CHAPTER 303 Posterior Lumbar Instrumentation
Indications for Instrumentation
Trauma
Lumbar spine trauma often requires spinal stabilization, particularly when two- or three-column injury is present. Multiple classification systems have been developed for spinal fractures, including those of Denis,1 McAfee and colleagues,2 Gertzbein and associates,3 and Magerl and coworkers.4 Most available systems suffer from one or more limitations, including a degree of complexity that limits routine clinical use, failure to include clinically relevant information such as the neurological status of the patient, and lack of guidance with regard to clinical management.5 Vaccaro and colleagues recently proposed a new classification of thoracolumbar injuries, the Thoracolumbar Injury Classification and Severity Score (TLICS), which attempts to overcome these limitations. TLICS focuses on three parameters, with points given for the various subcategories.5 First, the injury morphology is assessed as compression without burst (1 point), compression with burst (1 point), translational-rotational (3 points), or distraction (4 points). Second, the integrity of the posterior ligamentous complex (PLC) is assessed as intact (0 points), suspected or indeterminate injury (2 points), or clearly injured (3 points). Third, the neurological status of the patient is assessed as intact (0 points), nerve root injury (2 points), complete cord or conus injury (2 points), incomplete cord or conus injury (3 points), or cauda equina injury (3 points). A TLICS of 3 or less suggests a nonoperative injury, and a score of 5 or more suggests that an operative procedure should be considered. A TLICS of 4 suggests a situation that might be managed conservatively or operatively. In addition, based on neurological status and the integrity of the PLC, Vaccaro and colleagues provided suggested surgical approaches.5
Degenerative Disease
Indications for fusion in the setting of lumbar degenerative spine disease remain matters of ongoing debate and study. Resnick and coworkers recently provided a series of recommendations regarding this population based on the best available medical literature (Table 303-1).6–22 In addition, lumbar instrumentation is typically necessary to maintain correction and stability in patients with degenerative scoliosis or iatrogenic flat back syndrome who fail conservative management and desire surgical treatment.23–26
TABLE 303-1 Summary of Guidelines for the Performance of Fusion Procedures for Degenerative Disease of the Lumbar Spine
| DISORDER | RECOMMENDATION |
|---|---|
| Intractable low back pain (LBP) without stenosis or spondylolisthesis | Class I medical evidence reported in support of use of lumbar fusion as a treatment standard for carefully selected patients with LBP intractable to best medical management.16 Although multiple reports suggest improved clinical outcomes with addition of pedicle screw fixation, there are conflicting findings from similarly classified sources (mostly class II and III). It is recommended that pedicle screw supplementation of posterolateral fusion (PLF) be reserved for patients with increased risk for nonunion when treated with PLF (e.g., smokers and those undergoing revision surgery or suffering from systemic conditions associated with poor bone healing).11 |
| Lumbar disk herniation and radiculopathy | No convincing evidence to support routine use of lumbar fusion (with or without instrumentation) at time of primary lumbar disk excision. Conflicting class III evidence suggests possible benefit with fusion in patients with preoperative lumbar instability, substantial chronic axial back pain, or recurrent disk herniation.15 |
| Lumbar stenosis and spondylolisthesis | Best medical evidence in literature confirms utility of fusion for improving patient outcome following decompression for stenosis associated with spondylolisthesis. Medical evidence related to use of pedicle screw fixation in this setting is class III and is inconsistent. Consistent benefit of pedicle screw fixation has been reported in setting of preoperative or iatrogenic instability or kyphosis.14 |
| Lumbar stenosis following decompression, without spondylolisthesis | Best medical evidence does not indicate that fusion (with or without instrumentation) provides any benefit over decompression alone for treatment of lumbar stenosis following decompression in the absence of preoperative deformity or instability. Class III evidence suggests that patients undergoing wide decompression resulting in iatrogenic instability may benefit from fusion.13 |
Dynamic stabilization devices are designed to maintain or restore normal spinal motion. Although the indications for these devices are still being clarified, many of the applications are related to degenerative disease.27–32 Khoueir and associates recently provided a salient overview of the disorders in which these devices are expected to play a role: (1) controlling motion in the iatrogenically destabilized spine such as may occur with decompression procedures, (2) augmentation of interbody fusion with promotion of increased anterior load sharing, (3) protection or restoration of degenerated facet joints and intervertebral disks, (4) as part of a 360-degree circumferential segment reconstruction with motion preservation, (5) reduction of destructive forces at instrumentation-bone interfaces in patients with osteopenia or osteoporosis, and (6) reduction of fusion-related sequelae such as adjacent-level degeneration and pseudarthosis.33
Infection
Vertebral osteomyelitis can frequently be treated with parenteral antibiotics. Failure of medical therapy may necessitate surgical débridement. Although no rigorously defined criteria exist, spinal stabilization may be necessary when more than 50% of the vertebral body has been eroded or if aggressive débridement would produce an unstable spine. Historically, débridement and stabilization were performed as two separate procedures to optimize infection clearance. However, several reports have demonstrated successful treatment with combined débridement and stabilization, with instrumentation including titanium cages and pedicle screw and rod systems, performed either through a combined anterior and posterior approach34–37 or entirely through a posterior approach.36,38 This combined approach has produced favorable results with regard to clearing infection, achieving ultimate spinal stability, and permitting earlier ambulation and rehabilitation. This approach has been similarly favorable for spinal tuberculosis.39–43
Tumor
Surgical treatment of primary and metastatic spine tumors may be indicated to alleviate pain, provide stability, and decompress the neural elements.44 The type, location, and degree of destruction of the tumor, as well as the overall treatment goals of surgery, determine whether the optimal approach is anterior, posterior, or combined anterior and posterior.45 For metastatic disease, it must be remembered that the primary goal of surgery is typically palliation, and the goal should be maximization of quality of life with minimization of surgical complications.46
Posterior Lumbar Instrumentation
Brief Overview of Historical Instrumentation
Modern attempts at spine instrumentation began in the 1940s with the Harrington system, which used a distraction-compression rod.47 Although Harrington rods represented a significant advancement, their limitations soon became evident. Overdistraction frequently produced flat back syndrome, and neural compression by the laminar hooks and hook failure were not uncommon. In addition, the Harrington system lacked the ability to apply segmental corrective forces, although the addition of segmental wiring provided limited segmental correction.47
The Luque rod system was developed as an alternative to the Harrington system.48 Luque rods require fixation points above and below the affected segment and employ sublaminar wires to provide segmental fixation. The limitations of this system included neurological deficits associated with sublaminar wire passage or migration of the rods through a laminectomy defect.49 In addition, this system provided very limited resistance to axial loads, limiting its utility in cases in which the anterior column was compromised.
The Cotrel-Dubousset (CD) system, developed in the late 1980s, consisted of multiple laminar hooks that were connected by a rod.50 The CD system improved on the Harrington and Luque systems by requiring fixation of fewer segments above and below the diseased level. Pedicle screws were used in place of hooks at levels requiring laminectomy.
Metallic Pedicle Screw-Rod Systems
Boucher51 initially reported the use of pedicle screws for spinal fixation in the early 1950s, and Roy-Camille52 later popularized their use for lumbar fracture, pseudarthrosis, metastases, primary spine tumor, lumbosacral fusion, and spondylolisthesis. Pedicle screws offer considerable segmental control and enable fusion of fewer levels than was required with Harrington or Luque rods. Polyaxial screw heads have been introduced to facilitate connection of the screws to the rod or plate system.53 Care must be used when placing pedicle screws in order to prevent injury to the neural structures and dura.
A variety of materials are used to construct pedicle screw-rod systems. Until the late 1990s, pedicle screw-rod systems were primarily composed of stainless steel. Given the considerable interference of stainless steel on computed tomography (CT) and magnetic resonance imaging (MRI) studies, titanium-alloy systems were developed. Titanium-alloy implants have been shown to be compatible with high-quality MR imaging and to result in a significant reduction of artifacts on CT imaging.54,55 In addition, titanium-alloy implants have been shown to produce greater bone ongrowth compared with stainless steel implants, resulting in greater bone-pedicle screw fixation.56 However, studies have suggested that the fatigue life of titanium spinal implants is inferior to that of steel, especially at notch sites resulting from rod contouring.57,58 Cobalt-chromium rods were designed to offer improved mechanical strength compared with titanium-alloy, while still maintaining improved imaging characteristics relative to stainless steel. Significantly elevated levels of cobalt and chromium ions have been identified in the serum of patients following implantation of metal-on-metal Maverick-type artificial lumbar disks.59 Whether elevated levels of these ions are encountered following implantation of cobalt-chromium rods and whether there are any long-term implications remain to be studied.
Polyetheretherketone Rods
Polyetheretherketone (PEEK), a synthetic semicrystalline thermoplastic polymer, has been used in cervical and lumbar interbody cages since the 1980s.32,60 The modulus of elasticity of PEEK is between that of cortical and cancellous bone, which simulates the load characteristics of the spine. PEEK rods have recently been developed and provide a semirigid alternative to metallic-based rods. PEEK rods are used with a modified top-loading metallic screw (CD Horizon Legacy; Medtronic Sofamor Danek, Inc., Memphis, TN.) and allow limited motion while still providing resistance to marked flexion, extension, axial loading, and lateral rotation.32
There are several potential applications for PEEK rods.32 First, they may be used in the treatment of spinal instability, such as spondylolisthesis or degenerative disk disease, to subject adjacent levels to less stress while still facilitating osseous fusion. Second, a PEEK rod and a domino connector may be used to treat adjacent-level disease following prior instrumented fusion. Third, a PEEK rod may be used to provide a tension band following decompression in a patient with spondylolisthesis in which arthrodesis is not the objective. Although there are several potential theoretical benefits of semirigid fixation with PEEK rods, supporting literature demonstrating these benefits remains very limited at this time.32
Dynamic Stabilization
Posterior dynamic stabilization is one of the most rapidly evolving fields in spinal surgery.33 As an alternative to fusion, dynamic stabilization devices are intended to maintain or restore intervertebral motion and simulate the behavior of the normal spine. Khoueir and colleagues recently described a classification system for posterior dynamic stabilization devices.33 This system divides these devices into three categories: interspinous spacer devices, pedicle screw-rod–based devices, and total facet replacement systems.
Implantation Techniques
During the past 5 to 10 years, rapid advances have been made in techniques for implantation of posterior lumbar instrumentation. One of the most significant advances has been the development of minimally invasive approaches. Although the standard open techniques remain in widespread use, minimally invasive techniques are gaining in popularity.61,62
Open Technique
The patient is first placed under general anesthesia, intubated, then positioned prone on a radiolucent surgical table, such as a Jackson table. Pressure points are appropriately padded, and the surgical field is prepared and draped in a sterile fashion. Unless contraindicated, use of a blood product recycling unit should be considered, although a recent cost-benefit analysis questioned its cost-effectiveness.63 Fluoroscopy is used to mark an incision that ends about 5 to 7 cm above and below the vertebral levels to be instrumented. A midline skin incision is made, and subperiosteal muscle dissection is performed to expose the segments to be instrumented. The dissection is extended to expose the lateral tips of the transverse processes.
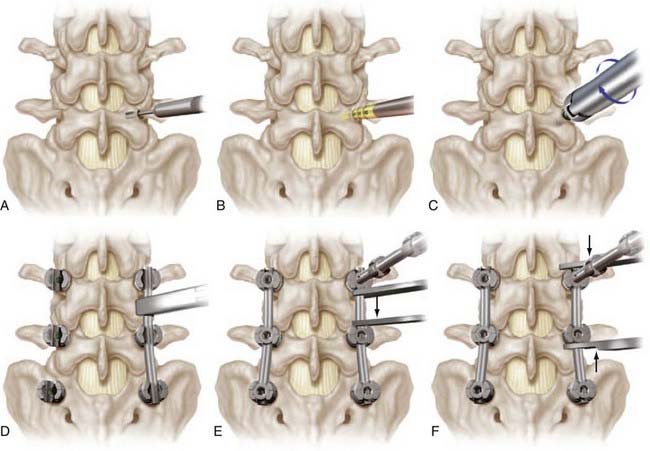
Once adequate bony exposure is achieved, the external landmarks for pedicle screw placement are identified. In the lumbar spine, the starting point for pedicle cannulation is typically defined as the intersection of the axial plane through the middle of the transverse process and the sagittal plane through the superior facet (Fig. 303-1A). The entry site for the first sacral pedicle is at the inferolateral portion of the superior S1 facet (Fig. 303-1D). Fluoroscopy is used to confirm each entry site, and a high-speed drill is then used to create a pilot hole just through the cortex. A pedicle finder is then gently advanced through the pilot hole to cannulate the pedicle and into the vertebral body (see Fig. 303-1A). Fluoroscopy can be used to determine the sagittal and axial trajectories. A ball-tip feeler is then used to palpate the trajectory created by the pedicle finder to assess for breaches. Recannulation of the pedicle using a modified trajectory may be necessary if a breach is identified. The trajectory is then prepared with a tap (Fig. 303-1B), followed by reassessment for evidence of breaches and introduction of the screw (Fig. 303-1C). Preoperative imaging can be used to preselect screw sizes, with the desired depth being about 70% to 80% of the vertebral body. To avoid injury to vascular and visceral structures, screws should not breach the anterior aspect of the vertebral body. Once all screws have been placed, the desired rod length is measured, cut, and contoured. The rod is secured into place with locking nuts, and levels may be distracted or compressed as indicated (Fig. 303-1D to F). Pedicle screws may be either monaxial or polyaxial, with the latter deigned to facilitate rod placement. One or more cross-linking devices may be used to link the rods horizontally. Triggered electromyographic stimulation has been suggested as an adjunct to optimize safe pedicle screw placement.64,65
Minimally Invasive Technique
Minimally invasive surgical techniques have been developed for posterior instrumentation of the lumbar spine to reduce the morbidity associated with the traditional open surgical approach.62 Potential advantages of using a minimally invasive approach for instrumentation include reduced blood loss, decreased infection risk, and less soft tissue and muscle trauma. Minimally invasive techniques for the placement of pedicle screw-rod systems in the lumbar spine have been previously described.66–69 The best documented approach uses the bull’s-eye technique for placement of pedicle screws.
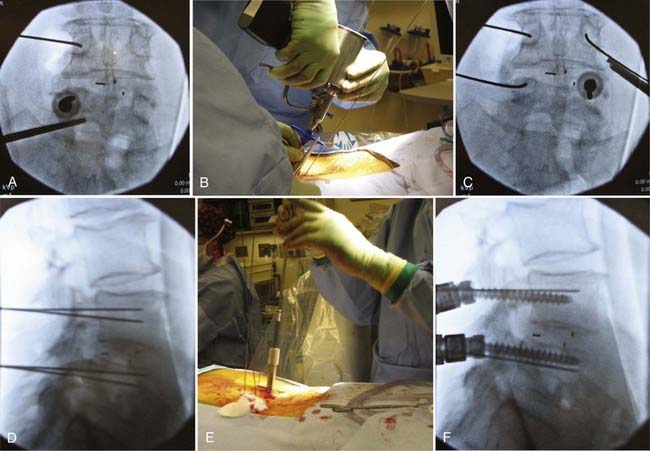
Similar to the open technique, the patient is first placed under general anesthesia, intubated, then positioned prone on a radiolucent surgical table, such as a Jackson table. Pressure points are appropriately padded, and the surgical field is prepared and draped in a sterile fashion. Anteroposterior fluoroscopy is then aligned to provide an en face view of the pedicles at the first desired level of instrumentation (Fig. 303-2A). Care must be taken to ensure that the pedicles are well aligned. At the working vertebral level, both the superior and inferior end plates should be aligned, and the spinous process should be in the midline. In addition, the pedicle should be visualized in the upper half of the vertebral body.
Using fluoroscopic imaging, the tip of a Jamshidi needle is placed on the skin overlying the center of the pedicle, and a scalpel is used to make approximately a 2-cm vertical skin incision, centered at the tip of the needle. The Jamshidi needle is then carefully advanced through the incision, directed toward the underlying pedicle. Fluoroscopy and tactile feedback are used to place the tip of the Jamshidi needle in the center of the pedicle. The needle is then aligned to provide an en face view of both the needle and the pedicle (see Fig. 303-2A). A K wire is then driven about 2 cm beyond the tip of the Jamshidi needle (Fig. 303-2B). Next, the Jamshidi needle is removed, leaving the K wire in place (Fig. 303-2A to C). A K wire is similarly placed in each pedicle planned for instrumentation. On lateral-view fluoroscopy, the K wires are then driven to a depth of about two thirds of the vertebral body (Fig. 303-2D). A cannulated tap is passed over each K wire and used to prepare the trajectory (Fig. 303-2E), followed by placement of cannulated pedicle screws (Fig. 303-2F). Efforts have been made to develop accurate navigation systems for minimally invasive pedicle screw placement to reduce radiation exposure for both the surgeon and patient.70–73
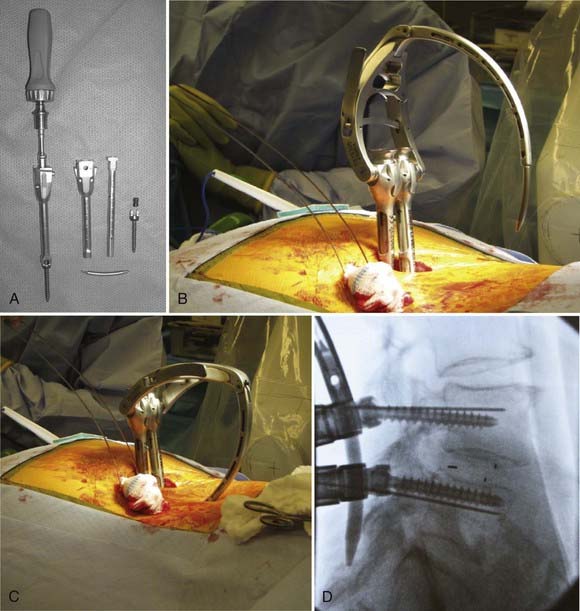
A variety of systems have been developed for minimally invasive rod passage following pedicle screw placement. Typically, minimally invasive rod passage is based on specific screw extenders that are attached to the screws, extend out of the wound, facilitate rod passage, and are then removed after the rod is secured in place (Fig. 303-3A and B). One of the first systems developed was the Sextant (Medtronic Sofamor Danek, Inc.), which can be used to instrument either one or two levels (see Fig. 303-3). After the pedicle screws have been placed, the extenders lock together, and a Sextant arm, with the rod attached at the end, is then connected to the extenders (see Fig. 303-3B). The Sextant arm then permits introduction of the rod through the screw heads (Fig. 303-3C and D). Locking caps are then placed through the extenders, and the extenders are removed.
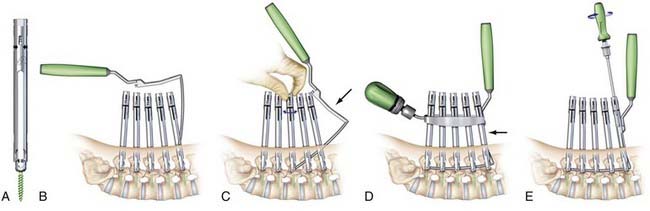
Because the rod passage technique employed by Sextant is limited to two levels, alternative techniques have been developed to pass rods minimally invasively for longer constructs. For example, the Viper2 (DePuy Spine, Inc., Raynham, Massachusetts) (Fig. 303-4) and the Longitude (Medtronic Sofamor Danek, Inc.) (Fig. 303-5) systems both enable minimally invasive rod passage following percutaneous placement of pedicle screws and can be used to instrument a single level or for substantially longer constructs.
Achieving Arthrodesis
With open instrumentation, arthrodesis is promoted by clearing the bony surfaces, including laminae, facet joints, and transverse processes, of soft tissue and decorticating the surface with a high-speed drill. Decortication is best performed before rod placement to maximize the bony surface that can be accessed with the high-speed drill. Cancellous bone graft is then packed into the joints and placed along the decorticated surfaces. Autogenous iliac crest bone graft may be used depending on surgeon preference, the amount of graft needed, and the level of risk for pseudarthrosis. Investigations are ongoing regarding the use of agents such as bone morphogenetic protein (BMP) to assist in the fusion process.35,74–76
Outcomes
Degenerative Spondylolisthesis
Spondylolisthesis, the forward slippage of one vertebra relative to the adjacent vertebra, can result from degenerative disease. Degenerative spondylolisthesis may warrant surgical treatment in the setting of significant back or leg pain that is unresponsive to nonsurgical measures, if neurological deficits develop, or if the listhesis is shown to be progressive. Several studies have been designed to compare treatment approaches.77–91 Martin and associates conducted a systematic review of the surgical management of degenerative lumbar spondylolisthesis.78 Based on 13 studies, that were generally of low methodologic quality, satisfactory outcomes were significantly more likely with fusion than with decompression alone (relative risk, 1.40; 95% confidence interval, 1.04 to 1.89; P < .05). In addition, there was a significantly greater probability of achieving fusion with the use of instrumentation (relative risk, 1.37; 95% confidence interval, 1.07 to 1.75; P < .05). However, use of instrumentation was not associated with a significant improvement in clinical outcome (relative risk, 1.19; 95% confidence interval, 0.92 to 1.54; P > .05). Nevertheless, the optimal treatment approach for degenerative spondylolisthesis continues to be controversial and remains an area of active study.
Degenerative Disk Disease
There are few rigorously designed, randomized prospective studies to address the effectiveness of fusion for the treatment of degenerative disk disease.16,92,93 Fritzell and colleagues reported a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group in 2001.92 This study included 294 patients who met the following criteria: (1) disabling low back pain for at least 2 years, (2) required to have radiographic and clinical evidence of spondylosis at either or both L4-5 and L5-S1, and (3) deemed to be surgical candidates. Patients were randomized to one of two arms, consisting of nonoperative or operative treatment. The nonoperative arm included physical therapy, education, and other pain-relieving modalities based on the discretion of the treating physician. Twenty-six surgeons provided operative treatment, which consisted of posterolateral fusion, posterolateral fusion and pedicle screw fixation, or interbody fusion supplemented with posterolateral fusion and pedicle screw fixation. The only significant demographic or clinical difference between the nonoperative and operative patient groups was that the latter had a greater degree of comorbidity. Two-year follow-up was achieved in 98% of patients and included interim evaluations at 6 months and 1 year. A battery of standardized outcomes measures were used, including the pain Visual Analog Scale (VAS) score, Oswestry Disability Index (ODI) for low back pain, the Million VAS, the General Function Scale (GFS), Work Status, a patient satisfaction survey, and an independent evaluation by a different spine surgeon. Rigorous statistical analyses were performed, including intention to treat and worst-case scenarios.
The data from Fritzell and colleagues clearly demonstrated that the surgical group had significantly improved outcome based on pain relief, disability, and degree of satisfaction compared with the nonsurgical group. All outcome measures assessed were significantly better in the surgically treated patients compared with the patients treated nonoperatively. The study by Fritzell and colleagues has been criticized as being biased against nonoperative therapy because patients had already failed a trial of nonoperative treatment before entering the study.94
Brox and associates conducted a randomized prospective study comparing operative and nonoperative treatment for low back pain. Inclusion criteria included the following: (1) low back pain for at least 1 year, (2) ODI score of 30 to 100, and (3) radiographic evidence of degeneration at either or both L4-5 and L5-S1.93 Patients randomized to operative treatment underwent instrumented posterolateral fusion. Patients randomized to nonoperative treatment underwent a very rigorous program designed for patients with low back pain. This included cognitive therapy, supervised physical therapy (average of 25 hours per week for 8 weeks), and a home program of exercises. Outcome measures included a modified ODI, pain VAS score, medication use, GFS, the Waddel Fear Avoidance Belief Questionnaire, and a patient satisfaction score. Of the 121 patients evaluated for inclusion, only 64 were ultimately randomized, with 37 to surgical treatment and 27 to nonsurgical treatment. Except for a greater number of men in the surgical group, the patients in the two treatment arms did not differ significantly.
Trauma
The most common traumatic lumbar spine fractures are compression and burst fractures. Although most compression fractures and stable burst fractures may be treated nonsurgically,95 for those cases that do warrant surgical treatment, instrumentation offers several advantages.96 These advantages include the ability to correct kyphotic deformity, to help protect neural elements from injury, to facilitate fusion through rigid immobilization, to reduce pain, and to facilitate earlier patient mobilization. Whether surgical treatment is best achieved through posterior, anterior, or combined approaches depends on the integrity of the anterior and posterior columns and remains a controversial topic.97–101 The superiority of pedicle screw systems over other forms of posterior instrumentation for unstable lumbar spine fractures has been demonstrated.102–105 Several authors have reported favorable outcomes using minimally invasive techniques in the management of thoracolumbar trauma.61,106,107
Although it is generally accepted that posterior instrumentation with pedicle screws can be an effective means of treating unstable lumbar fractures,108 there continues to be considerable controversy about whether short-segment pedicle instrumentation is adequate treatment.100,109–114 McLain and coworkers provided a preliminary report on instrumentation failure in 10 of 19 patients treated with short-segment pedicle instrumentation for thoracolumbar fractures.109 These failures included progressive kyphosis related to bending of the screws in 6 patients, kyphosis due to bony collapse or vertebral translation in 3 patients, and segmental kyphosis following fracture of a caudad screw in 1 patient. The authors suggested that posterior screw fixation alone may be inadequate.
In contrast, Parker and colleagues reported very favorable results for 46 patients who were treated with short-segment pedicle screw fixation (one level above the fracture to one level below the fracture) for thoracolumbar spine fractures.110 One patient died of unrelated causes. All the remaining 45 patients were followed at least 40 months and had successful fracture healing in virtual anatomic alignment. In deciding which patients should be treated with short-segment posterior fixation, the authors used the Load-Sharing Classification.115
Afzal and associates evaluated the use of short-segment pedicle screw instrumentation and augmentation vertebroplasty in 16 patients with lumbar burst fractures.111 The central and anterior height of the vertebral body were restored to 72% and 82% of the estimated intact height on average, respectively. They noted four complications, including three cases of cement leakage without clinical consequence and one superficial wound infection. All patients demonstrated good fracture reduction. The authors concluded that posterior short-segment pedicle fixation in conjunction with balloon vertebroplasty is a feasible option to address all three vertebral columns through a single approach.
Infection
Operative stabilization may be indicated for lumbar vertebral osteomyelitis and diskitis. However, there are several areas of controversy and active research regarding the optimal timing and approach for these surgical procedures. Traditional teaching suggests that, if spinal reconstruction is indicated, clearing the infection, which may include an open débridement, should precede spinal instrumentation. Multiple recent studies have suggested that débridement and instrumented stabilization of the infected and unstable spine may be performed in a single stage. Carragee reported a retrospective review of 17 cases of spinal instrumentation for active pyogenic vertebral osteomyelitis with greater than 2-years of follow-up.37 Twelve of the 17 patients were immunocompromised. Eight patients had significant postoperative complications, which include two instrumentation failures and one wound dehiscence. All the patients were mobilized directly after instrumentation, and none of the patients had evidence of persistent infection at last follow-up. The author concluded that instrumentation did not appear to compromise the ability to clear infection. Other reports have demonstrated similar success of instrumentation in the setting of active infection, including both pyogenic36,38 and tuberculous.38–40,42,43 The use of titanium implants, including pedicle screws, laminar hooks, rods, and cages, has been specifically investigated and does not appear to negatively affect infection clearance, although this remains controversial.34–3641
In cases of spinal osteomyelitis that warrant operative stabilization, both anterior and posterior approaches may be required. With the use of modern posterior instrumentation, several authors have suggested that a posterior-only approach may be adequate. Güzey and coworkers reported on 19 patients with thoracic or lumbar tuberculous spondylitis who were treated with a single-stage posterior decompression and débridement, followed by placement of interbody grafts if necessary, instrumentation, and posterior or posterolateral grafts.42 The only instrumentation failure was fracture of a single pedicle screw at 3 months of follow-up, and all patients demonstrated at least some improvement of neurological status at a mean follow-up of 53 months. Thirteen patients had a kyphotic deformity, with a mean angulation of 18.2 degrees (range, 0 to 42 degrees). Although this did not significantly change following surgery (mean, 17.3 degrees; range, 0 to 42 degrees), there was only a 2.8-degree loss of correction (range, 2 to 5 degrees) at a mean follow-up of 44 months, and there was no progression of kyphosis beyond 15 months in any patient.
Lee and associates reported 16 patients with lumbar tuberculous spondylitis who were treated by posterior lumbar interbody fusion with autogenous iliac bone grafting and pedicle screw instrumentation.43 Bony fusion was achieved within 1 year in 15 patients. They noted a preoperative lordotic angle of 27.8 degrees (range 9 to 45 degrees) that improved to 35.8 degrees (range 28 to 48 degrees) at a mean follow-up of 33 months.
Ames CP, Smith JS, Preul MC, et al. Effect of recombinant human bone morphogenetic protein-2 in an experimental model of spinal fusion in a radiated area. Spine. 2005;30:2585-2592.
Anderson DG, Samartzis D, Shen FH, et al. Percutaneous instrumentation of the thoracic and lumbar spine. Orthop Clin North Am. 2007;38:401-408.
Aryan HE, Lu DC, Acosta FLJr, et al. Corpectomy followed by the placement of instrumentation with titanium cages and recombinant human bone morphogenetic protein-2 for vertebral osteomyelitis. J Neurosurg Spine. 2007;6:23-30.
Bono CM, Lee CK. The influence of subdiagnosis on radiographic and clinical outcomes after lumbar fusion for degenerative disc disorders: an analysis of the literature from two decades. Spine. 2005;30:227-234.
Fayazi AH, Ludwig SC, Dabbah M, et al. Preliminary results of staged anterior debridement and reconstruction using titanium mesh cages in the treatment of thoracolumbar vertebral osteomyelitis. Spine J. 2004;4:388-395.
Fessler RG, O’Toole JE, Eichholz KM, et al. The development of minimally invasive spine surgery. Neurosurg Clin N Am. 2006;17:401-409.
Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807-2812.
Fritzell P, Hagg O, Wessberg P, et al. 2001 Volvo Award winner in clinical studies. Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521-2532.
Highsmith JM, Tumialan LM, Rodts GEJr. Flexible rods and the case for dynamic stabilization. Neurosurg Focus. 2007;22:E11.
Isaacs RE, Podichetty VK, Santiago P, et al. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine. 2005;3:98-105.
Khoueir P, Kim KA, Wang MY. Classification of posterior dynamic stabilization devices. Neurosurg Focus. 2007;22:E3.
Klimo PJr, Dailey AT, Fessler RG. Posterior surgical approaches and outcomes in metastatic spine-disease. Neurosurg Clin N Am. 2004;15:425-435.
Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine. 2007;32:E111-E120.
Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845-4869.
Lenke LG, Padberg AM, Russo MH, et al. Triggered electromyographic threshold for accuracy of pedicle screw placement. An animal model and clinical correlation. Spine. 1995;20:1585-1591.
Martin CR, Gruszczynski AT, Braunsfurth HA, et al. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine. 2007;32:1791-1798.
Parker JW, Lane JR, Karaikovic EE, et al. Successful short-segment instrumentation and fusion for thoracolumbar spine fractures: a consecutive 41/2-year series. Spine. 2000;25:1157-1170.
Rampersaud YR, Annand N, Dekutoski MB. Use of minimally invasive surgical techniques in the management of thoracolumbar trauma: current concepts. Spine. 2006;31:S96-S102.
Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 7: intractable low-back pain without stenosis or spondylolisthesis. J Neurosurg Spine. 2005;2:670-672.
Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 8: lumbar fusion for disc herniation and radiculopathy. J Neurosurg Spine. 2005;2:673-678.
Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: fusion in patients with stenosis and spondylolisthesis. J Neurosurg Spine. 2005;2:679-685.
Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 10: fusion following decompression in patients with stenosis without spondylolisthesis. J Neurosurg Spine. 2005;2:686-691.
Thomsen K, Christensen FB, Eiskjaer SP, et al. 1997 Volvo Award winner in clinical studies. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine. 1997;22:2813-2822.
Vaccaro AR, Lehman RAJr, Hurlbert RJ, et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005;30:2325-2333.
1 Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
2 McAfee PC, Yuan HA, Fredrickson BE, et al. The value of computed tomography in thoracolumbar fractures. An analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am. 1983;65:461-473.
3 Gertzbein SD, Court-Brown CM. Rationale for the management of flexion-distraction injuries of the thoracolumbar spine based on a new classification. J Spinal Disord. 1989;2:176-183.
4 Magerl F, Aebi M, Gertzbein SD, et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184-201.
5 Vaccaro AR, Lehman RAJr, Hurlbert RJ, et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005;30:2325-2333.
6 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 17: bone growth stimulators and lumbar fusion. J Neurosurg Spine. 2005;2:737-740.
7 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: bone graft extenders and substitutes. J Neurosurg Spine. 2005;2:733-736.
8 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine. 2005;2:725-732.
9 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 14: brace therapy as an adjunct to or substitute for lumbar fusion. J Neurosurg Spine. 2005;2:716-724.
10 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 13: injection therapies, low-back pain, and lumbar fusion. J Neurosurg Spine. 2005;2:707-715.
11 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 12: pedicle screw fixation as an adjunct to posterolateral fusion for low-back pain. J Neurosurg Spine. 2005;2:700-706.
12 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine. 2005;2:692-699.
13 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 10: fusion following decompression in patients with stenosis without spondylolisthesis. J Neurosurg Spine. 2005;2:686-691.
14 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: fusion in patients with stenosis and spondylolisthesis. J Neurosurg Spine. 2005;2:679-685.
15 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 8: lumbar fusion for disc herniation and radiculopathy. J Neurosurg Spine. 2005;2:673-678.
16 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 7: intractable low-back pain without stenosis or spondylolisthesis. J Neurosurg Spine. 2005;2:670-672.
17 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 6: magnetic resonance imaging and discography for patient selection for lumbar fusion. J Neurosurg Spine. 2005;2:662-669.
18 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 5: correlation between radiographic and functional outcome. J Neurosurg Spine. 2005;2:658-661.
19 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion. J Neurosurg Spine. 2005;2:653-657.
20 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 3: assessment of economic outcome. J Neurosurg Spine. 2005;2:647-652.
21 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 2: assessment of functional outcome. J Neurosurg Spine. 2005;2:639-646.
22 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 1: introduction and methodology. J Neurosurg Spine. 2005;2:637-638.
23 Marchesi DG, Aebi M. Pedicle fixation devices in the treatment of adult lumbar scoliosis. Spine. 1992;17:S304-S309.
24 Lu DC, Chou D. Flatback syndrome. Neurosurg Clin N Am. 2007;18:289-294.
25 Wiggins GC, Ondra SL, Shaffrey CI. Management of iatrogenic flat-back syndrome. Neurosurg Focus. 2003;15:E8.
26 Daffner SD, Vaccaro AR. Adult degenerative lumbar scoliosis. Am J Orthop. 2003;32:77-82.
27 Cheng BC, Gordon J, Cheng J, et al. Immediate biomechanical effects of lumbar posterior dynamic stabilization above a circumferential fusion. Spine. 2007;32:2551-2557.
28 Schulte TL, Hurschler C, Haversath M, et al. The effect of dynamic, semi-rigid implants on the range of motion of lumbar motion segments after decompression. Eur Spine J. 2008;17:1057-1065.
29 Grob D, Benini A, Junge A, et al. Clinical experience with the Dynesys semirigid fixation system for the lumbar spine: surgical and patient-oriented outcome in 50 cases after an average of 2 years. Spine. 2005;30:324-331.
30 Schnake KJ, Schaeren S, Jeanneret B. Dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolisthesis. Spine. 2006;31:442-449.
31 Scott-Young M. Posterior dynamic stabilization devices in the coming age of lumbar disc replacement. Neurosurg Focus. 2007;22:E14.
32 Highsmith JM, Tumialan LM, Rodts GEJr. Flexible rods and the case for dynamic stabilization. Neurosurg Focus. 2007;22:E11.
33 Khoueir P, Kim KA, Wang MY. Classification of posterior dynamic stabilization devices. Neurosurg Focus. 2007;22:E3.
34 Fayazi AH, Ludwig SC, Dabbah M, et al. Preliminary results of staged anterior debridement and reconstruction using titanium mesh cages in the treatment of thoracolumbar vertebral osteomyelitis. Spine J. 2004;4:388-395.
35 Aryan HE, Lu DC, Acosta FLJr, et al. Corpectomy followed by the placement of instrumentation with titanium cages and recombinant human bone morphogenetic protein-2 for vertebral osteomyelitis. J Neurosurg Spine. 2007;6:23-30.
36 Suess O, Weise L, Brock M, et al. Debridement and spinal instrumentation as a single-stage procedure in bacterial spondylitis/spondylodiscitis. Zentralbl Neurochir. 2007;68:123-132.
37 Carragee EJ. Instrumentation of the infected and unstable spine: a review of 17 cases from the thoracic and lumbar spine with pyogenic infections. J Spinal Disord. 1997;10:317-324.
38 Rath SA, Neff U, Schneider O, et al. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery. 1996;38:926-933.
39 Lee SH, Sung JK, Park YM. Single-stage transpedicular decompression and posterior instrumentation in treatment of thoracic and thoracolumbar spinal tuberculosis: a retrospective case series. J Spinal Disord Tech. 2006;19:595-602.
40 Moon MS, Woo YK, Lee KS, et al. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20:1910-1916.
41 Kim DJ, Yun YH, Moon SH, et al. Posterior instrumentation using compressive laminar hooks and anterior interbody arthrodesis for the treatment of tuberculosis of the lower lumbar spine. Spine. 2004;29:E275-E279.
42 Güzey FK, Emel E, Bas NS, et al. Thoracic and lumbar tuberculous spondylitis treated by posterior debridement, graft placement, and instrumentation: a retrospective analysis in 19 cases. J Neurosurg Spine. 2005;3:450-458.
43 Lee JS, Moon KP, Kim SJ, et al. Posterior lumbar interbody fusion and posterior instrumentation in the surgical management of lumbar tuberculous spondylitis. J Bone Joint Surg Br. 2007;89:210-214.
44 Steinmetz MP, Mekhail A, Benzel EC. Management of metastatic tumors of the spine: strategies and operative indications. Neurosurg Focus. 2001;11:e2.
45 Kostuik JP, Errico TJ, Gleason TF, et al. Spinal stabilization of vertebral column tumors. Spine. 1988;13:250-256.
46 Klimo PJr, Dailey AT, Fessler RG. Posterior surgical approaches and outcomes in metastatic spine-disease. Neurosurg Clin N Am. 2004;15:425-435.
47 White AH, Zucherman JF, Hsu K. Lumbosacral fusions with Harrington rods and intersegmental wiring. Clin Orthop Relat Res. 1986;203:185-190.
48 Luque ER. The anatomic basis and development of segmental spinal instrumentation. Spine. 1982;7:256-259.
49 Quint DJ, Salton G. Migration of Luque rods through a laminectomy defect causing spinal cord compression. AJNR Am J Neuroradiol. 1993;14:395-398.
50 Gurr KR, McAfee PC. Cotrel-Dubousset instrumentation in adults. A preliminary report. Spine. 1988;13:510-520.
51 Boucher HH. A method of spinal fusion. J Bone Joint Surg Br. 1959;41:248-259.
52 Roy-Camille R, Saillant G, Mazel C. Internal fixation of the lumbar spine with pedicle screw plating. Clin Orthop Relat Res. 1986;203:7-17.
53 Shepard MF, Davies MR, Abayan A, et al. Effects of polyaxial pedicle screws on lumbar construct rigidity. J Spinal Disord Tech. 2002;15:233-236.
54 Ebraheim NA, Rupp RE, Savolaine ER, et al. Use of titanium implants in pedicular screw fixation. J Spinal Disord. 1994;7:478-486.
55 Wang JC, Sandhu HS, Yu WD, et al. MR parameters for imaging titanium spinal instrumentation. J Spinal Disord. 1997;10:27-32.
56 Christensen FB, Dalstra M, Sejling F, et al. Titanium-alloy enhances bone-pedicle screw fixation: mechanical and histomorphometrical results of titanium-alloy versus stainless steel. Eur Spine J. 2000;9:97-103.
57 Lindsey C, Deviren V, Xu Z, et al. The effects of rod contouring on spinal construct fatigue strength. Spine. 2006;31:1680-1687.
58 Chen PQ, Lin SJ, Wu SS, et al. Mechanical performance of the new posterior spinal implant: effect of materials, connecting plate, and pedicle screw design. Spine. 2003;28:881-886.
59 Zeh A, Planert M, Siegert G, et al. Release of cobalt and chromium ions into the serum following implantation of the metal-on-metal Maverick-type artificial lumbar disc (Medtronic Sofamor Danek). Spine. 2007;32:348-352.
60 Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845-4869.
61 Rampersaud YR, Annand N, Dekutoski MB. Use of minimally invasive surgical techniques in the management of thoracolumbar trauma: current concepts. Spine. 2006;31:S96-S102.
62 Fessler RG, O’Toole JE, Eichholz KM, et al. The development of minimally invasive spine surgery. Neurosurg Clin N Am. 2006;17:401-409.
63 Reitman CA, Watters WC3rd, Sassard WR. The Cell Saver in adult lumbar fusion surgery: a cost-benefit outcomes study. Spine. 2004;29:1580-1583.
64 Raynor BL, Lenke LG, Bridwell KH, et al. Correlation between low triggered electromyographic thresholds and lumbar pedicle screw malposition: analysis of 4857 screws. Spine. 2007;32:2673-2678.
65 Lenke LG, Padberg AM, Russo MH, et al. Triggered electromyographic threshold for accuracy of pedicle screw placement. An animal model and clinical correlation. Spine. 1995;20:1585-1591.
66 Anderson DG, Samartzis D, Shen FH, et al. Percutaneous instrumentation of the thoracic and lumbar spine. Orthop Clin North Am. 2007;38:401-408.
67 Foley KT, Gupta SK. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg. 2002;97:7-12.
68 Ringel F, Stoffel M, Stuer C, et al. Minimally invasive transmuscular pedicle screw fixation of the thoracic and lumbar spine. Neurosurgery. 2006;59:ONS361-ONS366.
69 Isaacs RE, Podichetty VK, Santiago P, et al. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine. 2005;3:98-105.
70 Resnick DK. Prospective comparison of virtual fluoroscopy to fluoroscopy and plain radiographs for placement of lumbar pedicle screws. J Spinal Disord Tech. 2003;16:254-260.
71 Rampersaud YR, Foley KT, Shen AC, et al. Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine. 2000;25:2637-2645.
72 Sasso RC, Garrido BJ. Computer-assisted spinal navigation versus serial radiography and operative time for posterior spinal fusion at L5-S1. J Spinal Disord Tech. 2007;20:118-122.
73 Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine. 2007;32:E111-E120.
74 Ames CP, Smith JS, Preul MC, et al. Effect of recombinant human bone morphogenetic protein-2 in an experimental model of spinal fusion in a radiated area. Spine. 2005;30:2585-2592.
75 Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008;62:ONS423-ONS431.
76 Tumialan LM, Pan J, Rodts GE, et al. The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenetic protein-2: a review of 200 patients. J Neurosurg Spine. 2008;8:529-535.
77 Bono CM, Lee CK. The influence of subdiagnosis on radiographic and clinical outcomes after lumbar fusion for degenerative disc disorders: an analysis of the literature from two decades. Spine. 2005;30:227-234.
78 Martin CR, Gruszczynski AT, Braunsfurth HA, et al. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine. 2007;32:1791-1798.
79 Booth KC, Bridwell KH, Eisenberg BA, et al. Minimum 5-year results of degenerative spondylolisthesis treated with decompression and instrumented posterior fusion. Spine. 1999;24:1721-1727.
80 Bridwell KH, Sedgewick TA, O’Brien MF, et al. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993;6:461-472.
81 Fernandez-Fairen M, Sala P, Ramirez H, et al. A prospective randomized study of unilateral versus bilateral instrumented posterolateral lumbar fusion in degenerative spondylolisthesis. Spine. 2007;32:395-401.
82 Majid K, Fischgrund JS. Degenerative lumbar spondylolisthesis: trends in management. J Am Acad Orthop Surg. 2008;16:208-215.
83 DeWald CJ, Vartabedian JE, Rodts MF, et al. Evaluation and management of high-grade spondylolisthesis in adults. Spine. 2005;30:S49-S59.
84 Jacobs WC, Vreeling A, De Kleuver M. Fusion for low-grade adult isthmic spondylolisthesis: a systematic review of the literature. Eur Spine J. 2006;15:391-402.
85 Moller H, Hedlund R. Instrumented and noninstrumented posterolateral fusion in adult spondylolisthesis—a prospective randomized study: part 2. Spine. 2000;25:1716-1721.
86 Rousseau MA, Lazennec JY, Bass EC, et al. Predictors of outcomes after posterior decompression and fusion in degenerative spondylolisthesis. Eur Spine J. 2005;14:55-60.
87 Schnee CL, Freese A, Ansell LV. Outcome analysis for adults with spondylolisthesis treated with posterolateral fusion and transpedicular screw fixation. J Neurosurg. 1997;86:56-63.
88 Thomsen K, Christensen FB, Eiskjaer SP, et al. 1997 Volvo Award winner in clinical studies. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine. 1997;22:2813-2822.
89 Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807-2812.
90 Jang JS, Lee SH. Minimally invasive transforaminal lumbar interbody fusion with ipsilateral pedicle screw and contralateral facet screw fixation. J Neurosurg Spine. 2005;3:218-223.
91 Scheufler KM, Dohmen H, Vougioukas VI. Percutaneous transforaminal lumbar interbody fusion for the treatment of degenerative lumbar instability. Neurosurgery. 2007;60:203-212.
92 Fritzell P, Hagg O, Wessberg P, et al. 2001 Volvo Award winner in clinical studies. Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521-2532.
93 Brox JI, Sorensen R, Friis A, et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine. 2003;28:1913-1921.
94 Mooney V. Point of view. Spine. 2001;26:2532-2533.
95 Wood K, Buttermann G, Mehbod A, et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85:773-781.
96 Mikles MR, Stchur RP, Graziano GP. Posterior instrumentation for thoracolumbar fractures. J Am Acad Orthop Surg. 2004;12:424-435.
97 Stambough JL. Posterior instrumentation for thoracolumbar trauma. Clin Orthop Relat Res. 1997;335:73-88.
98 Danisa OA, Shaffrey CI, Jane JA, et al. Surgical approaches for the correction of unstable thoracolumbar burst fractures: a retrospective analysis of treatment outcomes. J Neurosurg. 1995;83:977-983.
99 Slosar PJJr, Patwardhan AG, Lorenz M, et al. Instability of the lumbar burst fracture and limitations of transpedicular instrumentation. Spine. 1995;20:1452-1461.
100 Korovessis P, Baikousis A, Zacharatos S, et al. Combined anterior plus posterior stabilization versus posterior short-segment instrumentation and fusion for mid-lumbar (L2-L4) burst fractures. Spine. 2006;31:859-868.
101 Acosta FLJr, Buckley JM, Xu Z, et al. Biomechanical comparison of three fixation techniques for unstable thoracolumbar burst fractures. Laboratory investigation. J Neurosurg Spine. 2008;8:341-346.
102 Sasso RC, Cotler HB. Posterior instrumentation and fusion for unstable fractures and fracture-dislocations of the thoracic and lumbar spine. A comparative study of three fixation devices in 70 patients. Spine. 1993;18:450-460.
103 Stambough JL. Cotrel-Dubousset instrumentation and thoracolumbar spine trauma: a review of 55 cases. J Spinal Disord. 1994;7:461-469.
104 Katonis PG, Kontakis GM, Loupasis GA, et al. Treatment of unstable thoracolumbar and lumbar spine injuries using Cotrel-Dubousset instrumentation. Spine. 1999;24:2352-2357.
105 Carl AL, Tromanhauser SG, Roger DJ. Pedicle screw instrumentation for thoracolumbar burst fractures and fracture-dislocations. Spine. 1992;17:S317-S324.
106 Korovessis P, Hadjipavlou A, Repantis T. Minimal invasive short posterior instrumentation plus balloon kyphoplasty with calcium phosphate for burst and severe compression lumbar fractures. Spine. 2008;33:658-667.
107 Wild MH, Glees M, Plieschnegger C, et al. Five-year follow-up examination after purely minimally invasive posterior stabilization of thoracolumbar fractures: a comparison of minimally invasive percutaneously and conventionally open treated patients. Arch Orthop Trauma Surg. 2007;127:335-343.
108 Whang PG, Vaccaro AR. Thoracolumbar fracture: posterior instrumentation using distraction and ligamentotaxis reduction. J Am Acad Orthop Surg. 2007;15:695-701.
109 McLain RF, Sparling E, Benson DR. Early failure of short-segment pedicle instrumentation for thoracolumbar fractures. A preliminary report. J Bone Joint Surg Am. 1993;75:162-167.
110 Parker JW, Lane JR, Karaikovic EE, et al. Successful short-segment instrumentation and fusion for thoracolumbar spine fractures: a consecutive 41/2-year series. Spine. 2000;25:1157-1170.
111 Afzal S, Akbar S, Dhar SA. Short segment pedicle screw instrumentation and augmentation vertebroplasty in lumbar burst fractures: an experience. Eur Spine J. 2008;17:336-341.
112 Wang XY, Dai LY, Xu HZ, et al. Kyphosis recurrence after posterior short-segment fixation in thoracolumbar burst fractures. J Neurosurg Spine. 2008;8:246-254.
113 Altay M, Ozkurt B, Aktekin CN, et al. Treatment of unstable thoracolumbar junction burst fractures with short- or long-segment posterior fixation in Magerl type a fractures. Eur Spine J. 2007;16:1145-1155.
114 Mahar A, Kim C, Wedemeyer M, et al. Short-segment fixation of lumbar burst fractures using pedicle fixation at the level of the fracture. Spine. 2007;32:1503-1507.
115 McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine. 1994;19:1741-1744.