CHAPTER 103 Postendograft Imaging of the Abdominal Aorta and Iliac Arteries
The primary complication of abdominal aortic aneurysm (AAA) is acute rupture, a frequently lethal event.1 As a rule, even large abdominal aortic aneurysms are asymptomatic until rupture occurs.2,3 In the United States, a ruptured abdominal aortic aneurysm is the 10th leading cause of death in patients older than 55 years. Risk of rupture is directly related to aneurysmal size, with a 5% to 10% annual risk of rupture for AAAs measuring between 5 and 6 cm. There is controversy regarding the exact size at which an aneurysm should be repaired. Surgical repair has been advocated for AAAs reaching 5.0 to 5.5 cm in size by several authors; however, open surgical repair is a major operation, with an overall operative mortality ranging between 4% and 8.4%.3,4 Endovascular repair of abdominal aortic aneurysms with the use of stent grafts has emerged as a new imaging-guided procedure (Fig. 103-1). The use of stent grafts for repair of AAA in humans was first described in 1991 by Parodi and colleagues,5 who constructed devices from Palmaz stents and a standard woven polyethylene terephthalate surgical graft material.
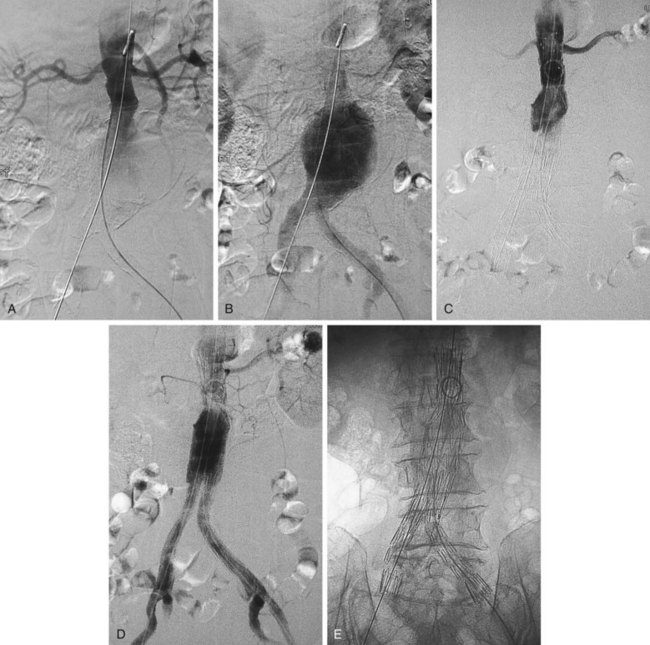
 FIGURE 103-1 Angiographic appearance of an abdominal aortic aneurysm before (A, B) and after (C, D, E) stent graft placement.
FIGURE 103-1 Angiographic appearance of an abdominal aortic aneurysm before (A, B) and after (C, D, E) stent graft placement.
Similarly, the natural course of an iliac artery aneurysm (IAA) consists of progressive expansion with eventual rupture. Involvement of iliac arteries is seen in 10% to 20% of patients with AAAs.6 IAA is defined as enlargement of the iliac artery to a diameter of more than 1.5 cm. The natural course of an IAA consists of progressive expansion with eventual rupture, and the risk of rupture increases with aneurysm size.7 Surgical repair should be recommended for isolated IAAs larger than 3.0 to 3.5 cm in diameter.8,9 A variety of minimally invasive therapeutic options have now become available (e.g., coil embolization, stent graft placement; Fig. 103-2), and choosing an appropriate option is essential for achieving optimal long-term results and reducing potential complications.10
The ultimate goal of endovascular repair of AAA and IAA with a stent graft is the same as for surgical repair—that is, depressurization and exclusion of the aneurysm sac from the circulation to prevent rupture. The dominant limiting factor in patient selection is the stent graft itself.11,12 Each device has specific and relatively restrictive requirements with regard to the diameter, length, and angulation of the proximal and distal attachment sites, and to the ability of the iliofemoral arteries to accommodate the stent graft delivery systems. A certain length of nonaneurysmal infrarenal aortic length, depending on the device used, must be present for proper placement of the proximal attachment of the stent graft. Aortic neck angles more than 45 degrees may pose problems with implantation and may stress the device. Trapezoidal, conical, or thrombus-filled aortic necks may cause device instability. Additionally, the iliofemoral vessels must have a certain (device-dependent) caliber and straightness. Patients who do not fit the device cannot be treated.13
POSTOPERATIVE ASSESSMENT
Endograft placement is relatively new, and studies regarding its long term efficacy are still being performed to define its clinical role better. In the Endovascular Aneurysm Repair (EVAR) trial 1,14 the 30-day mortality rate for endovascular repair was 1.7% and the corresponding rate for open repair was 4.7% (P < .001). At 4 years, the aneurysm-related mortality rate in the group of patients who underwent endovascular repair was half that of the group of patients who underwent open repair (P = .04), but there was no significant difference in mortality from any cause (26% for endovascular repair and 29% for open repair). However, a higher percentage of patients (20%) who underwent endovascular repair required reinterventions (compared with 6% in the open-repair group). Therefore, although perioperative mortality was higher in patients who underwent open repair, a higher percentage of patients in the endovascular repair group required reinterventions, and there was no significant difference in 4-year mortality rates between the two groups.
In the Dutch Randomized Endovascular Aneurysm Management (DREAM) trial,15 the 30-day mortality rate was significantly lower in patients who had undergone endovascular repair compared with those who had undergone open repair. However, the overall survival rate was not significantly different between the two groups at 2 years. Although aneurysm-related death was more frequent in patients who had undergone open repair, reinterventions were required more often in patients who had undergone endovascular repair. These results corroborate those of the EVAR trial 1. In the EVAR trial 2,16 338 patients with AAA who were not candidates for open repair were randomly assigned to undergo endovascular repair or no intervention. No benefit of endovascular repair was apparent during follow-up. Complications and subsequent interventions were more frequent in the group of patients who underwent endovascular repair. Therefore, the benefit of endovascular repair in patients who are not surgical candidates appears unclear.
Successful endovascular repair of AAA and IAA remains technically challenging despite continued improvement in device design. As noted, additional interventions may be required before or during stent graft placement.13 Primary technical success is defined on an intent to treat basis and requires the following: (1) successful insertion and deployment of the graft without the need for surgical conversion; (2) no perioperative mortality; (3) absence of type I or III endoleak; and (4) freedom from limb obstruction or occlusion, up to 24 hours postoperatively. Clinical success of stent grafting is defined as technical success in conjunction with freedom from aneurysm-related death, type I or III endoleak, graft infection or thrombosis, aneurysm rupture, conversion to open repair, or graft migration during the life of the patient.17
Occlusion of branch vessels arising from the proximal neck, aneurysm sac, or iliac arteries is the most common preprocedural intervention.13,18 This is done to provide appropriate attachment sites or prevent retrograde perfusion of the aneurysm sac after placement of the stent graft. Contralateral common iliac artery occlusion is required when inserting an aortounilateral iliac artery stent graft. In a patient with a common or internal IAA, embolization of the internal iliac artery is required before stent graft is deployed across its origin into the ipsilateral external iliac artery (Fig. 103-3). Interventions such as angioplasty or stent placement may also be required during insertion of a stent graft to accommodate large delivery systems better.13 Angioplasty to dilate conduit artery stenoses should be performed at the time of the stent graft procedure, not before. At times, the main body of the graft may have to be inserted through the contralateral side from the one initially planned if difficulties are encountered during its insertion because of a stenosis (Fig. 103-4). The threshold for stent placement to repair a traumatic dissection (Figs. 103-5 to 103-7) caused by a large delivery system or to buttress the iliac limb of an unsupported stent graft should be low. Aortounilateral iliac artery stent grafts require a surgical femoral to femoral bypass graft to perfuse the pelvis and limb contralateral to the stent graft. A vascular plug to occlude flow through the contralateral common iliac artery should be placed immediately prior to the surgical femoral to femoral bypass.
Successful insertion of an aortic stent graft is achieved in more than 95% of procedures in carefully selected patients and appropriately chosen devices. The most common cause of a failed procedure is the inability to insert a device through diseased or tortuous iliac arteries, especially with devices with large profiles. Misplacement of the stent graft, failure to deploy, or acute and irreversible occlusion (Fig. 103-8) are unusual events but may require urgent open surgical repair. This is termed a surgical conversion and is associated with a higher morbidity than conventional open surgical repair.
Death during a stent graft procedure is very rare. Acute intraprocedural rupture of an AAA during stent graft placement with successful outcome has been reported but is also rare. Complications such as multiorgan system failure, myocardial infarction, bowel infarction, stroke, pulmonary embolism, and peripheral arterial embolism have all been described after stent graft procedures, but most early complications are minor and mainly consist of injuries to access arteries or issues related to groin incisions.13
As noted, complete exclusion of the aneurysm sac is the goal of stent graft placement and the definition of early clinical success.19 Figure 103-9 demonstrates the expected anatomic appearance after successful deployment of a stent graft on CT angiography.
Endoleak
Persistent opacification of the aneurysm sac after insertion of a stent graft is termed an endoleak and is classified by cause and time of occurrence.20 This classification of endoleaks is described in Table 103-1. Most early endoleaks are currently types II and IV. Type I leaks can be minimized by careful patient selection and preprocedural measurements. Tube stent grafts may have a higher rate of type I leak, particularly at the distal attachment site, than bifurcated stent grafts and today are used for selected patients only. Large attachment leaks that result in continued pressurization of the aneurysm sac indicate a failed procedure and may leave the patient at risk for subsequent AAA rupture. Because more than 50% of small endoleaks will resolve spontaneously, their management is usually expectant.13 Although many early endoleaks disappear within 6 months, reappearance of leaks and delayed appearance of new leaks can occur at any time. Stabilization of aneurysm size or even shrinkage and avoidance of secondary endovascular procedures or open surgery are considered measures of long-term success.
| Type of Endoleak | Features |
|---|---|
| I | Lack of complete seal between stent graft and vessel wall at attachment sites |
| II | Back-filling of aneurysm sac via branch vessels such as lumbar or inferior mesenteric arteries |
| III | Leaks at connections of modular components, device disruption, fabric tears |
| IV | Extravasation of contrast material through interstices in the graft material |
| V | Endotension |
| Primary endoleak | Immediate or less than 30 days postimplantation |
| Secondary endoleak | More than 30 days postimplantation |
Modified from Kaufman JA, Geller SC, Brewster DC, et al. Endovascular repair of abdominal aortic aneurysms: Current status and future directions. AJR Am J Roentgenol 2000; 175:289-302.
The presence of an endoleak is correlated with less reduction in the diameter of the sac and occasionally even with slight enlargement. After endovascular repair, an increase in the aneurysm sac diameter of less than 0.5 cm is considered acceptable. A greater degree of enlargement should prompt treatment of the endoleak using endovascular means or surgical conversion. The currently reported rate of long-term surgical conversion caused by persistent endoleak and aneurysm expansion is low.13 Conversion caused by aneurysm expansion with no visible leak is even less frequent, but both indications may become more common as long-term follow-up experience is accumulated. Additional complications such as thromboses, stent migration, and visceral organ infarctions can also be demonstrated. Finally, because atherosclerotic disease can progress over time, it is imperative to evaluate the aortic visceral tributaries for disease progression.
Imaging Techniques and Findings
Imaging plays a pivotal role in preprocedural patient evaluation, during the procedure of stent graft implantation, and for patient follow-up. The follow-up of patients with stent grafts requires evaluation of AAA sac size (Fig. 103-10) and perfusion, stent graft patency, changes in diameter of the vessels at the sites of endograft attachment (Fig. 103-11), changes in stent graft morphology, changes in stent graft position (Fig. 103-12), and detection of new aneurysms. The imaging requirements are different from those after surgical repair of an AAA, in which imaging is limited in scope and frequency.
Techniques
The single most useful imaging test that allows rapid assessment of patients with stent grafts is contrast-enhanced multidetector CT (MDCT).13 A sample CTA protocol used is shown in Table 103-2. The crucial issue after stent graft placement is correlation of sac opacification from any cause with pressure within the aneurysm sac, because continued pressurization results in continued risk of AAA rupture.21 There is no easy way to obtain sac pressures in a noninvasive manner and, furthermore, it has been suggested that lack of visualization of contrast material within the aneurysm sac does not guarantee lack of pressurization.13 Contrast-enhanced CT scans are sensitive for the detection of endoleaks, although use of color flow Doppler sonography has been advocated. Essentially, all patients with stent grafts should be followed up for life with contrast-enhanced CT. In addition, although abdominal radiographs are invaluable tools for assessment of the integrity of the metallic components of the stent graft, the integrity of the metallic components can be assessed on CT studies through volume-rendered and maximum intensity projection images.
| Parameter | Protocol |
|---|---|
| Anatomic region |
Complications and Their Detection
Endoleaks
Complete exclusion of the aneurysm sac is the goal of stent graft placement and the definition of early clinical success. The most common complication after stent graft implantation is leakage of blood into the aneurysm sac. Endoleak is defined as blood flow outside the lumen of the stent graft and contained within the aneurysm sac as demonstrated on contrast-enhanced CT, contrast-enhanced MR, duplex ultrasonography, or conventional x-ray catheter arteriography, and are classified by type (see Table 103-1). Rates of endoleak after endovascular repair of aortic aneurysms range between 2.4% to 45.5%.13,22
Type I endoleaks include those caused by inadequate proximal or distal graft fixation, resulting in lack of complete seal between the stent graft and vessel wall (Figs. 103-13 and 103-14). Type II endoleaks are defined by retrograde flow originating from aneurysm sac side branches, such as lumbar or inferior mesenteric arteries, back into the aneurysm sac (Figs. 103-15 to 103-18). Type III endoleaks include grafts that developed prosthetic graft holes after implantation or that sustained a separation of one or more of the modular components that formed the completed graft (Fig. 103-19). Type IV endoleaks include extravasation of contrast material through interstices in the graft material. Endotension, or a type V endoleak, is evident when the aneurysmal sac increases in size without a definite demonstration of an endoleak on imaging studies.
Graft Thrombosis
On contrast-enhanced CT scans, graft thrombosis is recognized as a nonenhancing intraluminal, parietal, circular, or semicircular area within the stent graft (Figs. 103-20 to 103-23; see Fig. 103-8). Parietal thrombi within the stent graft are reported to occur at rates of 3% to 19%. The prognosis for these parietal thrombi varies from spontaneous shrinkage to development of complete thrombosis, thus necessitating follow-up studies at short intervals.23
Graft Infection
Graft infection, a rare event after endovascular stent graft implantation, is associated with considerable mortality and morbidity rates. Although fever, leukocytosis, elevation of C-reactive protein, and perigraft air are frequently observed immediately after implantation, these findings do not represent evidence of systemic or graft infections. Periprosthetic thickening with soft tissue attenuation at follow-up CT does not always indicate graft infection, and the diagnosis of graft infection may be difficult to establish. Graft infection is occasionally observed some time after the procedure and is suspected on the basis of clinical symptoms and CT findings. A combination of administration of systemic antibiotics and surgery is typically necessary to obtain successful therapeutic results. The outcome of no treatment of aortic stent graft infection or treatment with exclusive antibiotics is invariably fatal. The principle in such surgical treatment is total excision of the infected graft.23
Graft Occlusion
Graft occlusion is a relatively rare event after stent graft implantation. Decreased blood flow into the stent graft, caused by the inability of the stent graft to bend along a tortuous infrarenal aorta, may lead to graft occlusion. Occlusion of the iliac graft limb caused by emboli from the left atrium has been reported. Occlusion of the aortic portion of a bifurcated stent graft 4 months after implantation has been described, associated with distal kinking of the left iliac limb.23
Shower Embolism
Shower embolism is one of the most serious, possibly fatal complications after conventional repair of infrarenal abdominal aortic aneurysm and is relatively rare. Shower embolism occurs more frequently after endovascular aneurysm repair than after conventional open surgery. Shower embolism after endovascular procedures is reported at rates of 4% to 17% and was fatal in all reported cases.24
Colon Necrosis
Colon ischemia, or necrosis, is a feared complication of endovascular stent graft implantation to treat infrarenal abdominal aortic aneurysm. This will occur if both internal iliac arteries and the inferior mesenteric artery are occluded by the stent graft, in which case colon ischemia necessitating sigmoid colon resection may be needed.25 Intraprocedural hypoperfusion of both internal iliac arteries and occlusion of a widely patent inferior mesenteric artery by the stent graft may result in sigmoid colon necrosis, with subsequent hemicolectomy.23 It has been reported that a widely patent inferior mesenteric artery may be an exclusion criterion for endovascular treatment.26 There is not, however, sufficient evidence to support this.23
Dissection
Aortic or iliac artery dissection caused by a retrograde injury from delivery system introduction has been described. However, no treatment is necessary in cases in which the patient is asymptomatic and hemodynamically stable. Alternatively, placement of a stent can be considered, if applicable (see Figs. 103-5 to 103-7).
POSTOPERATIVE MANAGEMENT
As noted, surveillance of stent graft patients is critical to determine long-term performance of the devices. This is usually accomplished with contrast-enhanced CT at 30 days, 6 months, and then annually for the life of the patient. The goals of these scans are to determine the response of the aneurysm sac to the placement of the endograft and to identify the presence or absence of endoleaks. An endoleak is defined as blood flow outside the lumen of the stent graft and contained within the aneurysm sac as demonstrated on contrast-enhanced CT, contrast-enhanced MR, duplex ultrasonography, or conventional x-ray catheter arteriography. Endoleaks after endovascular repair of aortic aneurysm range from 2.4% to 45.5%.13 Persistent endoleak is considered a therapeutic failure. Its detection, therefore, is of primary importance because it is associated with increased pressure within the aneurysmal sac, thus increasing the risk of aneurysmal rupture. Endoleaks can appear during the first 30 days after implantation, in which case they are termed primary. Secondary endoleaks are the ones that occur during subsequent follow-up. The reappearance of an endoleak after its spontaneous resolution or after it was treated is termed a recurrent endoleak.13,28 Type I endoleaks, also called attachment site endoleaks, are repaired immediately after diagnosis because this represents a direct communication between the aneurysm sac and arterial blood under systemic pressure. These leaks are usually corrected by further securing the attachment sites with angioplasty balloons, stents, or stent graft extensions. They can be minimized by careful patient selection and accurate preprocedural measurements.
Type II endoleaks are the most commonly identified endoleaks and there is controversy regarding optimal management. A type II endoleak is retrograde flow into the aneurysmal sac from patent side branches, usually the lumbar arteries and/or a patent inferior mesenteric artery. A risk factor that predisposes to type II endoleak formation is the number of patent side branch vessels, including lumbar arteries and the inferior mesenteric artery. The greater the number of patent side branches, the greater the likelihood of leak formation. Preprocedural embolization of patent lumbar or inferior mesenteric arteries is not performed routinely. Treatment of all type II endoleaks has been advocated because the increasing pressure within the aneurysm sac may promote sac rupture. Others, however, have recommended that unless the aneurysm sac is expanding, clinical observation is sufficient because many small type II endoleaks will seal spontaneously; this practice is most widely accepted.29 The treatment of type II endoleaks can be performed using percutaneous embolization, such as with a microcatheter that is advanced through collateral vessels and into the vessel that communicates with the aneurysm sac, usually the inferior mesenteric artery or a lumbar artery, if possible. Metallic coils are then used to embolize the vessel near its communication with the aneurysm. However, recurrences using this technique are very common. A percutaneous translumbar approach has also been described; this involves direct puncture of the aneurysm sac at the level of the endoleak using a 19-gauge, 20-cm needle. Embolic agents are then administered through the access needle. The most commonly used embolic agents are stainless steel or platinum coils. Liquid embolic agents such as n-butyl cyanoacrylate have also been successfully used.30
KEY POINTS
 Endovascular treatment of abdominal aortic aneurysms has been accepted as an alternative to conventional open surgery in carefully selected patients.
Endovascular treatment of abdominal aortic aneurysms has been accepted as an alternative to conventional open surgery in carefully selected patients. Contrast-enhanced CT (typically CTA) is the preferred imaging modality for follow-up of these patients.
Contrast-enhanced CT (typically CTA) is the preferred imaging modality for follow-up of these patients. Various complications may occur after endovascular implantation of a stent graft, and many of these can be treated by interventionalists.
Various complications may occur after endovascular implantation of a stent graft, and many of these can be treated by interventionalists.Bashir MR, Ferral H, Jacobs C, et al. Endoleaks after endovascular abdominal aortic aneurysm repair: management strategies according to CT findings. AJR Am J Roentgenol. 2009;192:W178-W186.
Chaikof EL, Blankensteijn JD, Harris PL, et al. Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048-1060.
Davis M, Taylor PR. Endovascular infrarenal abdominal aortic aneurysm repair. Heart. 2008;94:222-228.
Eliason JL, Clouse WD. Current management of infrarenal abdominal aortic aneurysms. Surg Clin North Am. 2007;87:1017-1033.
Eliason JL, Upchurch GRJr. Endovascular abdominal aortic aneurysm repair. Circulation. 2008;117:1738-1744.
Kaufman JA, Geller SC, Brewster DC, et al. Endovascular repair of abdominal aortic aneurysms: current status and future directions. AJR Am J Roentgenol. 2000;175:289-302.
Lovegrove RE, Javid M, Magee TR, Galland RB. A meta-analysis of 21,178 patients undergoing open or endovascular repair of abdominal aortic aneurysm. Br J Surg. 2008;95:677-684.
1 Dardik A, Burleyson G, Bowman H, et al. Surgical repair of ruptured abdominal aortic aneurysms in the state of Maryland: factors influencing outcome among 527 recent cases. J Vasc Surg. 1998;28:413-421.
2 Hallett JJ, Bower T, Cherry K, et al. Selection and preparation of high-risk patients for repair of abdominal aortic aneurysms. Mayo Clin Proc. 1994;69:763-768.
3 Ernst C. Abdominal aortic aneurysm. N Engl J Med. 1993;328:1167-1172.
4 Hodges TC, Cronenwett J. Abdominal aortic and iliac artery aneurysms: clinical presentation, natural history, and indications for intervention. In: Perler BA, Becker GJ, editors. Vascular Intervention: A Clinical Approach. New York: Thieme; 1998:339-350.
5 Parodi J, Palmaz J, Barone H. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491-499.
6 Krupski WC, Selzman CH, Floridia R, et al. Contemporary management of isolated iliac aneurysms. J Vasc Surg. 1998;28:1-11.
7 Sekkal S, Cornu E, Christides C, et al. Iliac artery aneurysms: sixty-seven cases in forty-eight patients. J Mal Vasc. 1993;18:13-17.
8 Brunkwall J, Hauksson N, Bengtsson H, et al. Solitary aneurysm of the iliac artery system: an estimate of their frequency of occurrence. J Vasc Surg. 1989;10:381-384.
9 Richardson JW, Greenfield LJ. Natural history and management of iliac aneurysms. J Vasc Surg. 1988;8:165-171.
10 Sakamoto I, Sueyoshi E, Hazama S, et al. Endovascular treatment of iliac artery aneurysms. Radiographics. 2005;25:S213-S227.
11 Armon M, Yusuf S, Latief K, et al. Anatomical suitability of abdominal aortic aneurysms for endovascular repair. Br J Surg. 1997;84:178-180.
12 Treiman G, Lawrence P, Edwards WJ, et al. An assessment of the current applicability of the EVT endovascular graft for treatment of patients with an infrarenal abdominal aortic aneurysm. J Vasc Surg. 1999;30:68-75.
13 Kaufman JA, Geller SC, Brewster DC, et al. Endovascular repair of abdominal aortic aneurysms: current status and future directions. AJR Am J Roentgenol. 2000;175:289-302.
14 EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179-2186.
15 Blankensteijn JD, de Jong SECA, Prinssen M, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352:2398-2405.
16 EVAR Trial Participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005;365:2187-2192.
17 Lee C, Kaufman J, Fan C-M, et al. Clinical outcome of internal iliac artery occlusions during endovascular treatment of aorto-iliac aneurysmal diseases. J Vasc Interv Radiol. 2000;11:567-571.
18 Ahn S, Rutherford R, Johnston K, et al. Reporting standards for infrarenal endovascular abdominal aortic aneurysm repair. J Vasc Surg. 1997;25:405-410.
19 White G, May J, Waugh R, et al. Type III and type IV endoleak: toward a complete definition of blood flow in the sac after endoluminal abdominal aortic aneurysm repair. J Endovasc Surg. 1998;5:305-309.
20 Marin ML, Hollier LH, Ellozy SH, et al. Endovascular stent graft repair of abdominal and thoracic aortic aneurysms: a ten-year experience with 817 patients. Ann Surg. 2003;238:586-595.
21 Rozenblit A, Marin M, Veith F, et al. Endovascular repair of abdominal aortic aneurysm: value of postoperative follow-up with helical CT. AJR Am J Roentgenol. 1995;165:1473-1479.
22 Görich J, Rilinger N, Soldner J, et al. Endovascular repair of aortic aneurysm: treatment of complications. J Endovasc Surg. 1999;6:136-146.
23 Mita T, Arita T, Matsunaga N, et al. complications of endovascular repair for thoracic and abdominal aortic aneurysm: an imaging spectrum. Radiographics. 2000;20:1263-1278.
24 Thompson MM, Smith J, Naylor AR, et al. Microembolization during endovascular and conventional aneurysm repair. J Vasc Surg. 1997;25:179-186.
25 Yusuf SW, Whitaker SC, Chuter TAM, et al. Early results of endovascular aortic aneurysm surgery with aortouniiliac graft, contralateral iliac occlusion, and femorofemoral bypass. J Vasc Surg. 1997;25:165-172.
26 Blum U, Langer M, Spillner G, et al. Abdominal aortic aneurysms: preliminary technical and clinical results with transfemoral placement of endovascular self-expanding stent grafts. Radiology. 1996;198:25-31.
27 Mialhe C, Amicabile C, Becquemin JP. Endovascular treatment of infrarenal abdominal aneurysms by the Stentor system: preliminary results of 79 cases. J Vasc Surg. 1997;26:199-209.
28 Chaikof EL, Blankensteijn JD, Harris PL, et al. Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048-1060.
29 Zarins CK, White RA, Hodgson KJ, et al. Endoleak as a predictor of outcome after endovascular aneurysm repair: AneuRx multicenter clinical trial. J Vasc Surg. 2000;32:90-107.
30 Baum RA, Stavropoulos SW, Fairman RM, Carpenter JP. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003;14:1111-1117.

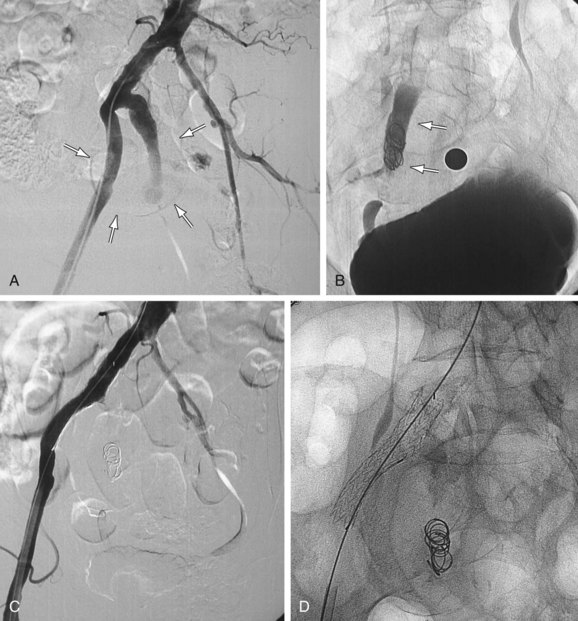
 FIGURE 103-2
FIGURE 103-2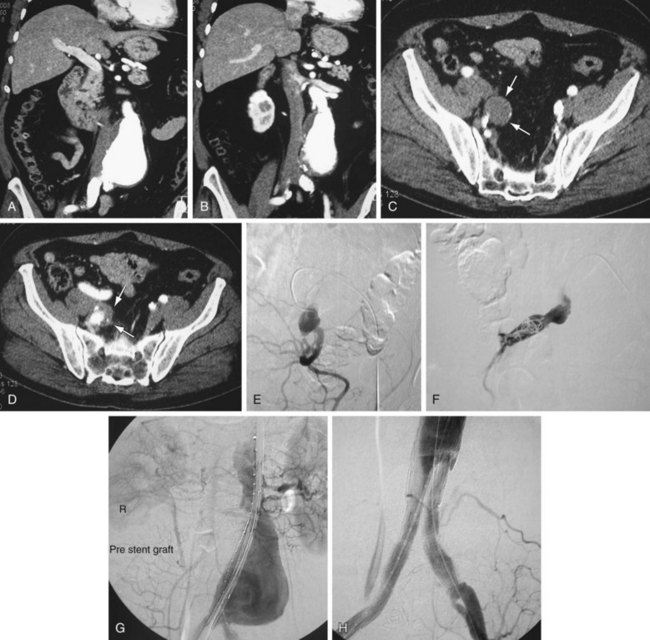
 FIGURE 103-3
FIGURE 103-3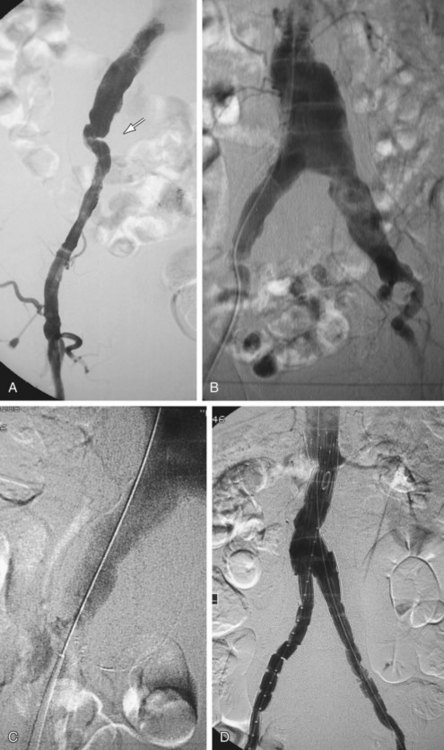
 FIGURE 103-4
FIGURE 103-4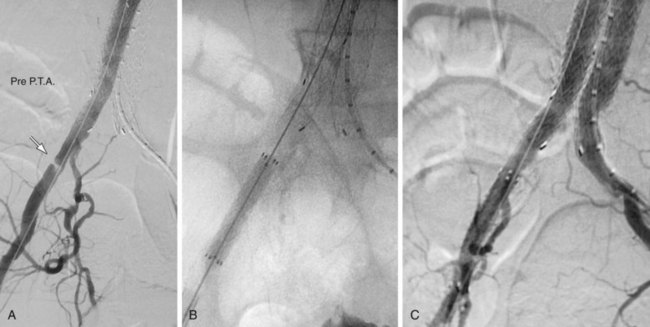
 FIGURE 103-5
FIGURE 103-5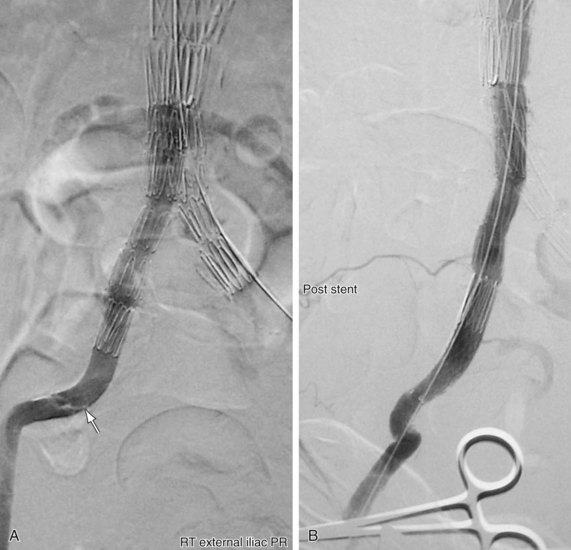
 FIGURE 103-6
FIGURE 103-6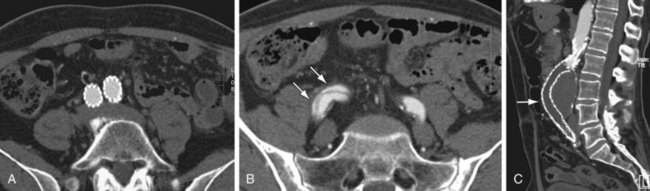
 FIGURE 103-7
FIGURE 103-7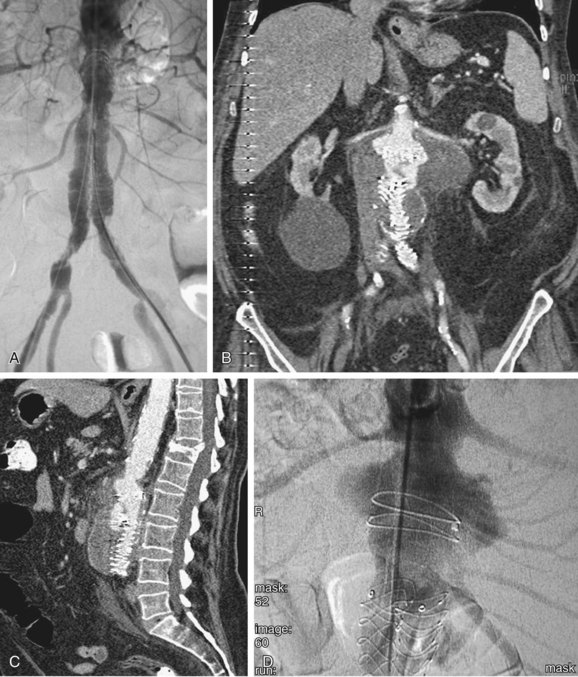
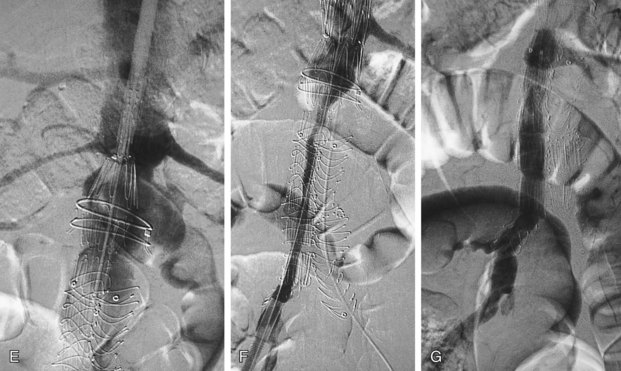
 FIGURE 103-8
FIGURE 103-8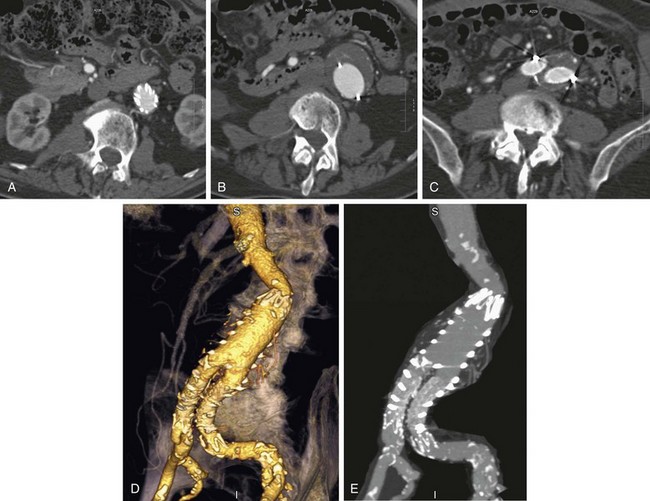
 FIGURE 103-9
FIGURE 103-9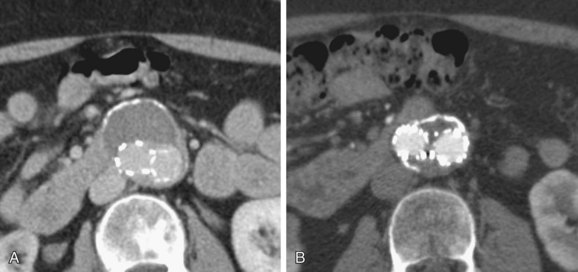
 FIGURE 103-10
FIGURE 103-10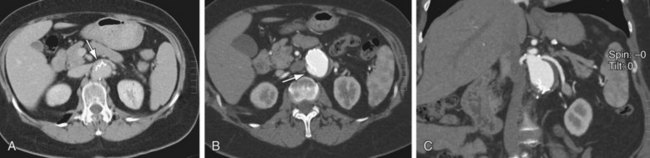
 FIGURE 103-11
FIGURE 103-11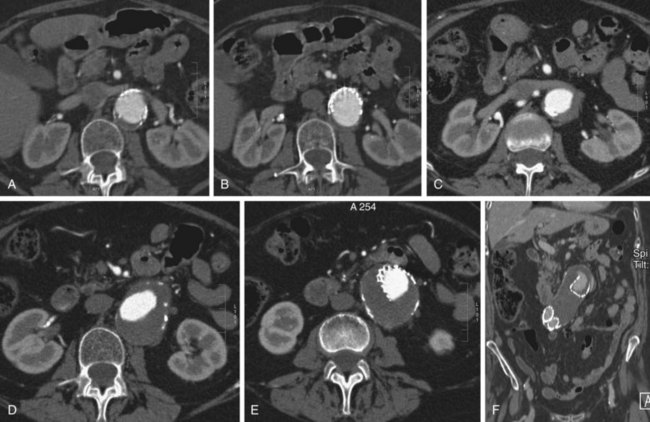
 FIGURE 103-12
FIGURE 103-12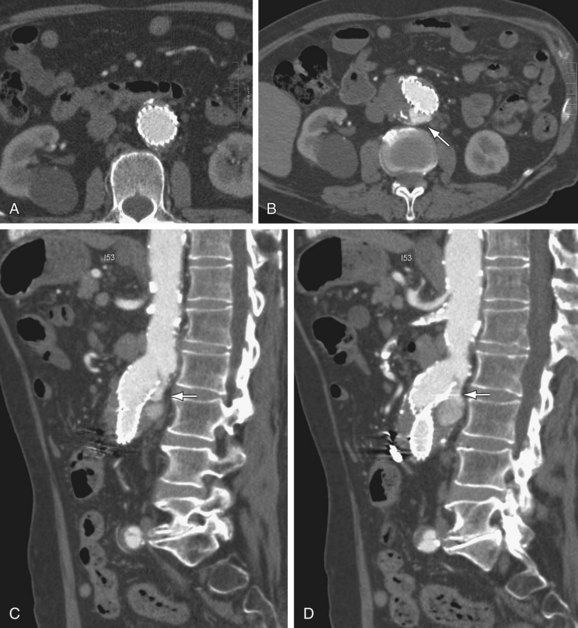
 FIGURE 103-13
FIGURE 103-13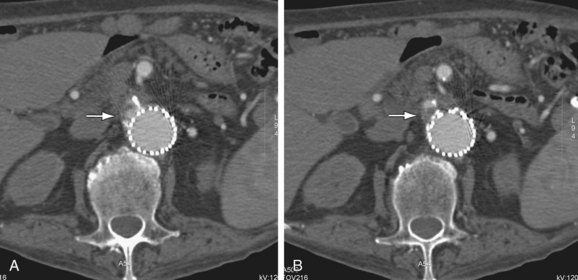
 FIGURE 103-14
FIGURE 103-14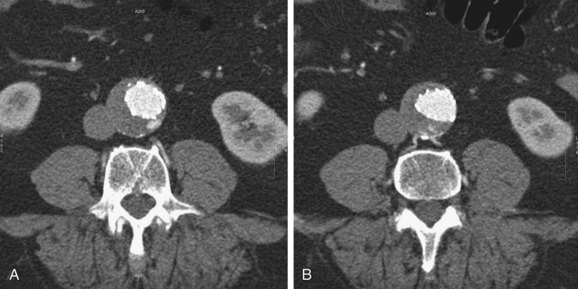
 FIGURE 103-15
FIGURE 103-15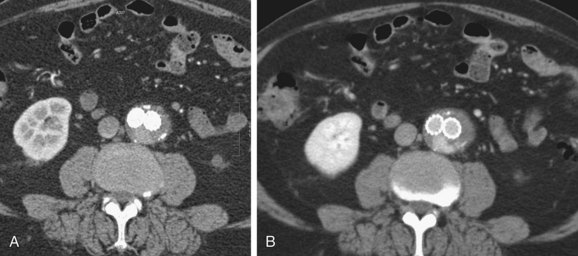
 FIGURE 103-16
FIGURE 103-16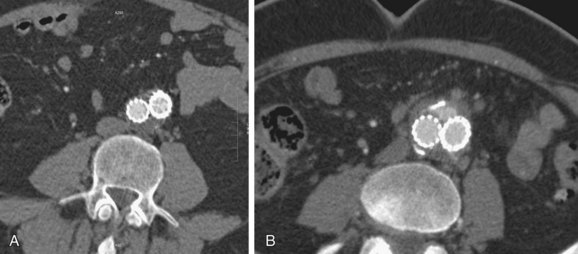
 FIGURE 103-17
FIGURE 103-17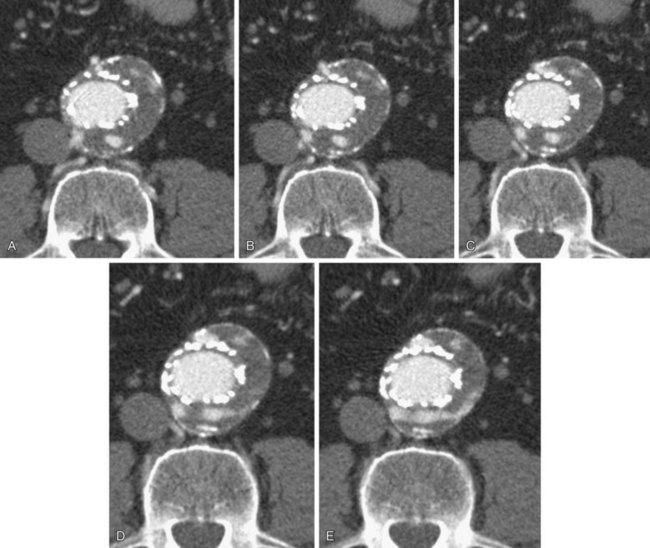
 FIGURE 103-18
FIGURE 103-18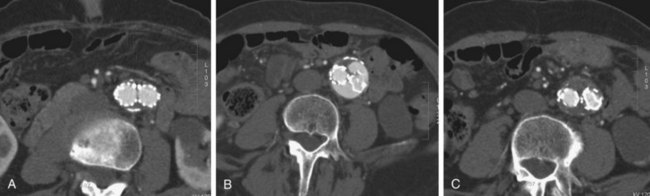
 FIGURE 103-19
FIGURE 103-19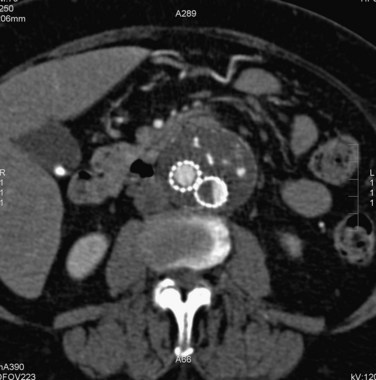
 FIGURE 103-20
FIGURE 103-20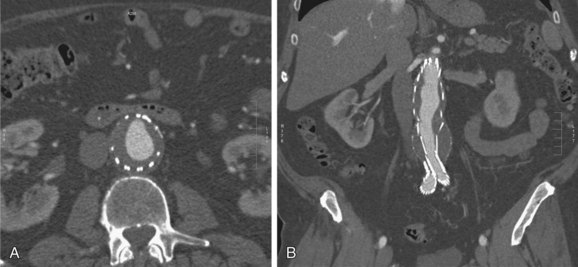
 FIGURE 103-21
FIGURE 103-21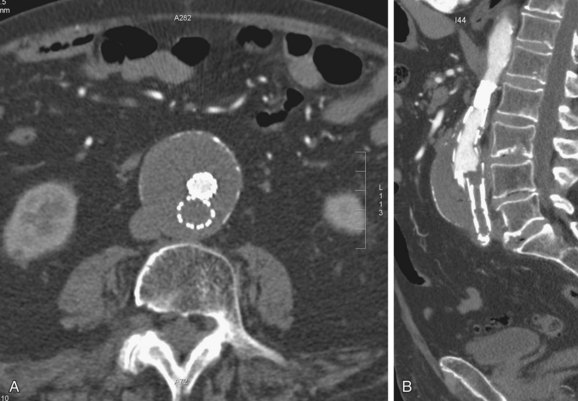
 FIGURE 103-22
FIGURE 103-22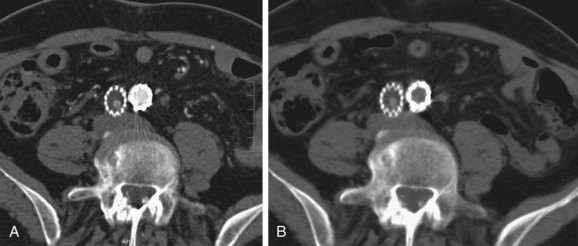
 FIGURE 103-23
FIGURE 103-23


