PV, Lin FJ, Haid RW, et al. Current indications and techniques for anterior approaches to the lumbar spine. Contemp Neurosurg. 2002;24:1-8.
2 Briggs H, Milligan P. Chip fusion of the low back following exploration of the spinal canal. J Bone Joint Surg. 1944;26:125-130.
3 Jaslow I. Intracorporeal bone graft in spinal fusion after disc removal. Surg Gynecol Obstet. 1946;82:215-222.
4 Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10:154-168.
5 Humphreys SC, Hodges SD, Patwardhan AG, et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine. 2001;26:567-571.
6 Cloward RB. Posterior lumbar interbody fusion updated. Clin Orthop Relat Res. 1985;193:16-19.
7 Collis JS. Total disc replacement: a modified posterior lumbar interbody fusion. Report of 750 cases. Clin Orthop Relat Res. 1985;193:64-67.
8 Fraser RD. Interbody, posterior, and combined lumbar fusions. Spine. 1995;20:167S-177S.
9 Hutter CG. Posterior intervertebral body fusion. A 25-year study. Clin Orthop Relat Res. 1983;179:86-96.
10 Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine. 1995;20:356-361.
11 Lin PM. Posterior lumbar interbody fusion technique: complications and pitfalls. Clin Orthop Relat Res. 1985;193:90-102.
12 Prolo DJ, Oklund SA, Butcher M. Toward uniformity in evaluating results of lumbar spine operations. A paradigm applied to posterior lumbar interbody fusions. Spine. 1986;11:601-606.
13 Steffee AD, Sitkowski DJ. Posterior lumbar interbody fusion and plates. Clin Orthop Relat Res. 1988;227:99-102.
14 Turner JA, Ersek M, Herron L, et al. Patient outcomes after lumbar spinal fusions. JAMA. 1992;268:907-911.
15 DeBowes RM, Grant BD, Bagby GW, et al. Cervical vertebral interbody fusion in the horse: a comparative study of bovine xenografts and autografts supported by stainless steel baskets. Am J Vet Res. 1984;45:191-199.
16 Wagner P, Grant B, Bagby G. Evaluation of cervical spinal fusion as a treatment in the equine “wobbler” syndrome. Vet Surg. 1979;8:84-89.
17 Bagby GW. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics. 1988;11:931-934.
18 Bagby GW. The Bagby and Kuslich (BAK) method of lumbar interbody fusion. Spine. 1999;24:1857.
19 Branch CL, Branch CLJr. Posterior lumbar interbody fusion with the keystone graft: technique and results. Surg Neurol. 1987;27:449-454.
20 Hutter CG. Spinal stenosis and posterior lumbar interbody fusion. Clin Orthop Relat Res. 1985;193:103-114.
21 Simmons JW. Posterior lumbar interbody fusion with posterior elements as chip grafts. Clin Orthop Relat Res. 1985;193:85-89.
22 Wiltberger BR. Intervertebral body fusion by the use of posterior bone dowel. Clin Orthop Relat Res. 1964;35:69-79.
23 Harms J, Rolinger H. A one-stage procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120:343-347.
24 Brantigan JW, Steffee AD, Geiger JM. A carbon fiber implant to aid interbody lumbar fusion. Mechanical testing. Spine. 1991;16:S277-S282.
25 Brodke DS, Dick JC, Kunz DN, et al. Posterior lumbar interbody fusion. A biomechanical comparison, including a new threaded cage. Spine. 1997;22:26-31.
26 Mummaneni PV, Haid RW, Rodts GE. Lumbar interbody fusion: state-of-the-art technical advances. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:24-30.
27 Barnes B, Rodts GEJr, Haid RWJr, et al. Allograft implants for posterior lumbar interbody fusion: results comparing cylindrical dowels and impacted wedges. Neurosurgery. 2002;51:1191-1198.
28 Kaiser MG, Haid RWJr, Subach BR, et al. Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: a retrospective review. Neurosurgery. 2002;51:97-105.
29 Whitecloud TSIII, Roesch WW, Ricciardi JE. Transforaminal interbody fusion versus anterior-posterior interbody fusion of the lumbar spine: a financial analysis. J Spinal Disord. 2001;14:100-103.
30 Lowe TG, Tahernia AD, O’Brien MF, et al. Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique, and 2-year results. J Spinal Disord Tech. 2002;15:31-38.
31 Mummaneni PV, Haid RW. Transforaminal lumbar interbody fusion. In: Haid RW, Fessler R, editors. Lumbar Interbody Fusion: Cages, Dowels, and Grafts. St Louis: Quality Medical; 2003:227-234.
32 Mummaneni PV, Haid RW, Lanman T, et al. Transforaminal lumbar interbody fusion: technical advances. Contemp Neurosurg. 2003;25:1-8.
33 Rodts GEJr, McLaughlin MR, Zhang J, et al. Laparoscopic anterior lumbar interbody fusion. Clin Neurosurg. 2000;47:541-556.
34 Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28:S26-S35.
35 Foley KT, Lefkowitz MA. Advances in minimally invasive spine surgery. Clin Neurosurg. 2002;49:499-517.
36 Holly LT, Schwender JD, Rouben DP, et al. Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus. 2006;30(3):E6.
37 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine. 2005;2:692-699.
38 Ames CP, Acosta FLJr, Chi J, et al. Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion performed at 1 and 2 levels. Spine. 2005;30:E562-E566.
39 Enker P, Steffee AD. Interbody fusion and instrumentation. Clin Orthop Relat Res. 1994;300:90-101.
40 Gertzbein SD, Hollopeter MR, Hall S. Pseudarthrosis of the lumbar spine. Outcome after circumferential fusion. Spine. 1998;23:2352-2356.
41 Slosar PJ, Reynolds JB, Schofferman J, et al. Patient satisfaction after circumferential lumbar fusion. Spine. 2000;25:722-726.
42 Weiner BK, Fraser RD. Spine update lumbar interbody cages. Spine. 1998;23:634-640.
43 Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18:2106-2107.
44 Jost B, Cripton PA, Lund T, et al. Compressive strength of interbody cages in the lumbar spine: the effect of cage shape, posterior instrumentation and bone density. Eur Spine J. 1998;7:132-141.
45 Vadapalli S, Sairyo K, Goel VK, et al. Biomechanical rationale for using polyetheretherketone (PEEK) spacers for lumbar interbody fusion: a finite element study. Spine. 2006;31:E992-E998.
46 Wang ST, Goel VK, Fu CY, et al. Comparison of two interbody fusion cages for posterior lumbar interbody fusion in a cadaveric model. Int Orthop. 2006;30:299-304.
47 Burkus JK, Heim SE, Gornet MF, Rodts GE. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16:113-122.
48 Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:19-23.
49 Lowe TG, Coe JD. Resorbable polymer implants in unilateral transforaminal lumbar interbody fusion. J Neurosurg. 2002;97:464-467.
50 Salehi SA, Tawk R, Ganju A, et al. Transforaminal lumbar interbody fusion: surgical technique and results in 24 patients. Neurosurgery. 2004;54:368-374.
51 Gibson S, McLeod I, Wardlaw D, et al. Allograft versus autograft in instrumented posterolateral lumbar spinal fusion: a randomized control trial. Spine. 2002;27:1599-1603.
52 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: bone graft extenders and substitutes. J Neurosurg Spine. 2005;2:733-736.
53 Rosenberg WS, Mummaneni PV. Transforaminal lumbar interbody fusion: technique, complications, and early results. Neurosurgery. 2001;48:569-574.
54 Gejo R, Matsui H, Kawaguchi Y, et al. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine. 1999;24:1023-1028.
55 Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21:941-944.
56 Mayer TG, Vanharanta H, Gatchel RJ, et al. Comparison of CT scan muscle measurements and isokinetic trunk strength in postoperative patients. Spine. 1989;14:33-36.
57 Sihvonen T, Herno A, Paljarvi L, et al. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575-581.
58 Styf JR, Willen J. The effects of external compression by three different retractors on pressure in the erector spine muscles during and after posterior lumbar spine surgery in humans. Spine. 1998;23:354-358.
59 Schwender JD, Holly LT, Rouben DP, et al. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(suppl 1):S1-S6.
60 Jang JS, Lee SH. Minimally invasive transforaminal lumbar interbody fusion with ipsilateral pedicle screw and contralateral facet screw fixation. J Neurosurg Spine. 2005;3:218-223.
61 Isaacs RE, Podichetty VK, Santiago P, et al. Minimally invasive microendoscopy-assisted transforaminal interbody fusion with instrumentation. J Neurosurg Spine. 2005;3:98-105.
62 Mummaneni PV, Rodts GEJr. The mini-open transforaminal lumbar interbody fusion. Neurosurgery. 2005;57:256-261.