Chapter 71 Polypoidal Choroidal Vasculopathy
Introduction
PCV is more common in non-white populations (including blacks, Hispanics, and Asians).1 The incidence of PCV in Chinese and Japanese patients in exudative AMD has been reported to be much higher than in Caucasians.2–7 The true prevalence and consequences may be underestimated if indocyanine green angiography (ICGA) is not performed. The widespread availability of ICGA has improved the diagnosis of PCV.
Polypoidal choroidal vasculopathy was first described as polypoidal, subretinal, vascular lesions associated with serous and hemorrhagic detachments of the retinal pigment epithelium (RPE) in a series of patients (10/11 were women) by Yannuzzi et al. at the Annual Meeting of the American Academy of Ophthalmology in 1982.8 The entitity was initially called idiopathic polypoidal choroidal vasculopathy (IPCV). Kleiner et al. in 19849 described a peculiar hemorrhagic disorder of the macula, characterized by recurrent subretinal and subretinal pigment epithelium bleeding in middle-aged black women, which they termed posterior uveal bleeding syndrome (PUBS).10 Later, a study from the same group of authors showed an expanded clinical spectrum for PCV, affecting various ages, both genders, and several racial populations.11 For the remainder of the chapter, I will use the PCV nomenclature; the IPCV and PUBS terms are mentioned for historical benefit.
The past decade has witnessed dramatic improvements in the understanding of exudative maculopathy and recognition of the importance of PCV, especially in the Asia–Pacific regions. A PCV Roundtable meeting panel of experts suggest that PCV is defined angiographically as the presence of single or multiple focal nodular areas of hyperfluorescence arising from the choroidal circulation within the first 6 minutes after injection of indocyanine green, with or without an associated choroidal interconnecting vascular network. The presence of orange–red subretinal nodules with corresponding indocyanine green hyperfluorescence is pathognomonic of PCV.12 The implication of the definition is obvious but bears repetition. The definition of PCV relies on ICGA. Retina specialists who do not generally utilize ICGA tend not to diagnose PCV. Later in the chapter, there is a clinical definition of PCV as well.
Pathogenesis
PCV is regarded as a primary abnormality of the choroidal circulation, characterized by an inner choroidal vascular network of vessels ending in an aneurysmal bulge or outward projection, often visible as a reddish-orange, spheroid, polyp-like structure. PCV primarily involves the inner choroidal vasculature that is well differentiated from the middle and larger choroid vessels by histology.13
Histopathological features have been reported by Kuroiwa from surgically excised specimens from five patients with PCV, who had been diagnosed by ICGA. The results indicated that arteriosclerosis appears to be an important pathological feature in the choroidal vessels of PCV subjects.14 In another histopathologic report, by Nakashizuka et al., who examined specimens surgically extracted from five eyes of five PCV patients, the pathologic findings revealed little granulation tissue formation in any of the specimens; on the other hand, all the specimens exhibited a massive exudative change and leaking, all the vessels exhibited hyalinization, and choriocapillaris had disappeared, even in the cases in which RPE had been preserved.15 This group also demonstrated by immunohistochemistry that PCV is not the same as choroidal neovascularization (CNV). CD-34 is a marker of vascular endothelial expression and CD-34 staining revealed discontinuity in the vascular endothelium, smooth muscle actin (SMA) was negative in hyalinized vessels, and there was disruption and injury of smooth muscle cells causing dilation of vessels. VEGF antibody was negative in vascular endothelial cells. These histopathologic findings indicated that hyalinization of choroidal vessels, like arteriosclerosis, was characteristic of PCV.15
The genetic studies with CNV and PCV have been controversial in published papers.16,17 Recently, we investigated the relationship between CNV of AMD and PCV by performing a series of meta-analyses. We found that many genes have common associations with PCV and CNV. For example, the pooled odds ratio (OR) between SNP rs10490924 (TT : GG, within the ARMS2 gene, previously identified as LOC387715)18 and CNV is 4.23 (95% CI 3.535.06), while the pooled OR between this SNP and PCV is 5.13 (95%CI 3.40–7.75) (data unpublished). SNP rs9332739 within complement factor 3 (C3) gene also have a common association with CNV (GG : CC, pooled OR 2.12, 95%CI 1.81–2.47) and PCV (pooled OR 3.52, 95%CI 1.43–8.69). Moreover, a similar trend was found in complement factor H (CFH, rs1061170, CC : TT) and SERPING1 (C1 inhibitor, rs2511989 GG : AA). The elastin gene identified by Kondo et al. in 2008 was shown to disrupt the elastic area of the Bruch’s membrane. He found that a common elastin gene (ELN) variant was significantly associated with susceptibility to PCV.19
Furthermore, a series of meta-analyses was performed to investigate the pooled OR between the risk factors for CNV and PCV. We found that CNV and PCV have many common risk factors, such as smoking and diabetes. For example, the pooled OR between smoking and CNV is 1.78 (95%CI 1.52–2.09),20 while the pooled OR between smoking and PCV is 1.51 (95% CI 1.06–2.16, data unpublished). The pooled OR between diabetes and CNV is 1.66 (95% CI 1.05–2.63),20 while the pooled OR between diabetes and PCV is 1.94 (95% CI 1.29–2.92, data unpublished). However, the pooled OR between hypertension and CNV is 1.02 (95% CI 0.77–1.35),20 while the pooled OR between hypertension and PCV is 1.60 (95% CI 1.17–2.18, data unpublished). Therefore, hypertension is only associated with PCV. This is consistent with pathology findings: the PCV lesion has thickened and hyalinized vessel walls, which is similar to that seen in hypertension.21
Clinical features
Demographics
The prevalence of PCV in presumed neovascular AMD was reported as 7.8% in US,1 4.0% in Belgian,22 9.8% in Italian,23 8.2% in Greeek.24 23.0–54.7% in Japanese,25 22.3% in Chinese,26 and 24.6%27 in Korean populations. The prevalence varies with age.28 In summary, PCV is more prevalent in blacks, Japanese and other Asians than in whites, while the incidence of AMD is very high in whites, and low in blacks. The incidence of both diseases is high in Asians.
Although early reports suggested that PCV was a condition predominantly of middle-aged women,11 and that typically PCV presents one to two decades earlier than classical AMD, it is most commonly diagnosed in patients between the ages of 50 and 65.29 However, the age of the subjects was reported with a mean of 66.1 ± 9.6 years in a Chinese population.28 Caucasian patients usually present with PCV at an older age.30 It has subsequently been established that PCV occurs in both genders (and more commonly in Asian men than women).27 Although women are involved more often than men in some reports, more men still have manifestations of the disorder among Chinese patients.26,28,30,31 PCV is usually a bilateral disease. The majority of the patients with evidence of PCV in one eye eventually develop similar lesions in the fellow eye.
The natural course of PCV is variable: it may be relatively stable or there may be repeated bleeding and leakage with vision loss and chorioretinal atrophy, with or without fibrotic scarring. Reddish-orange nodules alone, or nodules and small subretinal hemorrhage and absence of hard exudates, may still allow a benign clinical course and stable vision.32,33
The association of PCV with other conditions is not certain. PCV with severe thrombocytopenia and massive hemorrhage has been reported,34 and PCV has also been associated with sickle cell disease and irradiation.35 Some experts believe it is advisable to screen PCV patients for hypertension and platelet count, but hypertension is common in older patients and is already monitored during medical visits, and the platelet data are not firmly established. The role of hereditary and environmental factors in its etiology is inconclusive and needs further study.
Clinical findings
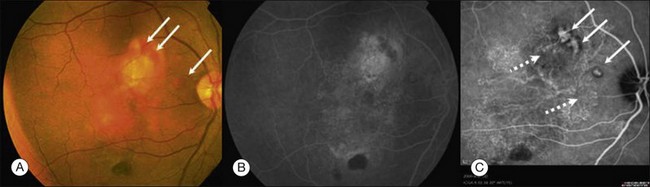
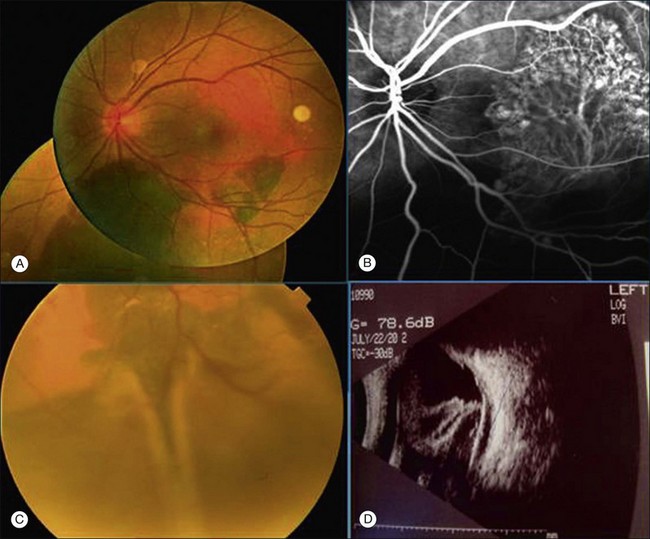
Clinically, PCV is characterized by protruding orange-red elevated lesions, often with nodular elevations of the RPE, that can be seen during routine fundus examination using ophthalmoscopy and contact lens slit-lamp biomicroscopy. The nodular elevations of the RPE are easily visible by optical coherence tomography (OCT). PCV is also characterized by polypoidal lesions only observed when using ICGA. The lesions appear as polyp-like or grape-like clusters (Fig. 71.1).11,15 The nodular lesions are associated with serous exudation and hemorrhage that may lead to detachment of the RPE and sometimes the neurosensory retina.32 Associated features are recurrent subretinal hemorrhage and vitreous hemorrhage (Fig. 71.2).
Polypoidal lesions are located mainly in the macular area, although this may be ascertainment bias since ICGA tends to look at that area. In one report, 69.5% polypoidal lesions were located in the macular area, 15% PCV lesions were located under the temporal retinal vascular arcade, and 4.5% PCV lesions were located peripapillary (within one disc diameter of the disc edge). PCV lesions were also located in the midperipheral area.28
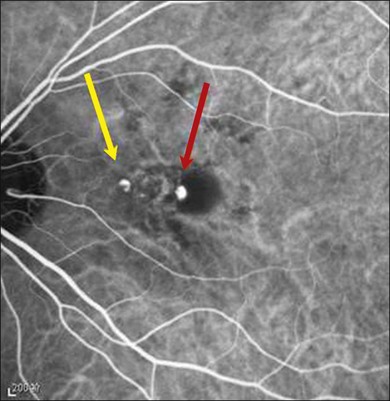
PCV lesions may be active or inactive (Fig. 71.3). PCV is considered as active if there is clinical, OCT or fluorescein angiography (FA) evidence of any one of the following: vision loss of 5 or more letters (ETDRS chart); subretinal fluid with or without intraretinal fluid; pigment epithelial detachment; subretinal hemorrhage; or fluorescein leakage (Box 71.1). There are currently no universally recognized criteria for defining disease activity in PCV; treatment should be initiated for active and symptomatic PCV and can be considered for active, asymptomatic PCV.12
Box 71.1
Characteristics of active PCV
Choroidal vascular hyperpermeability, reportedly a characteristic finding in central serous chorioretinopathy (CSC), might play a role in the pathogenesis of PCV. PCV with angioid streaks secondary to pseudoxanthoma elasticum has also been reported in one patient.36,37
Some patients with PCV may develop CNV. PCV can induce ischemic changes, inflammation, sick RPE and breaks in Bruch’s membrane. These changes can contribute to the development of CNV, and fibrosis and scarring may ensue.38
Angiographic features
PCV is better visualized by ICGA than by FA, because indocyanine green absorbs and emits near-infrared light, which readily penetrates the RPE, enhancing viewing of choroidal lesions. Also the binding affinity of indocyanine green to plasma proteins means that it does not leak from the choriocapillaris in the same way as fluorescein, so choroidal lesions are less obscured.39 PCV primarily involves the inner choroidal vasculature.22,40 In recent years ICGA has been accepted as the gold standard for the diagnosis of PCV, and as one of the specific criteria to distinguish PCV, retinal angiomatous proliferation, and typical AMD as types of neovascular AMD.3 The ICGA characteristics of PCV (summarized in Box 71.2) include: a branching network of inner choroidal vessels,3,21,40–43 and nodular polypoidal aneurysms or dilations at the edge of these abnormal vessel networks, which correspond to orange subretinal nodules;3,11,20,31,32,40–42 and the presence of single or multiple focal nodular areas of hyperfluorescence (hypofluorescent halo, see Figure 71.1C) arising from the choroidal circulation within the first 6 minutes. Pulsation in polyps and/or associated vasculature has been less extensively reported and can be observed only using video ICGA.44,45
Classification
PCV was classified by the Japanese Study Group on PCV.42 This panel of experts proposed three categories to subclassify PCV:12
• Quiescent: polyps in the absence of subretinal or intraretinal fluid or hemorrhage.
• Exudative: exudation without hemorrhage, which may include sensory retinal thickening, neurosensory detachment, PED, and subretinal lipid exudation.
• Hemorrhagic: any hemorrhage with or without other exudative characteristics.
Differential diagnosis
Neovascular age-related macular degeneration
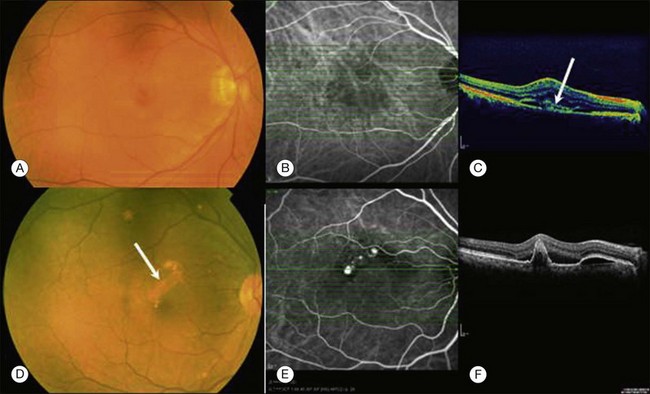
Some authors categorize PCV as a subtype of neovascular AMD.3 PCV has been considered to be part of or similar to AMD CNV due to similar features (Fig. 71.4A), elevated levels of vascular endothelial growth factor (VEGF), similar histology, and expression of growth factors and receptor antibodies. We view PCV as a distinct condition. It has been documented that the increased levels of VEGF in PCV are lower compared with AMD CNV or myopic CNV. The increase in VEGF levels in PCV is mild to moderate. The clinical features of the PCV are different from those of neovascular age-related macular degeneration. PCV is associated with polyp-like structures projecting from the plane of the inner choroid towards the outer retina or RPE, the RPE is mostly intact, while in neovascular AMD the neovascular tufts arising from the choroid may either invade and perforate Bruch’s membrane or grow throw defects in Bruch’s membrane and proliferate in either sub-RPE space (type I CNV) or in the subsensory retinal space (type II CNV).46 The ingrowth of new vessels extending from the choroid into the sub-RPE space is the most important histopathologic change of occult CNV (Fig. 71.4B).
OCT findings (Fig. 71.4C and F) can assist in understanding the differences in the retinal structures that are affected by these two clinical entities and also may help in the differential diagnosis of PCV and neovascular AMD.13
Central serous chorioretinopathy
Central serous chorioretinopathy (CSC) is characterized by accumulation of transparent fluid and round, serous detachment of the macular retina. It has been thought to be due to a focal leakage from one or more defects at the level of the RPE, which allow serous fluid from the choriocapillaris to diffuse into the subretinal space. It has been hypothesized that a protracted disturbance in the microcirculation of the choriocapillaris leads to leakage of fluid into the sub-RPE space. A combination of choroidal hyperpermeability and impaired RPE function leads to pooling of fluid in the sub-RPE space with eventual leakage through the RPE into the subretinal space. ICGA has expanded our knowledge of CSC. A consistent finding is the hyperpermeability of the choroid during ICGA in CSC. The multifocal choroid hyperfluorescence patches in the ICG demonstrate multifocal choroid vascular hyperpermeable areas.41
PCV masquerading as CSC has been reported. In CSC with persistent and/or recurrent exudation, a myriad of retinal pigment epithelial changes may evolve that make it difficult to differentiate it from PCV. In such patients, ICGA may be useful in differentiating these two entities. An accurate clinical diagnosis is important since CSC and PCV differ in terms of their risk factors, natural course, visual prognosis and treatment.47
Treatment
Thermal laser photocoagulation
Laser photocoagulation has been suggested to be beneficial, albeit it with short-term follow-up. The greatest benefit may be for extrafoveal PCV.48 In some studies that analyzed reported vision outcomes, ICGA-guided laser photocoagulation was successful in stabilizing or improving vision in 55–100% of eyes; however, vision loss occurred in 13–45% of eyes.31,48–50 Photocoagulation of the whole lesion, compared with polyps only, appears to be more efficacious.49
Photodynamic therapy
Verteporfin photodynamic therapy (PDT) causes regression or resolution of polyps by its angio-occlusive mechanism of action. It has been shown to achieve complete occlusion of polyps and resolution of exudative changes after less than three treatments, restricts loss of letters on the ETDRS chart to less than 15, or improved vision in 80–100% of patients after 1 year.51,52 For treatment-naïve patients the entire PCV lesion as indicated by ICGA (polyps plus the BVN) should be treated.
Common complications reported with verteporfin-PDT therapy are subretinal hemorrhage, recurrence of PCV with leakage from the BVN, and fibrous scarring. Subretinal hemorrhage is common and can lead to vitreous hemorrhage and consequently a poor outcome.51,52 Larger lesion size and a leaking vascular net51 are risk factors for bleeding after PDT.
Anti-VEGF therapy
Recent studies have demonstrated that anti-VEGF agents are useful in the treatment of CNV in neovascular AMD. Since increased VEGF levels have been observed in PCV patients,45, anti-VEGF therapy might be theoretically beneficial for treating PCV. It was shown that intravitreal anti-VEGF helped in resolving the macular edema, polypoidal complexes decreased in 4/12 (33%) eyes by intravitreal ranibizumab,53 and 1/11 (9.09%) eyes by intravitreal bevacizumab.54
Combination therapy
EVEREST is a multicenter, double-masked, ICGA-guided randomized controlled trial studying patients with symptomatic PCV. Eyes were treated with verteporfin PDT monotherapy, 0.5 mg ranibizumab monotherapy or a combination of these treatments. Both combination therapy and verteporfin PDT monotherapy were superior to ranibizumab monotherapy in achieving complete polyp regression at month 6. Improvements in best-corrected visual acuity (BCVA) and central retinal thickness (CRT) also favored combination therapy.12
The authors of a recent relatively large comparative case series reported that with combination verteporfin PDT plus bevacizumab there were better early BCVA outcomes than with verteporfin PDT alone (P = 0.0016 for the difference between treatments in mean BCVA change from baseline at 3 months and P = 0.048 at 12 months).52 Combination therapy also decreased the rate of PDT-related hemorrhages compared with verteporfin PDT monotherapy (3/61 [4.9%] vs 15/85 [17.6%], respectively), but did not impact the resolution and recurrence of lesions.55
1 Yannuzzi LA, Wong DW, Sforzolini BS, et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol. 1999;117:1503–1510.
2 Wen F, Chen C, Wu D, et al. Polypoidal choroidal vasculopathy in elderly Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2004;242:625–629.
3 Maruko I, Iida T, Saito M, et al. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthal. 2007;144:15–22.
4 Hwang DK, Yang CS, Lee FL, et al. Idiopathic polypoidal choroidal vasculopathy. J Chin Med Assoc. 2007;70:84–88.
5 Liu Y, Wen F, Huang S, et al. Subtype lesions of neovascular age-related macular degeneration in Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2007;245:1441–1445.
6 Yi C, Ou J, Yian H, et al. A case report of 360 idiopathic polypoidal choroidal vasculopathy. Yan ke xue bao/Eye Science. 2001;17:126–129.
7 Japanese Study Group of Polypoidal Choroidal Vasculopathy. [Criteria for diagnosis of polypoidal choroidal vasculopathy.]. Nippon Ganka Gakkai Zasshi. 2005;109:417–427.
8 Yannuzzi LA. Idiopathic polypoidal choroidal vasculopathy. Presented at the February 1982 Macula Society Meeting, Miami, Florida.
9 Kleiner RC, Bruckner AJ, Johnson RL. Posterior uveal bleeding syndrome. Ophthalmology. 1984;91:110.
10 Kleiner RC, Bruckner AJ, Johnson RL. The posterior uveal bleeding syndrome. Retina. 1990;10:9–17.
11 Yannuzzi LA, Sorenson J, Spaide RF, et al. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10:1–8.
12 PCV Roundtable Participants. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Submitted to Retina.
13 Ozawa S, Ishikawa K, Ito Y. Differences in macular morphology between polypoidal choroidal vasculopathy and exudative age-related macular degeneration detected by optical coherence tomography. Retina. 2009;29:793–802.
14 Kuroiwa S, Tateiwa H, Hisatomi T, et al. Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin Exp Ophthalmol. 2004;32:297–302.
15 Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49:4729–4737.
16 Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389.
17 Dewan A, Liu M, Hartman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–992.
18 Kondo N, Honda S, Ishibashi K, et al. LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population. Am J Ophthalmol. 2007;144:608–612.
19 Kondo N, Honda S, Ishibashi K, et al. Elastin gene polymorphisms in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49:1101–1105.
20 Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31.
21 Nakashizuka H, Yuzawa M. Hyalinization of choroidal vessels in polypoidal choroidal vasculopathy. Surv Ophthalmol. 2011;56:278–279. author reply 279
22 Lafaut BA, Leys AM, Snyers B, et al. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000;238:752–759.
23 Scassellati-Sforzolini B, Mariotti C, Bryan R, et al. Polypoidal choroidal vasculopathy in Italy. Retina. 2001;21:121–125.
24 Ladas ID, Rouvas AA, Moschos MM, et al. Polypoidal choroidal vasculopathy and exudative age-related macular degeneration in Greek population. Eye (Lond). 2004;18:455–459.
25 Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003;121:1392–1396.
26 Wen F, Chen C, Wu D, et al. Polypoidal choroidal vasculopathy in elderly Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2004;242:625–629.
27 Byeon SH, Lee SC, Oh HS, et al. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol. 2008;52:57–62.
28 Hou J, Tao Y, Li XX, et al. Clinical characteristics of polypoidal choroidal vasculopathy in Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2011;249:975–979.
29 Ciardella AP, Donsoff IM, Yannuzzi LA. Polypoidal choroidal vasculopathy. Ophthalmol Clin North Am. 2002;15:537–554.
30 Gomi F, Sawa M, Wakabayashi T, et al. Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2010;150:48–54.
31 Kwok AK, Lai TY, Chan CW, et al. Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol. 2002;86:892–897.
32 Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002;133:639–648.
33 Okubo A, Arimura N, Abematsu N, et al. Predictable signs of benign course of polypoidal choroidal vasculopathy: based upon the long-term observation of non-treated eyes. Acta Ophthalmol. 2010;88:107–114.
34 Lip PL, Hope-Ross MW, Gibson JM. Idiopathic polypoidal choroidal vasculopathy: a disease with diverse clinical spectrum and systemic associations. Eye (Lond). 2000;14(Pt 5):695–700.
35 Smith RE, Wise K, Kingsley RM. Idiopathic polypoidal choroidal vasculopathy and sickle cell retinopathy. Am J Ophthalmol. 2000;129:544–546.
36 Sasahara M, Tsujikawa A, Musashi K, et al. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006;142:601–607.
37 Baillif-Gostoli S, Quaranta-El Maftouhi M, Mauget-Fa?sse M. Polypoidal choroidal vasculopathy in a patient with angioid streaks secondary to pseudoxanthoma elasticum. Graefes Arch Clin Exp Ophthalmol. 2010;248:1845–1848.
38 Chen Yanli, Wen Feng, Sun Zuhua, Wu Dezheng. Polypoidal choroidal vasculopathy coexisting with exudative age-related macular degeneration. Int Ophthalmol. 2008;28:119–123.
39 Desmettre T, Devoisselle JM, Mordon S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv Ophthalmol. 2000;45:15–27.
40 Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2005;89:602–607.
41 Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16:203–213.
42 Japanese Study Group of Polypoidal Choroidal Vasculopathy. [Criteria for diagnosis of polypoidal choroidal vasculopathy.]. Nippon Ganka Gakkai Zasshi. 2005;109:417–427.
43 Costa RA, Navajas EV, Farah ME, et al. Polypoidal choroidal vasculopathy: angiographic characterization of the network vascular elements and a new treatment paradigm. Prog Retin Eye Res. 2005;24:560–586.
44 Okubo A, Hirakawa M, Ito M, et al. Clinical features of early and late stage polypoidal choroidal vasculopathy characterized by lesion size and disease duration. Graefes Arch Clin Exp Ophthalmol. 2008;246:491–499.
45 Byeon SH, Lew YJ, Lee SC, et al. Clinical features and follow-up results of pulsating polypoidal choroidal vasculopathy treated with photodynamic therapy. Acta Ophthalmol. 2010;88:660–668.
46 Gass JDM. Biomicroscopic and histopatholic considerations regarding the feasibility of surgical excision of subfoveal neovascular membrane. Am J Ophthalmol. 1994;118:285–298.
47 Yannuzzi LA, Freund KB, Goldbaum M, et al. Polypoidal choroidal vasculopathy masquerading as central serous chorioretinopathy. Ophthalmology. 2000;107:767–777.
48 Lee MW, Yeo I, Wong D, Ang CL. Argon laser photocoagulation for the treatment of polypoidal choroidal vasculopathy. Eye (Lond). 2009;23:145–148.
49 Yuzawa M, Mori R, Haruyama M. A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003;47:379–384.
50 Nishijima K, Takahashi M, Akita J, et al. Laser photocoagulation of indocyanine green angiographically identified feeder vessels to idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2004;137:770–773.
51 Hirami Y, Tsujikawa A, Otani A, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27(3):335–341.
52 Chan WM, Lam DS, Lai TY, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology. 2004;111:1576–1584.
53 Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010;94:297–301.
54 Gomi F, Ohji M, Sayanagi K, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008;115:141–146.
55 Gomi F, Sawa M, Wakabayashi T, et al. Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2010;150:48–54.