Site of presentation may depend partially on transplanted organ
• Imaging findings of post-transplant lymphoproliferative disorder parallel those of non-Hodgkin lymphoma (NHL) in immunocompetent patients
• GI tract: Imaging findings are similar to NHL, including mass-like bowel wall thickening, aneurysmal dilatation, ulcerated polyploid mass, or submucosal nodules
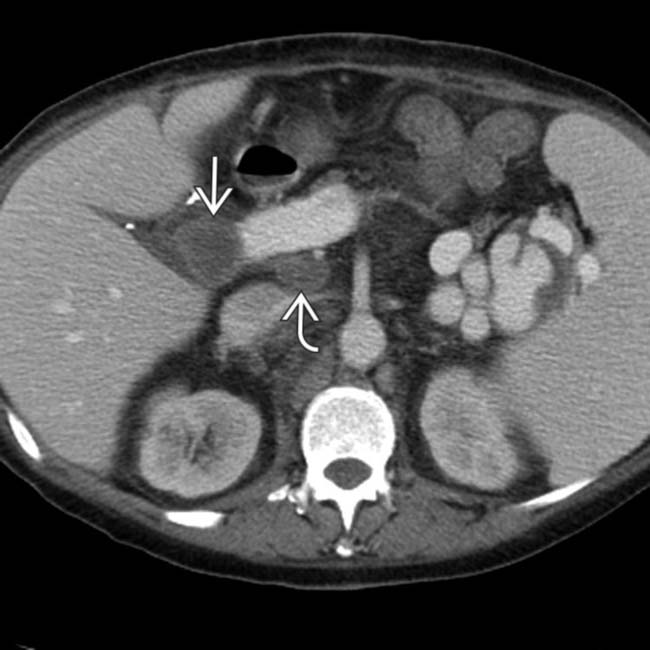
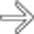
 in the porta hepatis, as well as an enlarging portacaval lymph node
in the porta hepatis, as well as an enlarging portacaval lymph node  .
.
 . The findings of post-transplant lymphoproliferative disorder (PTLD) in this case are indistinguishable from traditional non-Hodgkin lymphoma (NHL) in an immunocompetent patient.
. The findings of post-transplant lymphoproliferative disorder (PTLD) in this case are indistinguishable from traditional non-Hodgkin lymphoma (NHL) in an immunocompetent patient.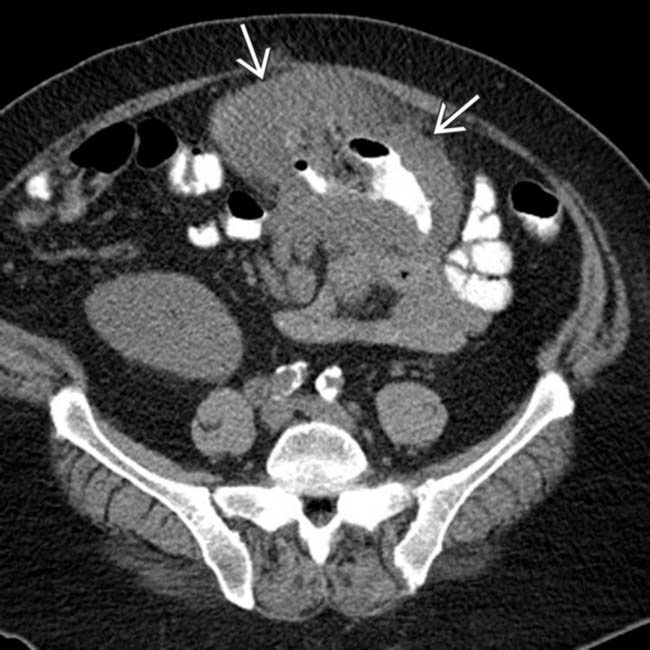
 of a segment of colon with aneurysmal dilatation.
of a segment of colon with aneurysmal dilatation.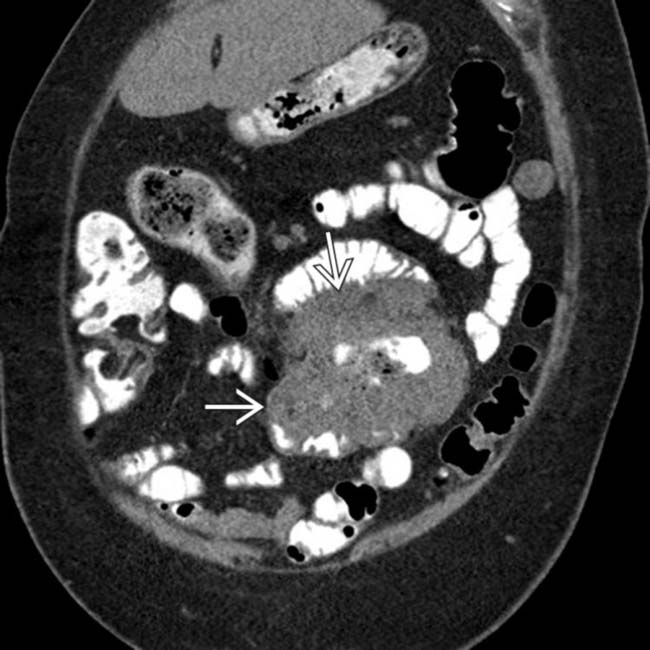
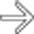
 of the bowel segment with dilatation. This is a common appearance for both NHL in immunocompetent patients and PTLD.
of the bowel segment with dilatation. This is a common appearance for both NHL in immunocompetent patients and PTLD.IMAGING
General Features
CT Findings
• Imaging findings of PTLD mostly parallel those of non-Hodgkin lymphoma (NHL) in immunocompetent patients
• Liver
• Kidney
 Most commonly involved site in renal transplant recipients and may affect native kidneys or allograft
Most commonly involved site in renal transplant recipients and may affect native kidneys or allograft
 Most commonly involved site in renal transplant recipients and may affect native kidneys or allograft
Most commonly involved site in renal transplant recipients and may affect native kidneys or allograftDIFFERENTIAL DIAGNOSIS
Recurrent or New Malignancy
• Patients with known prior malignancy are at high risk of recurrent malignancy after transplantation
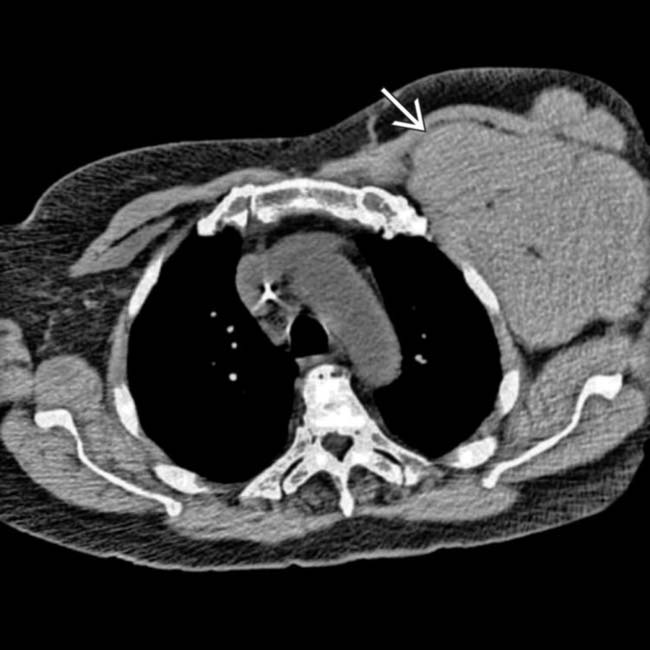
 in the left axilla, representing dramatically enlarged left axillary lymph nodes.
in the left axilla, representing dramatically enlarged left axillary lymph nodes.
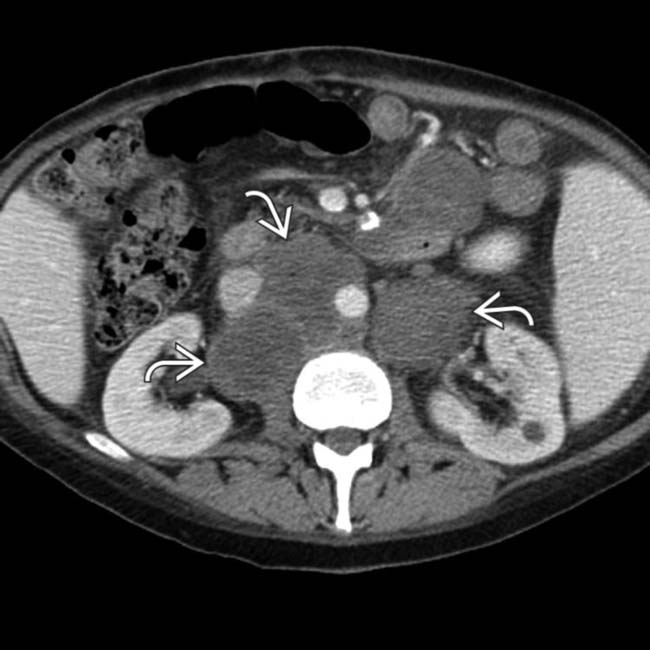
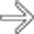
 and a right adrenal or retroperitoneal mass
and a right adrenal or retroperitoneal mass 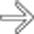 , compatible with the patient’s PTLD. PTLD can affect essentially any organ or lymph node group in the body, and any soft tissue mass in a transplant recipient must be regarded as worrisome for PTLD.
, compatible with the patient’s PTLD. PTLD can affect essentially any organ or lymph node group in the body, and any soft tissue mass in a transplant recipient must be regarded as worrisome for PTLD.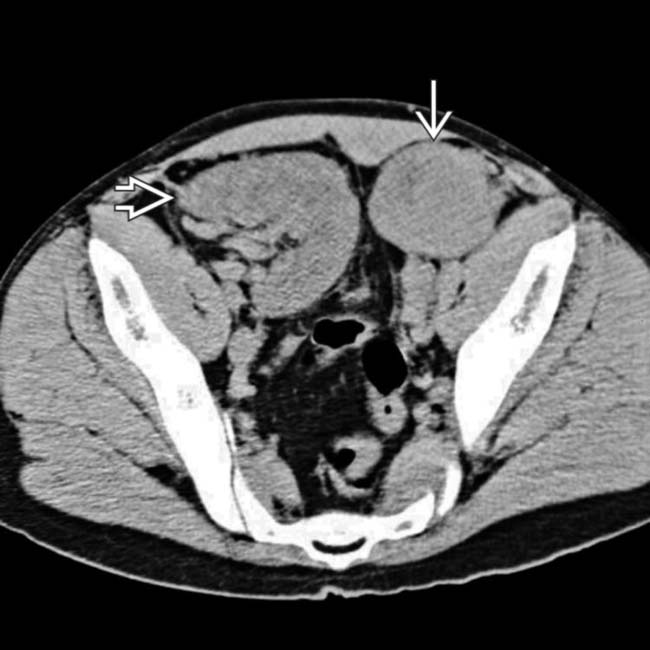
 . There is massive pelvic lymphadenopathy
. There is massive pelvic lymphadenopathy  . Both the lymphadenopathy and the adrenal masses represent manifestations of PTLD.
. Both the lymphadenopathy and the adrenal masses represent manifestations of PTLD.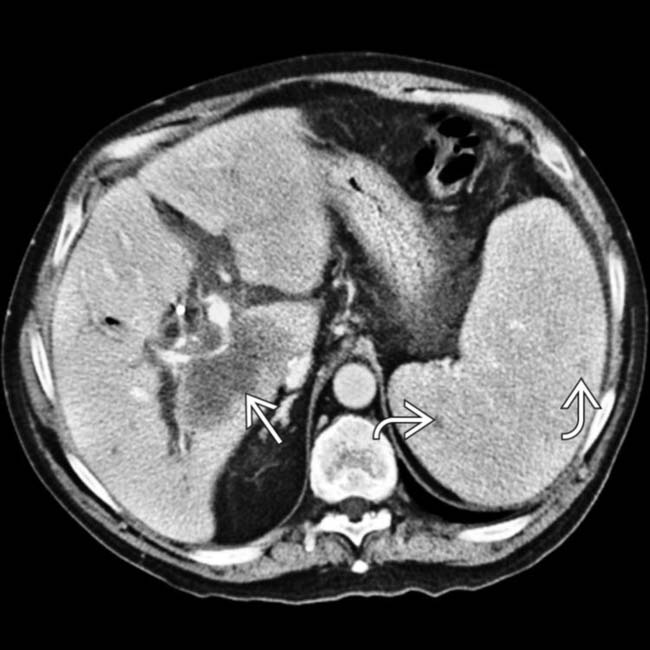
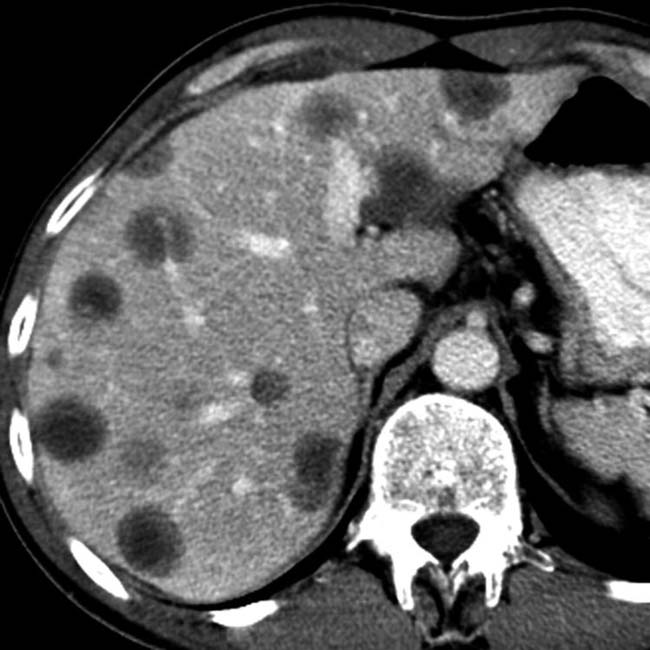
 within the liver allograft that envelopes the portal vein and bile ducts. The spleen is also involved, with splenomegaly and dozens of small hypodense nodules
within the liver allograft that envelopes the portal vein and bile ducts. The spleen is also involved, with splenomegaly and dozens of small hypodense nodules  .
.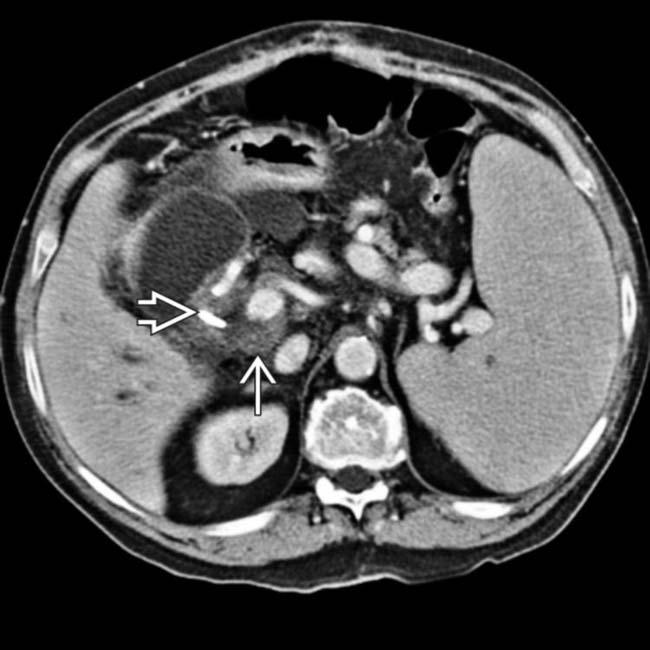
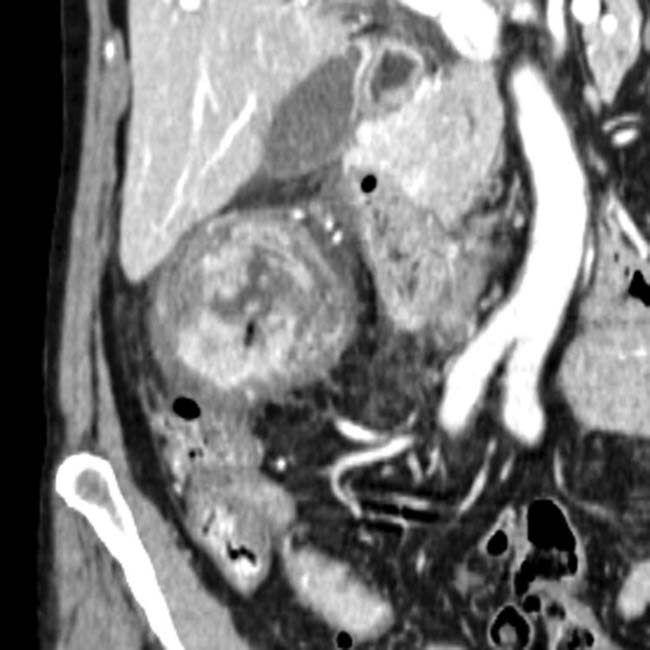
 present as manifestations of PTLD. Note the biliary stent
present as manifestations of PTLD. Note the biliary stent  placed to treat the extrinsic compression of the common bile duct by enlarged nodes and the liver mass.
placed to treat the extrinsic compression of the common bile duct by enlarged nodes and the liver mass.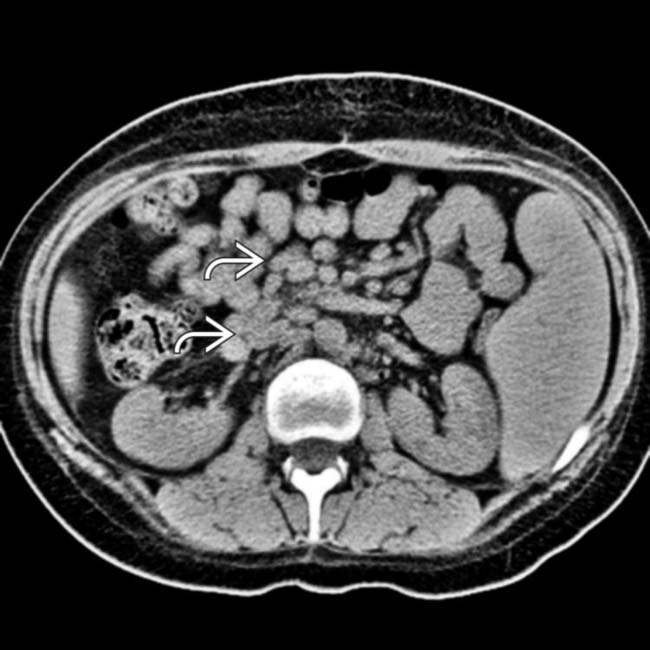
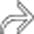 . The lack of oral or IV contrast makes it more difficult to distinguish the mesenteric and retroperitoneal nodes from unopacified vessels and bowel. Patients who have undergone heart transplantation are at markedly increased risk of developing PTLD.
. The lack of oral or IV contrast makes it more difficult to distinguish the mesenteric and retroperitoneal nodes from unopacified vessels and bowel. Patients who have undergone heart transplantation are at markedly increased risk of developing PTLD.












































































 .
.
 .
.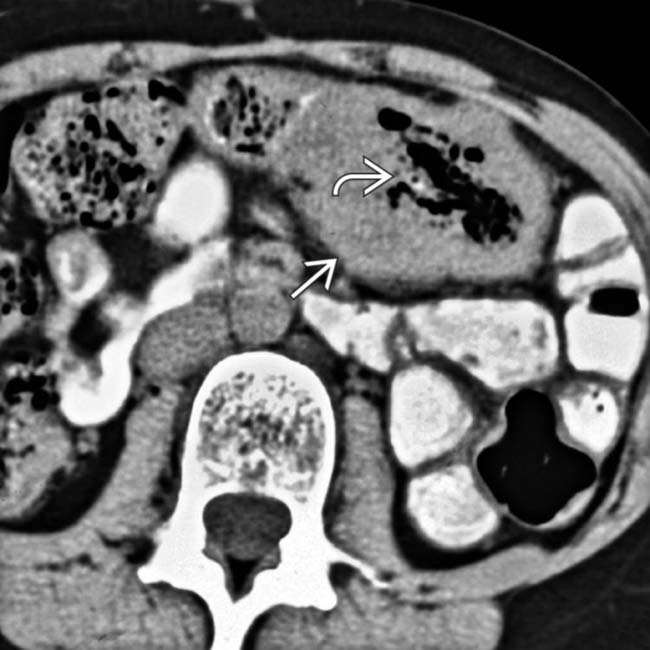
 encasing the jejunum. In the center of the mass is an irregular gas-filled lumen
encasing the jejunum. In the center of the mass is an irregular gas-filled lumen  . There is no evidence of bowel obstruction, as more distal segments of bowel fill with enteric contrast medium.
. There is no evidence of bowel obstruction, as more distal segments of bowel fill with enteric contrast medium.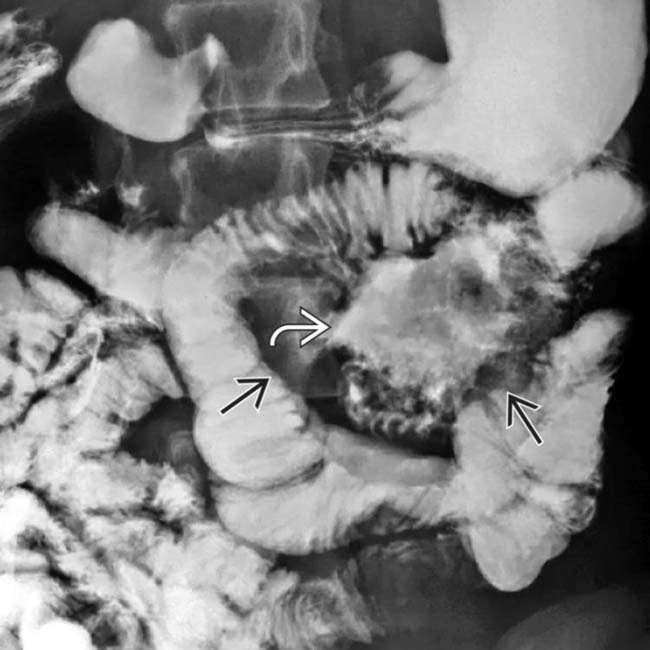
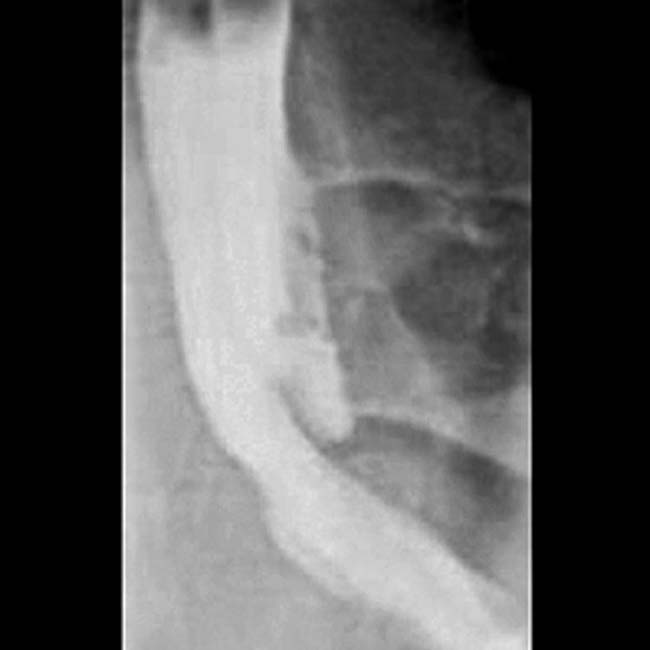
 with displaced bowel loops. The cavitated lumen of the involved jejunal segment
with displaced bowel loops. The cavitated lumen of the involved jejunal segment  is evident as a collection of barium lacking any sign of intact mucosa.
is evident as a collection of barium lacking any sign of intact mucosa.