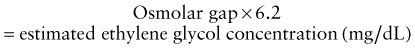Chapter 58 Poisonings
More than 85% of poisoning exposures in children <6 yr can be managed without direct medical intervention, either because the product involved is not inherently toxic or the quantity of the material involved is not sufficient to produce clinically relevant toxic effects (Table 58-1). However, a number of substances are potentially highly toxic to toddlers in small doses (Table 58-2). Fatalities often result from carbon monoxide, iron, analgesics, hydrocarbons, cardiovascular drugs, antidepressants, and pesticides. Although the majority of exposures occur in children <6 yr, only 2.8% of the reported deaths occur in this age group. In addition to the exploratory nature of ingestions in young children, product safety measures, poison prevention education, early recognition of exposures, and around-the-clock access to regionally based poison control centers all contribute to the favorable outcomes in this age group.
Table 58-1 COMMON NONTOXIC AND MINIMALLY TOXIC* PRODUCTS
* The potential for toxicity depends on the magnitude and amount of exposure. These agents are considered nontoxic or minimally toxic for mild to moderate exposure. The potential for toxicity increases with increased amount of exposure.
Table 58-2 MEDICATIONS POTENTIALLY TOXIC TO YOUNG CHILDREN IN SMALL DOSES*
| SUBSTANCE | TOXICITY |
|---|---|
| Antimalarials (chloroquine, quinine) | Seizures, cardiac arrhythmias |
| Benzocaine | Methemoglobinemia |
| β-Blockers (lipid-soluble β-blockers [e.g., propranolol] are more toxic than water-soluble β-blockers [e.g., atenolol]) | Bradycardia, hypotension, hypoglycemia |
| Calcium channel blockers | Bradycardia, hypotension, hyperglycemia |
| Camphor | Seizures |
| Clonidine | Lethargy, bradycardia, hypotension |
| Diphenoxylate and atropine (Lomotil) | CNS depression, respiratory depression |
| Hypoglycemics, oral (sulfonylureas and meglitinides) | Hypoglycemia, seizures |
| Lindane | Seizures |
| Monoamine oxidase Inhibitors | Hypertension followed by delayed cardiovascular collapse |
| Methyl salicylates | Tachypnea, metabolic acidosis, seizures |
| Opioids (especially methadone, lomotil and suboxone) | CNS depression, respiratory depression |
| Phenothiazines (chlorpromazine, hioridazine) | Seizures, cardiac arrhythmias |
| Theophylline | Seizures, cardiac arrhythmias |
| Tricyclic antidepressants | CNS depression, seizures, cardiac arrhythmias, hypotension |
CNS, central nervous system.
* “Small dose” typically implies 1 or 2 pills or 5 mL.
Poisoning exposures in children 6-12 yr old are much less common, involving only ~ 6% of all reported pediatric exposures. A second peak in pediatric exposures occurs in adolescence. Exposures in the adolescent age group are primarily intentional (suicide or abuse or misuse of substances) and thus often result in more severe toxicity (see Chapter 108). Families should be informed and given anticipatory guidance that over-the-counter (OTC) and prescription medications and even household products (e.g., inhalants) are common sources of adolescent exposures. Adolescents (ages 13-19 yr) accounted for 56 of the 102 reported poison-related pediatric deaths in 2007. Pediatricians should be aware of the signs of drug abuse or suicidal ideation in this population and should aggressively intervene (Chapter 108).
Approach to the Poisoned Patient
The initial approach to the patient with a witnessed or suspected poisoning should be no different than that in any other sick child, starting with stabilization and rapid assessment of the airway, breathing, circulation, and mental status (Chapter 62). A serum dextrose concentration should be obtained early in the evaluation of any patient with altered mental status. A targeted history and physical examination serves as the foundation for a thoughtful differential diagnosis, which can then be further refined through laboratory testing and other diagnostic studies.
Initial Evaluation
History
Symptoms
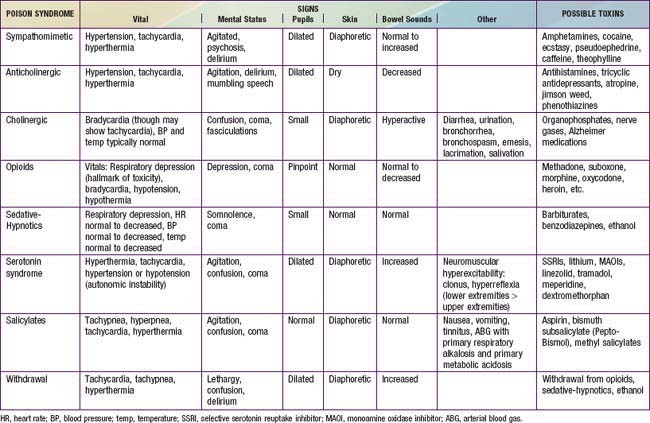
Obtaining a description of symptoms experienced after ingestion, including their timing of onset relative to the time of ingestion and their progression, can help to generate a list of potential toxins and to predict the severity of the ingestion. Coupled with physical exam findings, reported symptoms assist practitioners in identifying toxidromes or recognized poisoning syndromes suggestive of poisoning from specific substances or classes of substances (Tables 58-3 and 58-4).
| SIGN | TOXIN |
|---|---|
| ODOR | |
| Bitter almonds | Cyanide |
| Acetone | Isopropyl alcohol, methanol, paraldehyde, salicylates |
| Alcohol | Ethanol |
| Wintergreen | Methyl salicylate |
| Garlic | Arsenic, thallium, organophosphates, selenium |
| OCULAR SIGNS | |
| Miosis | Opioids (except propoxyphene, meperidine, and pentazocine), organophosphates and other cholinergics, clonidine, phenothiazines, sedative-hypnotics, olanzapine |
| Mydriasis | Atropine, cocaine, amphetamines, antihistamines, TCAs, carbamazepine, serotonin syndrome, PCP, LSD, post-anoxic encephalopathy |
| Nystagmus | Phenytoin, barbiturates, sedative-hypnotics, alcohols, carbamazepine, PCP, ketamine, dextromethorphan |
| Lacrimation | Organophosphates, irritant gas or vapors |
| Retinal hyperemia | Methanol |
| CUTANEOUS SIGNS | |
| Diaphoresis | Organophosphates, salicylates, cocaine and other sympathomimetics, serotonin syndrome, withdrawal syndromes |
| Alopecia | Thallium, arsenic |
| Erythema | Boric acid, elemental mercury, cyanide, carbon monoxide, disulfuram, scombroid, anticholinergics |
| Cyanosis (unresponsive to oxygen) | Methemoglobinemia (e.g., benzocaine, dapsone, nitrites, phenazopyridine), amiodarone, silver |
| ORAL SIGNS | |
| Salivation | Organophosphates, salicylates, corrosives, ketamine, PCP, strychnine |
| Oral Burns | Corrosives, oxalate-containing plants |
| Gum lines | Lead, mercury, arsenic, bismuth |
| GASTROINTESTINAL SIGNS | |
| Diarrhea | Antimicrobials, arsenic, iron, boric acid, cholinergics, colchicine, withdrawal |
| Hematemesis | Arsenic, iron, caustics, NSAIDs, salicylates |
| CARDIAC SIGNS | |
| Tachycardia | Sympathomimetics (e.g., amphetamines, cocaine), anticholinergics, antidepressants, theophylline, caffeine, antipsychotics, atropine, salicylates, cellular asphyxiants (cyanide, carbon monoxide, hydrogen sulfide), withdrawal |
| Bradycardia | β-Blockers, calcium channel blockers, digoxin, clonidine and other central α2 agonists, organophosphates, opioids, sedative-hypnotics |
| Hypertension | Sympathomimetics (amphetamines, cocaine, LSD), anticholinergics, clonidine (early), monoamine oxidase inhibitors |
| Hypotension | β blockers, calcium channel blockers, cyclic antidepressants, iron, phenothiazines, barbiturates, clonidine, theophylline, opioids, arsenic, amatoxin mushrooms, cellular asphyxiants (cyanide, carbon monoxide, hydrogen sulfide), snake envenomation |
| RESPIRATORY SIGNS | |
| Depressed respirations | Opioids, sedative-hypnotics, alcohol, clonidine, barbiturates |
| Tachypnea | Salicylates, amphetamines, caffeine, metabolic acidosis (ethylene glycol, methanol, cyanide), carbon monoxide, hydrocarbons |
| CENTRAL NERVOUS SYSTEM SIGNS | |
| Ataxia | Alcohol, anticonvulsants, benzodiazepines, barbiturates, lithium, dextromethorphan, carbon monoxide, inhalants |
| Coma | Opioids, sedative-hypnotics, anticonvulsants, cyclic antidepressants, antipsychotics, ethanol, anticholinergics, clonidine, GHB, alcohols, salicylates, barbiturates |
| Seizures | Sympathomimetics, anticholinergics, antidepressants (especially TCAs, bupropion, venlafaxine), isoniazid, camphor, lindane, salicylates, lead, organophosphates, carbamazepine, tramadol, lithium, ginkgo seeds, water hemlock, withdrawal |
| Delirium/psychosis | Sympathomimetics, anticholinergics, LSD, PCP, hallucinogens, lithium, dextromethorphan, steroids, withdrawal |
| Peripheral neuropathy | Lead, arsenic, mercury, organophosphates |
PCP, phencyclidine; LSD, lysergic acid diethylamide; TCA, tricylic antidepressants; NSAID, nonsteroidal anti-inflammatory drug; GHB, gamma hydroxybutyrate.
Physical Examination
In the poisoned patient, the key features of the physical exam are the vital signs, mental status, pupils (size, reactivity, nystagmus), skin, bowel sounds, and odors. Together, these findings might suggest a toxidrome (see Tables 58-3 and 58-4) that can guide the differential diagnosis and initial management.
Laboratory Evaluation
Based on the clinical presentation, additional labs tests that may be helpful include electrolytes and renal function (an elevated anion gap suggests a number of ingestions), serum osmolarity (toxic alcohols), complete blood count, liver function tests, urinalysis (crystals), co-oximetry, and a serum creatine kinase level (Table 58-5).
Table 58-5 SCREENING LABORATORY CLUES IN TOXICOLOGIC DIAGNOSIS
ANION GAP METABOLIC ACIDOSIS (MNEMONIC = MUDPILES)
ELEVATED OSMOLAR GAP
Alcohols: ethanol, isopropyl, methanol, ethylene glycol
HYPOGLYCEMIA (MNEMONIC = HOBBIES)
HYPERGLYCEMIA
HYPOCALCEMIA
RHABDOMYOLYSIS
RADIOPAQUE SUBSTANCE ON KUB (MNEMONIC = CHIPPED)
KUB, kidney-ureter-bladder radiograph.
Additional Diagnostic Testing
An electrocardiogram (ECG) is a quick and noninvasive bedside test that can yield important clues to diagnosis and prognosis. Toxicologists pay particular attention to the ECG intervals (Table 58-6). A widened QRS interval suggests blockade of fast sodium channels, as may be seen after ingestion of tricyclic antidepressants, diphenhydramine, cocaine, propoxyphene, and carbamazepine, among others. A widened QTc interval suggests effects at the potassium rectifier channels and portends a risk of torsades de pointes.
Table 58-6 ELECTROCARDIOGRAPHIC FINDINGS IN POISONING
PR INTERVAL PROLONGATION
QRS PROLONGATION
QTc PROLONGATION*
SSRI, selective serotonin reuptake inhibitor.
* This is a select list of important toxins, but other medications are also associated with QTc prolongation.
Chest x-ray may reveal signs of pneumonitis (e.g., hydrocarbon ingestion), pulmonary edema (e.g., salicylate toxicity), or a foreign body. Abdominal x-ray can suggest the presence of a bezoar, demonstrate radiopaque tablets, or reveal drug packets in a body packer. Endoscopy may be useful after significant caustic ingestions. Further diagnostic testing is based on the differential diagnosis and pattern of presentation (Table 58-7).
Table 58-7 DRUGS ASSOCIATED WITH MAJOR MODES OF PRESENTATION
COMMON TOXIC CAUSES OF CARDIAC ARRHYTHMIA
CAUSES OF COMA
COMMON AGENTS CAUSING SEIZURES (MNEMONIC = CAPS)
* Causes of methemoglobinemia: amyl nitrite, aniline dyes, benzocaine, bismuth subnitrate, dapsone, primaquone, quinones, spinach, sulfonamides.
From Kliegman RM, Mascdante KJ, Jenson HB, editors: Nelson essentials of pediatrics, ed 5, Philadelphia, 2006, Elsevier, p 208.
Principles of Management
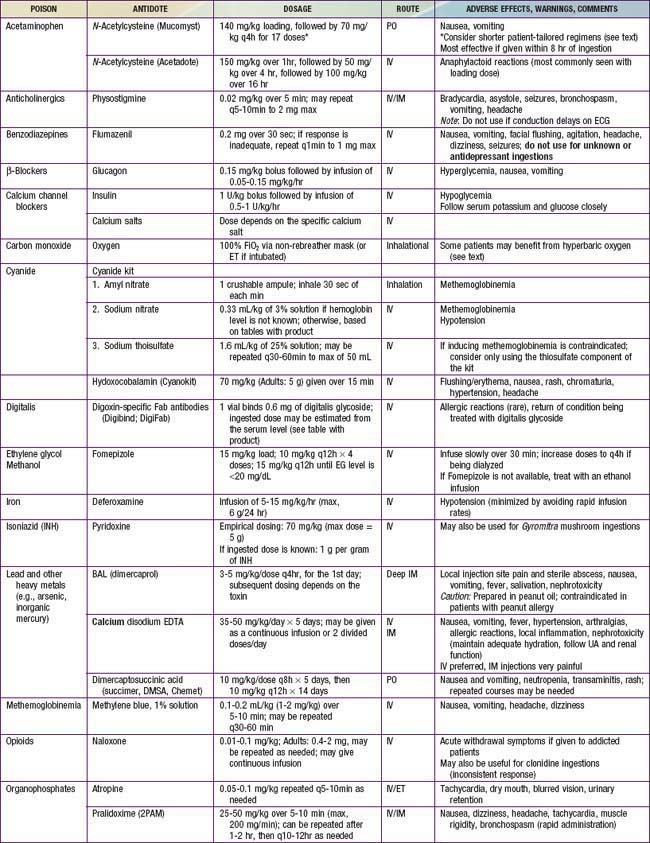
The four principles of management of the poisoned patient are decontamination, enhanced elimination, antidotes, and supportive care. Few patients meet criteria for all of these interventions, though clinicians should consider each option in every poisoned patient so as not to miss a potentially lifesaving therapy. Antidotes are available for relatively few poisons (Table 58-8), thus emphasizing the importance of meticulous supportive care and close clinical monitoring.
Decontamination
Syrup of Ipecac
Single-Dose Activated Charcoal
Of all the described modalities of gastric decontamination, activated charcoal is thought to potentially be the most useful, though clinical data to support this claim is somewhat limited. Charcoal is “activated” via heating to extreme temperatures, creating an extensive network of pores that provides a very large adsorptive surface area. Many, but not all, toxins are adsorbed onto its surface, thus preventing absorption from the GI tract. Charcoal is most likely to be effective when given within 1 hr of ingestion. Some toxins, including heavy metals, iron, lithium, hydrocarbons, cyanide, and low-molecular-weight alcohols, are not significantly bound to charcoal (Table 58-9). Charcoal administration should also be avoided after ingestion of a caustic substance, because the presence of charcoal can impede subsequent endoscopic evaluation.
Whole-Bowel Irrigation
Careful attention should be paid to assessment of the airway and abdominal exam before initiating WBI. Given the rate of administration and volume needed to flush the system, WBI is typically administered via a nasogastric tube. WBI is continued until the rectal effluent is clear. Complications of WBI include vomiting, abdominal pain, and abdominal distention. Bezoar formation might respond to WBI or might require endoscopy or surgery (Table 58-10).
Table 58-10 COMMON MEDICATIONS IMPLICATED IN BEZOAR FORMATION
ANTACIDS
Aluminum hydroxide
BULK-FORMING LAXATIVES
EXTENDED-RELEASE PRODUCTS
ION-EXCHANGE RESINS
VITAMIN AND NATURAL PRODUCTS
OTHER MEDICATIONS
Antidotes
Antidotes are available for relatively few toxins (Table 58-11, and see Table 58-8), but early and appropriate use of an antidote is a key element in managing the poisoned patient. Consensus guidelines indicate the important antidotes to stock in facilities that provide emergency care.
| ANTIDOTES | TOXIN OR POISON |
|---|---|
| Latrodectus antivenin | Black widow spider |
| Botulin antitoxin | Botulinum toxin |
| Glucagon and/or insulin and glucose | Calcium channel antagonists |
| Diphenhydramine and/or benztropine | Dystonic reactions |
| Calcium salts | Fluoride, calcium channel blockers |
| Protamine | Heparin |
| Folinic acid | Methotrexate, trimethoprim, pyrimethamine |
| Crotab-specific Fab antibodies | Rattlesnake envenomation |
| Sodium bicarbonate | Sodium channel blockade (tricyclic antidepressants, type 1 antiarrhythmics) |
Selected Compounds Commonly Involved in Pediatric Poisonings
Herbal medicines (Chapter 59), drugs of abuse (Chapter 108), and environmental health hazards (Chapters 699-706) are covered elsewhere.
Pharmaceuticals
Analgesics
Acetaminophen
Clinical and Laboratory Manifestations
Classically, four stages of acetaminophen toxicity have been described (Table 58-12). The initial signs of acetaminophen toxicity are nonspecific, including nausea and vomiting, and are often followed by an asymptomatic period. Thus, the diagnosis of acetaminophen toxicity cannot be based on clinical symptoms alone, but instead requires consideration of the combination of the patient’s history, symptoms, and laboratory findings.
Table 58-12 CLASSIC STAGES IN THE CLINICAL COURSE OF ACETAMINOPHEN TOXICITY
| STAGE | TIME AFTER INGESTION | CHARACTERISTICS |
|---|---|---|
| I | 0.5-24 hr |
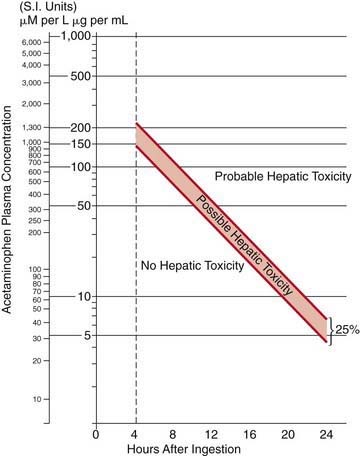
Any patient with a serum acetaminophen level in the possible or probable hepatotoxicity range per the Rumack-Matthew nomogram (see Fig. 58-1) should be treated with N-acetylcysteine (NAC). This nomogram is only intended for use in patients who present within 24 hr of a single acute acetaminophen ingestion with a known time of ingestion. Patients who have an initially nontoxic level and have ingested combination products or co-ingestants that can slow GI motility (e.g., diphenhydramine, opioids) should have a second acetaminophen level drawn 6-8 hr after ingestion to ensure that ongoing absorption in the setting of poor motility has not caused the acetaminophen level to cross the line into the possible or probable hepatotoxicity range.
Treatment
NAC is available in oral and intravenous forms, and both forms are equally efficacious (see Table 58-8 for the dosing regimens of the oral vs. IV form). The intravenous form is generally preferred, especially in patients with intractable vomiting, those with evidence of hepatic failure, and pregnant patients. NAC has an unpleasant taste and smell, and it should be mixed in soft drink or fruit juice or given via nasogastric tube to improve tolerability of the oral regimen. Administration of IV NAC (as a standard 3% solution to avoid administering excess free water, typically in 5% dextrose), especially the initial loading dose, is associated in some patients with the development of anaphylactoid reactions (non–immunoglobulin E [IgE] mediated). These reactions are typically managed by stopping the infusion; treating with diphenhydramine, albuterol, and/or epinephrine as indicated; and restarting the infusion at a slower rate once symptoms have resolved. IV NAC is also associated with mild elevation in measured INR (1.2-1.5 range).
Ibuprofen and Other Nonsteroidal Anti-inflammatory Drugs
Oral Opioids
Opioids are a commonly abused class of medications (see Chapter 108), both in their IV and oral forms. Two specific oral opioids, suboxone and methadone, merit particular mention given their potential for life-threatening toxicity in toddlers with ingestion of even 1 pill. Suboxone, a combination of buprenorphine and naloxone, and methadone are primarily used in managing opioid dependence. However, methadone is also used in the treatment of chronic pain, and both drugs are readily available for illicit purchase and potential abuse. In contrast to methadone in most dependence-treatment programs, suboxone is prescribed in a multiday supply, meaning it is available in homes and particularly susceptible to unintentional ingestion by toddlers.
Treatment
Patients with significant respiratory depression or CNS depression should be treated with the opioid antidote, naloxone (see Table 58-8). In pediatric patients who are not chronically on opioids, the full reversal dose of 0.1 mg/kg (max, 2 mg/dose) should be used. In contrast, opioid-dependent patients should be treated with smaller initial doses (0.01 mg/kg), which can then be repeated as needed to achieve the desired clinical response, hopefully avoiding abrupt induction of withdrawal. Because the half-lives of methadone and suboxone are far longer than that of naloxone, patients can require multiple doses of naloxone. These patients might benefit from a continuous infusion of naloxone, typically started at 2/3 of the reversal dose/hr and titrated to maintain an adequate respiratory rate and level of consciousness. Patients who have ingested methadone should have serial ECGs to monitor for the development of a prolonged QTc interval. If a patient does develop a prolonged QTc, management includes close cardiac monitoring, repletion of electrolytes (potassium, calcium, and magnesium), and having magnesium readily available should the patient develop torsades de pointes.
Cardiovascular Medications
β-Adrenergic Receptor Blockers
Treatment
In addition to supportive care and GI decontamination as indicated, glucagon is the antidote of choice for β-blocker toxicity (see Table 58-8). Glucagon stimulates adenyl cyclase and increases levels of cyclic AMP independent of the β receptor. Glucagon is typically given as a bolus and, if this is effective, followed by a continuous infusion. Other potentially useful interventions include atropine, calcium, vasopressors, and high-dose insulin. Seizures are managed with benzodiazepines, and QRS widening should be treated with sodium bicarbonate. Children who ingest 1 or 2 water-soluble β-blockers are unlikely to develop toxicity and can typically be discharged to home if they remain asymptomatic over a 6-hr observation period. Children who ingest sustained-release products, highly lipid soluble agents, and sotalol can require longer periods of observation before safe discharge. Any symptomatic child should be admitted for ongoing monitoring and directed therapy.
Calcium Channel Blockers
Treatment
Once initial supportive care has been instituted, GI decontamination should begin with activated charcoal as appropriate. WBI may be beneficial after ingestion of a sustained-release product. Calcium channel blockade in the smooth muscles of the GI tract can lead to greatly diminished motility; thus, any form of GI decontamination should be undertaken with careful attention to serial abdominal exams. High-dose insulin therapy is considered the antidote of choice for CCB toxicity. An initial bolus of 1 U/kg of regular insulin is followed by an infusion at 0.5-1 U/kg/hr (see Table 58-8). Blood glucose levels should be closely monitored, and supplemental glucose may be given to maintain euglycemia, though this is rarely necessary in the severely poisoned patient. Calcium salts are typically administered in an overdose setting, although they might not provide substantial clinical benefit. Additional therapies include IV fluid boluses, vasopressors, and cardiac pacing. In extreme cases, extracorporeal membrane oxygenation (ECMO), cardiac assist devices, and lipid emulsion therapy may be lifesaving. Given the potential for profound and sometimes delayed toxicity in toddlers after ingestion of 1 or 2 CCB tablets, hospital admission and 24 hr of monitoring for all of these patients is strongly recommended.
Digoxin
Treatment
Initial treatment includes good general supportive care and gastric decontamination with activated charcoal if the ingestion was recent. An antidote for digoxin, digoxin-specific Fab antibody fragments (Digibind or Digifab) is available (see Table 58-8). Fab fragments bind free digoxin in both the intravascular and the interstitial spaces to form a pharmacologically inactive complex that is subsequently renally eliminated. Indications for Fab fragments include life-threatening dysrhythmias, K+ value of >5-5.5 mEq/L in the setting of acute overdose, serum digoxin level of >15 ng/mL at any time or >10 ng/mL 6 hr after ingestion, and ingestion of >4 mg in children or >10 mg in adults. If Digibind or Digifab are not readily available, phenytoin or lidocaine may be beneficial in managing ventricular irritability. Atropine is potentially useful in managing symptomatic bradycardia. Consultation with a cardiologist is recommended in the management of patients chronically on digoxin, because administration of Fab fragments can lead to recurrence of the patient’s underlying dysrhythmias or dysfunction.
Iron
Treatment
Close clinical monitoring, combined with aggressive supportive and symptomatic care, is essential to the management of iron poisoning. Activated charcoal does not adsorb iron, and WBI remains the decontamination strategy of choice. Deferoxamine, a specific chelator of iron, is the antidote for moderate to severe iron intoxication (see Table 58-8). Indications for deferoxamine treatment include a serum iron concentration of >500 mg/dL or moderate to severe symptoms of toxicity, regardless of serum iron concentration. Deferoxamine is preferably given via continuous IV infusion at a rate of 15 mg/kg/hr. Hypotension is a common side effect of deferoxamine infusion and is managed by slowing the rate of the infusion and administering fluids and/or vasopressors as needed. Prolonged deferoxamine infusion (>24 hr) has been associated with pulmonary toxicity (acute respiratory distress syndrome) and Yersinia sepsis. The deferoxamine-iron complex can color the urine reddish (“vin rosé”), though this is an unreliable indicator of iron excretion. Clear endpoints for deferoxamine chelation are not well defined, but therapy is typically continued until clinical symptoms resolve. Consultation with a poison control center or medical toxicologist can yield guidelines for discontinuing deferoxamine.
Oral Hypoglycemics
Treatment
Patients with symptomatic hypoglycemia should be promptly treated with dextrose. In patients with mild symptoms, oral dextrose may be sufficient. However, patients with severe symptoms or profound hypoglycemia should be treated with a bolus of IV dextrose. Continuous dextrose infusions and repeated IV dextrose boluses should be avoided if possible, because this can stimulate further insulin release and lead to recurrent and prolonged hypoglycemia. Instead, the preferred antidote for symptomatic sulfonylurea toxicity is octreotide (see Table 58-8). Octreotide is a somatostain analogue that works via inhibiting insulin release. Octreotide is given IV or SC, typically in doses of 1-2 µg/kg (50-100 µg in adults) every 6-8 hr.
Psychiatric Medications: Antidepressants
Tricyclic Antidepressants
Clinical and Laboratory Manifestations
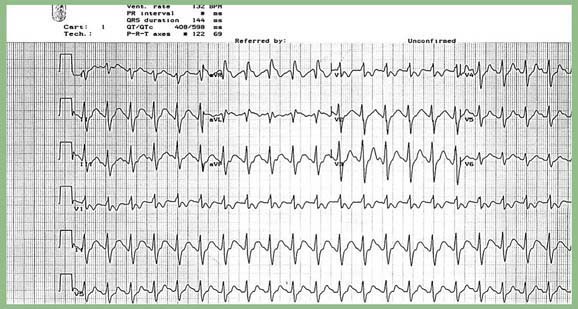
An ECG is a readily available bedside test that can help determine the diagnosis and prognosis of the TCA-poisoned patient (see Fig. 58-2). A QRS duration of >100 ms identifies patients who are at risk for seizures and cardiac arrhythmias. An R wave in lead aVR of >3 mm is also an independent predictor of toxicity. Both of these ECG parameters are superior to measured serum TCA concentrations in identifying patients at risk for serious toxicity, and obtaining levels is rarely helpful in management of the acutely ill patient.
Selective Serotonin Reuptake Inhibitors
In overdose, SSRIs are considerably less toxic than TCAs. SSRIs are unlikely to cause significant toxicity in exploratory ingestions. Some data suggest that initiating SSRI therapy is associated with an increased risk of suicidal ideation and behavior (Chapter 19).
Clinical and Laboratory Manifestations
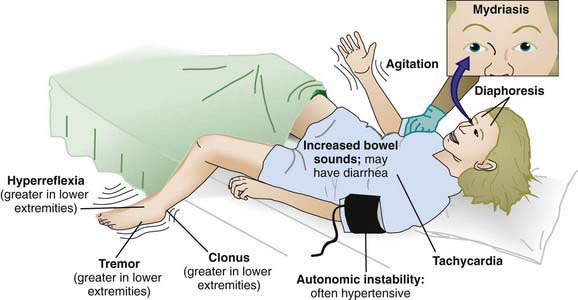
Though development of the serotonin syndrome is seen more often after therapeutic use or overdose of several serotoninergic agents in combination, it has also been described in ingestions of SSRIs alone (Table 58-13). Clinically, serotonin syndrome is a triad of altered mental status, autonomic instability, and neuromuscular hyperactivity (hyperreflexia, tremors, clonus in the lower extremities more than the upper extremities) (see Figure 58-3).
| DRUG TYPE | DRUGS |
|---|---|
| Selective serotonin reuptake inhibitors | Sertraline, fluoxetine, fluvoxamine, paroxetine, citalopram |
| Antidepressant drugs | Trazodone, nefazodone, buspirone, clomipramine, venlafaxine |
| Monoamine oxidase inhibitors | Phenelzine, moclobemide, clorgiline, isocarboxazid |
| Anticonvulsants | Valproate |
| Analgesics | Meperidine, fentanyl, tramadol, pentazocine |
| Antiemetic agents | Ondansetron, granisetron, metoclopramide |
| Antimigraine drugs | Sumatriptan |
| Bariatric medications | Sibutramine |
| Antibiotics | Linezolid (a monoamine oxidase inhibitor), ritonavir (through inhibition of cytochrome P450 enzyme isoform 3A4) |
| Over-the-counter cough and cold remedies | Dextromethorphan |
| Drugs of abuse | Methylenedioxymethamphetamine (MDMA, or “ecstasy”), lysergic acid diethylamide (LSD), 5-methoxydiisopropyltryptamine (“foxy methoxy”), Syrian rue (contains harmine and harmaline, both monoamine oxidase inhibitors) |
| Dietary supplements and herbal products | Tryptophan, Hypericum perforatum (St. John’s wort), Panax ginseng (ginseng) |
| Other | Lithium |
From Boyer EW, Shannon M: The serotonin syndrome, N Engl J Med 352:1112–1120, 2005.
Psychiatric Medications: Antipsychotics
Clinical and Laboratory Manifestations
Though the presentation of atypical antipsychotic toxicity can vary based on the receptor affinities of the specific agent, sedation, tachycardia, and QTc prolongation are common. Overdose of agents with muscarinic receptor activity leads to features of the anticholinergic toxidrome (see Table 58-4). Peripheral α-receptor blockade (e.g., with quetiapine) is associated with hypotension. In therapeutic use, clozapine is associated with agraulocytosis.
Household Products
Cholinesterase-Inhibiting Insecticides
Treatment
Two antidotes are useful in treating cholinesterase inhibitor poisoning: atropine and pralidoxime (see Table 58-8). Atropine, which antagonizes the muscarinic acetylcholine receptor, is useful for both organophosphate and carbamate intoxication. Often, large doses of atropine must be administered by intermittent bolus or via continuous infusion to control symptoms. Atropine dosing is primarily targeted to resolving respiratory secretions and bronchospasm. Heart rate is not an appropriate endpoint because tachycardia can result from nicotinic effects. Pralidoxime breaks the bond between the organophosphate and the enzyme, reactivating acetylcholinesterase. Pralidoxime is only effective if it is used before the bond ages and becomes permanent. Pralidoxime is not necessary for carbamate poisonings because the bond between the insecticide and the enzyme degrades spontaneously.
Hydrocarbons
Pathophysiology
Certain hydrocarbons have unique toxicities and can cause symptoms after ingestion, inhalation, or dermal exposures. Several chlorinated solvents, most notably carbon tetrachloride, can produce hepatic toxicity. Methylene chloride, found in some paint removers, is metabolized to carbon monoxide. Benzene is known to cause cancer, most commonly acute myelogenous leukemia, after long-term exposure. Nitrobenzene, aniline, and related compounds can produce methemoglobinemia. Methemoglobinemia is suggested by the classic “chocolate brown” blood and confirmed via co-oximetry. Methemoglobinemia is treated with methylene blue (see Table 58-8).
A number of volatile hydrocarbons, including toluene, propellants, refrigerants, and volatile nitrites, are commonly abused by inhalation. Some of these substances, principally the halogenated hydrocarbons (which contain a chlorine, bromine, or fluorine), can sensitize the myocardium to the effects of endogenous catecholamines. This can result in dysrhythmias and “sudden sniffing death.” Chronic abuse of these agents can lead to cerebral atrophy, neuropsychological changes, peripheral neuropathy, and kidney disease (see Chapter 108.4).
Toxic Alcohols
Isopropyl alcohol (rubbing alcohol, hand sanitizers) causes intoxication similar to that associated with ethanol but can also cause a hemorrhagic gastritis and myocardial depression in massive ingestions. Unlike ethylene glycol and methanol, isopropyl alcohol is metabolized to a ketone and does not cause a metabolic acidosis. Management is similar to that of ethanol ingestions (see Chapter 108.1) and is not further discussed here.
Ethylene Glycol
Treatment
Because methanol and ethylene glycol are rapidly absorbed, gastric decontamination is generally not of value. The classic antidote for methanol and ethylene glycol poisoning was ethanol, a preferential substrate for alcohol dehydrogenase, thus preventing the metabolism of parent compounds to toxic metabolites. Fomepizole (see Table 58-8), a potent competitive inhibitor of alcohol dehydrogenase, has almost entirely replaced the use of ethanol owing to its ease of administration, lack of CNS and metabolic effects, and overall excellent patient tolerability profile. Indications for fomepizole include ethylene glycol or methanol level >20 mg/dL, history of potentially toxic ingestion and an elevated osmolar gap, or history of ingestion with evidence of acidosis. There are few disadvantages to giving the initial dose of fomepizole to patients with a concerning history of ingestion or lab findings, and given the dosing schedule of fomepizole (every 12 hr), this strategy buys the clinician time to confirm or exclude the diagnosis before giving a second dose. Adjunctive therapy includes folate (methanol toxicity) and pyridoxine (ethylene glycol toxicity).
Plants
Exposure to plants, both inside the home and outside in backyards and fields, is one of the most common causes of unintentional poisoning in children. Fortunately, the majority of ingestions of plant parts (leaves, seeds, flowers) result in either no toxicity or mild, self-limiting effects (Table 58-14). However, ingestion of certain plants (Table 58-15) outlines some of the most toxic plants) can lead to serious toxicity.
Table 58-14 NONTOXIC AND MINIMALLY TOXIC PLANTS*
* The potential for toxicity depends on the magnitude and amount of exposure. These agents are considered nontoxic or minimally toxic for mild to moderate exposure. The potential for toxicity increases with increased amount of exposure. Many plants contain substances that can be irritating to the mucosa (dermal/oroesophageal) and/or can precipitate allergic responses.
Table 58-15 COMMONLY INGESTED PLANTS WITH SIGNIFICANT TOXIC POTENTIAL
| PLANT | SYMPTOMS | MANAGEMENT |
|---|---|---|
| Autumn crocus (Colchicum autumnlae) |
pt, patient; ECG, electrocardiogram; AV, atrioventricular; Fab, fragment, antigen binding; CNS, central nervous system; GI, gastrointestinal; CV, cardiovascular.
For potentially toxic plant ingestions, consider decontamination with activated charcoal in patients who present within 1-2 hr of ingestion; otherwise, treatment is primarily supportive and symptomatic. The most common manifestation of toxicity after plant ingestion is GI upset, which can be managed with antiemetics and fluid and electrolyte support. Management strategies for a few specific toxicities are outlined in Table 58-15.
Toxic Gases
Hydrogen Cyanide
Treatment
Treatment includes removal from the source of exposure, rapid administration of high concentrations of oxygen, and antidotal therapy. The cyanide antidote kit includes nitrites (amyl nitrite and sodium nitrite) used to produce methemoglobin, which then reacts with cyanide to form cyanmethemoglobin (see Table 58-8). The third part of the kit is sodium thiosulfate, given to hasten the metabolism of cyanmethemoglobin to hemoglobin and the less-toxic thiocyanate. In patients for whom induction of methemoglobinemia could produce more risk than benefit, the sodium thiosulfate component of the kit may be given alone. In 2006, the FDA approved hydroxocobalamin (a form of vitamin B12) for use in known or suspected cyanide poisoning. This antidote, used for many years in Europe, reacts with cyanide to form the nontoxic cyanocobalamin, which is then excreted in urine. Side effects of hydroxocobalamin include red discoloration of the skin and urine, transient hypertension, and interference with colorimetric lab assays. Overall, the safety profile of hydoxocobalamin appears superior to that of the cyanide antidote kit; thus this is becoming the preferred antidote for cyanide poisoning.
2003 American Academy of Pediatrics Committee on Injury, Violence, and Poison Prevention: Poison treatment in the home. Pediatrics. 2003;112:1182-1185.
Boehnert MT, Lovejoy FHJr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313:474-479.
Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
Brent J. Fomepizole for ethylene glycol and methanol poisoning. N Engl J Med. 2009;360:2216-2223.
Bronstein AC, Spyker DA, Cantilena LRJr, et al. 2007 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 25th annual report. Clin Toxicol (Phila). 2008;46(10):927-1057.
Carlsson M, Cortes D, Jepsen S, et al. Severe iron intoxication treated with exchange transfusion. Arch Dis Child. 2008;93:321-322.
Centers for Disease Control and Prevention. Fatal poisoning among young children from diethylene glycol–contaminated acetaminophen—Nigeria, 2008–2009. MMWR Morb Mortal Wkly Rep. 2009;58:1345-1346.
Centers for Disease Control and Prevention. Carbon monoxide exposures after Hurricane Ike: Texas, September 2008. MMWR Morb Mortal Wkly Rep. 2009;58:845-849.
Centers for Disease Control and Prevention. Carbon monoxide related deaths—United States, 1999–2004. MMWR Morb Mortal Wkly Rep. 2007;56:1309-1312.
Dart RC, Rumack BH. Patient-tailored acetylcysteine administration. Ann Emerg Med. 2007;50:280-281.
Dart RC, Borron SW, Caravati EM, et al. Expert consensus guidelines for stocking of antidotes in hospitals that provide emergency care. Ann Emerg Med. 2009;54:386-391.
Eddleston M, Buckley NA, Eyer P, et al. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597-606.
Eddleston M, Juszczak E, Buckley NA, et al. Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet. 2008;371:579-587.
Geib AJ, Babu K, Ewald MB, et al. Adverse effects in children after unintentional buprenorphine exposure. Pediatrics. 2006;118:1746-1751.
Geller RJ, Barthold C, Saiers JA, et al. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006;118:2146-2158.
Heard K. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285-292.
Khine H, Weiss D, Graber N, et al. A cluster of children with seizures caused by camphor poisoning. Pediatrics. 2009;123:1269-1272.
Kivistö JE, Mattilia VM, Arvola T, et al. Secular trends in poisoning leading to hospital admission among Finnish children and adolescents between 1971 and 2005. J Pediatr. 2008;153:820-824.
Klein-Schwartz W. Trends and toxic effects from pediatric clonidine exposures. Arch Pediatr Adolesc Med. 2002;156:392-396.
Levine M, Boyer EW, Pozner CN, et al. Assessment of hyperglycemia after calcium channel blocker overdoses involving diltiazem or verapamil. Crit Care Med. 2007;35:2071-2075.
Liebelt EL, Francis PD, Woolf AD. ECG lead aVR versus QRS interval in predicting seizures and arrhythmias in acute tricyclic antidepressant toxicity. Ann Emerg Med. 1995;26:195-201.
Osterhoudt KC, Durbin D, Alpern ER, et al. Risk factors for emesis after therapeutic use of activated charcoal in acutely poisoned children. Pediatrics. 2004;113:806-810.
Pawar KS, Bhoite RR, Pillay CP, et al. Continuous pralidoxime infusion versus repeated bolus injection to treat organophosphorus pesticide poisoning: a randomized controlled trial. Lancet. 2006;368:2136-2140.
Rimsza ME, Newberry S. Unexpected infant deaths associated with use of cough and cold medications. Pediatrics. 2008;122:e318-e322.
Roberts DM, Aaron CK. Managing acute organophosphorus pesticide poisoning. BMJ. 2007;334:629-634.
Schillie SF, Shehab N, Thomas KE, et al. Medication overdoses leading to emergency department visits among children. Am J Prev Med. 2009;37:181-187.
Weaver LK. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1224.
Williams JF, Kokotailo PK. Abuse of proprietary (over-the-counter) drugs. Adolesc Med Clin. 2006;17(3):733-750. abstract xiii