Chapter 241 Polioviruses
Epidemiology
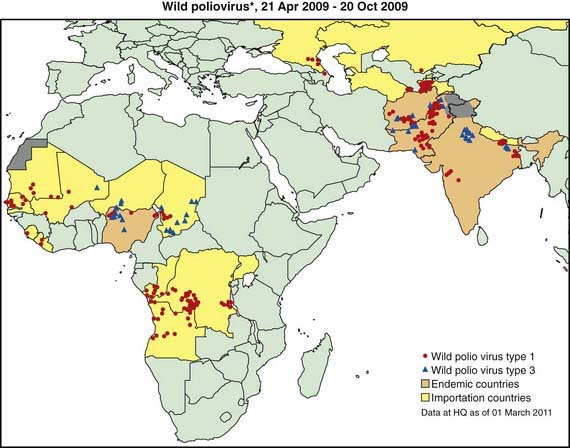
The most devastating result of poliovirus infection is paralysis, although 90-95% of infections are inapparent but induce protective immunity. Clinically apparent but nonparalytic illness occurs in about 5% of all infections, with paralytic polio occurring in about 1/1,000 infections among infants to about 1/100 infections among adolescents. In developed countries prior to universal vaccination, epidemics of paralytic poliomyelitis occurred primarily in adolescents. Conversely, in developing countries with poor sanitation, infection early in life results in infantile paralysis. Improved sanitation explains the virtual eradication of polio from the USA in the early 1960s, when only about 65% of the population was immunized with the Salk vaccine, which contributed to the disappearance of circulating wild-type poliovirus in the USA and Europe. Poor sanitation and crowding have permitted the continued transmission of poliovirus in certain poor countries in Africa and Asia, despite massive global efforts to eradicate polio, which in some areas involve an average of 12-13 doses of polio vaccine administered to children in the 1st 5 yr of life (Fig. 241-1).
Differential Diagnosis
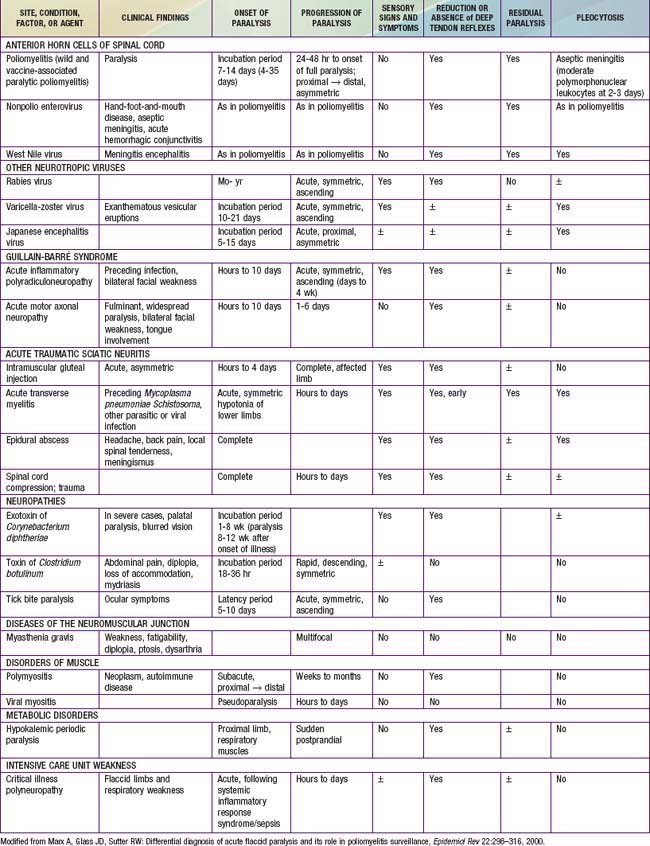
Poliomyelitis should be considered in the differential diagnosis of any case of paralysis, and is only 1 of many causes of acute flaccid paralysis in children and adults. There are numerous other causes of acute flaccid paralysis (Table 241-1). In most conditions, the clinical features are sufficient to differentiate between these various causes, but in some cases nerve conduction studies and electromyograms in addition to muscle biopsies may be required.
Prevention
Several countries are global priorities because they face challenges in eradication of the disease (see Fig. 241-1). Polioviruses are endemic in India, Pakistan, Afghanistan, and Nigeria. The Global Polio Eradication Initiative began in 1988. By 2006, transmission of virulent polio virus type 2 had been interrupted globally and transmission of indigenous type 1 and type 3 (WPV1 and WPV3) had been interrupted in all but four countries world wide (Afghanistan, India, Nigeria and Pakistan). During 2002-2006, 20 previously polio-free countries were infected by importations of WPV1 originating from Nigeria, and 3 polio-free African countries by WPV1 imported from India. Relatively few importations occurred in 2007, but during 2008-2009 additional WPV1 and WPV3 occurred in 15 countries in Africa, including 5 that had interrupted outbreaks resulting from earlier outbreaks (see Fig. 241-1). In April 2010, Tajikistan reported an outbreak of poliomyelitis in 20 districts with 458 cases. This strain (WPV1 from northern India) has spread to other central Asian countries and to the Russian Federation.
Centers for Disease Control and Prevention. Progress toward interrupting wild poliovirus circulation in countries with reestablished transmission—Africa, 2009-2010. MMWR. 2011;60(10):306-311.
Centers for Disease Control and Prevention. Wild poliovirus type 1 and type 3 importations—15 countries, Africa, 2008–2009. MMWR Morb Mortal Wkly Rep. 2009;58:357-360.
Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission—worldwide, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:545-550.
Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses—worldwide, January 2008–June 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1002-1006.
Centers for Disease Control and Prevention. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding routine poliovirus vaccination. MMWR Morb Mortal Wkly Rep. 2009;58:829-830.
Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—India, January 2007–May 2009. MMWR Morb Mortal Wkly Rep. 2009;58:719-723.
Ehrenfeld E, Glass RI, Agol VI, et al. Immunization against poliomyelitis: moving forward. Lancet. 2008;371:1385-1387.
el-Sayed N, el-Gamal Y, Abbassy AA, et al. Monovalent type 1 oral poliovirus vaccine in newborns. N Engl J Med. 2008;359:1655-1665.
Grassly NC, Jafari H, Bahl S, et al. Mucosal immunity after vaccination with monovalent and trivalent oral poliovirus vaccine in India. J Infect Dis. 2009;200:794-801.
Grassly NC, Fraser C, Wenger J, et al. New strategies for the elimination of polio from India. Science. 2006;314:1150-1153.
Jenkins HE, Aylward RB, Gasasira A, et al. Effectiveness of immunization against paralytic poliomyelitis in Nigeria. N Engl J Med. 2008;359:1666-1674.
Jenkins HE, Aylward RB, Gasasira A, et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N Engl J Med. 2010;362:2360-2368.
Modlin JF. The bumpy road to polio eradication. N Engl J Med. 2010;362:2346-2349.
Mohammed AJ, AlAwaidy S, Bawikar S, et al. Fractional doses of inactivated poliovirus vaccine in Oman. N Engl J Med. 2010;362:2351-2359.