Point-of-care pelvic ultrasound
Overview
This chapter illustrates the basic features of pelvic ultrasound by outlining the exploration of the female genital system, concluding thus the holistic approach (HOLA) ultrasound concept of the abdominal examination (see Chapters 1 and 44). Clinician-performed point-of-care pelvic ultrasound is an integral skill in a growing number of medical subspecialties. History and physical examination are not always reliable in the evaluation of possible pelvic pathology, especially in the critically ill patient. The ability to diagnose life-threatening conditions at the bedside can decrease time to diagnosis. Ultrasonography is the preferred imaging modality for female pelvic anatomy in both the nonpregnant and pregnant patient. The accuracy of clinician-performed bedside ultrasound in identifying pelvic pathology has been well established.
Indications
Abdominal pain, pelvic pain, and vaginal bleeding are common complaints for which pelvic ultrasound is used. Although less common, pelvic ultrasound may also be indicated for syncope, dizziness, abdominal or pelvic mass on physical examination, trauma, fever, and hemodynamic instability. In the setting of pregnancy, the first and foremost use of pelvic ultrasound is in the diagnosis of an intrauterine pregnancy (IUP). Once an IUP has been established, ultrasonography can be further used to document gestational age, fetal heart rate, subchorionic hemorrhage, abnormal pregnancies, pregnancy loss, or to follow normal pregnancy. For the nonpregnant, critically ill patient, other pertinent applications include the evaluation of life-threatening ovarian pathology, such as ovarian torsion, ovarian cyst rupture with associated hemorrhage, and tubo-ovarian abscess. In any female who is hypotensive, septic, febrile, or suffering from severe abdominal pain, pelvic ultrasound is an extremely useful tool to direct appropriate treatment.1,2
Technique and normal anatomy
Imaging of the pelvic organs is assisted by the use of both transabdominal sonography (TAS) and transvaginal sonography (TVS). Each technique has its own advantages in the assessment of pelvic organs. A transabdominal approach gives the examiner spatial orientation of the pelvis and defines the relationship of the uterus with adjacent pelvic and abdominal structures. It is also helpful to delineate large masses, free fluid, and other pathology extending outside of the true pelvis. A transvaginal approach is useful because of probe proximity to the pelvic organs and the higher frequency resulting in images with superior resolution. This approach provides more anatomic detail of pelvic structures, allowing early identification of intrauterine contents and adnexal abnormalities. In addition, the probe can be used to detect the mobility of the pelvic organs. This can reveal adhesions and highlight pelvic pathology. These two modalities are complimentary, and in the undifferentiated patient, we recommend using both approaches.1–4
Transabdominal technique
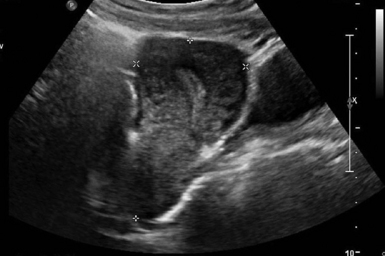
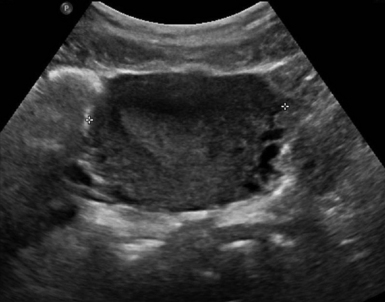
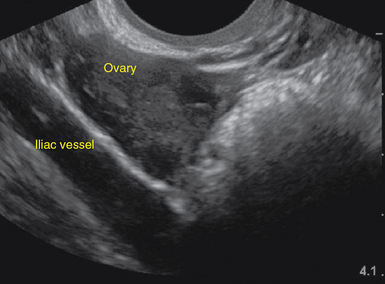
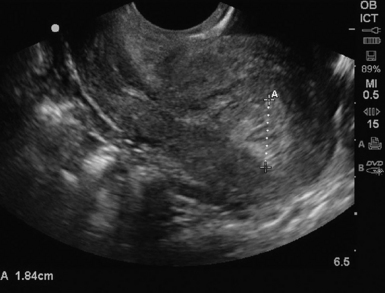
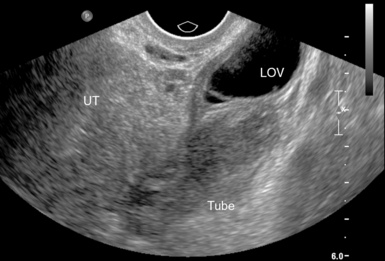
For TAS, scanning starts in the suprapubic area, using a low-frequency curvilinear transducer. The examination is best performed with a full bladder, although an overly distended bladder will decrease image quality. The bladder acts as an acoustic window and also displaces bowel loops from the pelvis for better imaging.1–4 With the indicator directed cephalad, sagittal images can be obtained by sweeping the transducer slowly from right to left. Transverse images can be obtained with the indicator to the patient’s right, by convention. By gently sweeping the transducer caudad and cephalad, all organs can be imaged in all three dimensions. The examiner should first start with an assessment of the uterus in both the sagittal and transverse planes. The position of the uterus, whether it is anteverted or retroverted, should be noted. Obesity and retroverted position can present challenges to the sonographer. The various portions of the uterus are then identified, namely, the cervix, body, fundus, and endometrial stripe. Sonographically, the normal uterus exhibits a low-gray homogenous texture. The endometrial stripe is typically seen as an echogenic line in the middle of the uterus (Figures 43 E-1 and 43 E-2). The appearance of uterine endometrium changes as the thickness of the endometrium changes with different phases of menstrual cycle. The cervix has similar sonographic appearance to that of the rest of the uterus. The thin linear hyperechoic vaginal canal is visualized between the anechoic urinary bladder (anterior) and the echogenic rectum (posterior). The cul-de-sac should be evaluated for free fluid or hematoma or mass, both in long and transverse planes. Next, the operator moves the transducer to the right or left lower quadrants to identify the adnexa. The identification of the ovaries is aided by identifying the iliac vessels, which define the borders of the pelvis. The ovaries are often adjacent to them. Ovaries are identified by their “chocolate chip cookie” appearance of hypoechoic follicles within the ovary. Again, they should be imaged in two planes, sagittal and transverse. Ovarian size can be evaluated by measuring the ovary’s dimensions in three planes. Normal ovarian measurements are approximately 5.1 ± 3.1 cm3 in the preovulation period, 3.2 ± 1.7 cm3 in the postovulation period, and 1.3 ± 0.6 cm3 in postmenopausal women. Ovaries may not always be seen on TAS but are visualized 65% to 99% of the time when performed in the radiology suite.3,4
Transvaginal technique
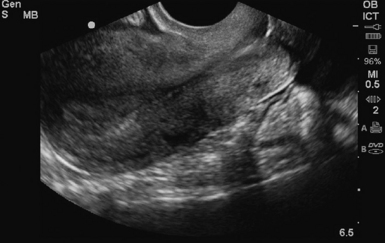
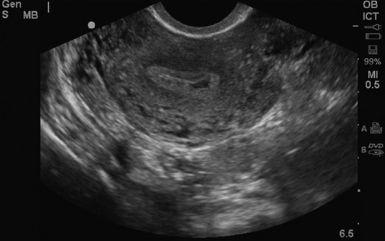
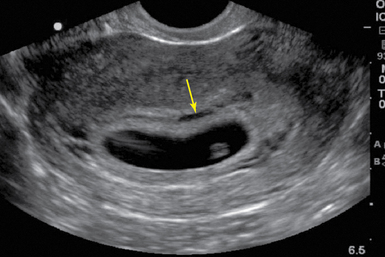
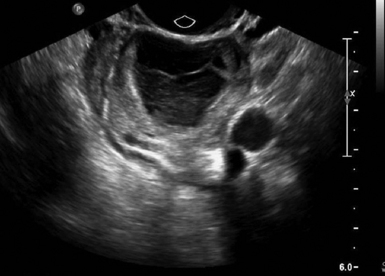
For TVS, consent should be obtained and the procedure explained, if at all possible. A chaperone should also be present. The patient should be placed in lithotomy position, and the bladder should be decompressed because a distended bladder can distort the normal pelvic anatomy and push the uterus posteriorly.3,4 The transducer should be prepared with an appropriate cover, with lubricating gel inside and outside of the cover. The endocavitary transducer is directed to different points of interest by using the midpoint on the probe as a fulcrum. To image anteriorly on the body, the transducer handle should be moved posteriorly, thus bringing the imaging of the probe anteriorly. This principle is one of the frequent missteps of novice sonographers but can be easily learned and mastered. Just as in TAS, assessment begins with the uterus imaged in two planes. With the indicator facing the ceiling, the sagittal images are obtained, and with the indicator pointed to the right side of the patient, the coronal plane images are obtained. The uterine contents should be screened thoroughly, and the posterior cul-de-sac should be evaluated at the level of the fundus, body, and cervix (Figures 43 E-3 to 43 E-5). The free fluid in the posterior cul-de-sac can be quantified into small (free fluid seen < 1⁄3 of the way up the posterior wall of the uterus), medium (free fluid seen 2⁄3 of the way up the posterior wall of the uterus), and large (free fluid seen > 2⁄3 tracking along the posterior wall of the uterus). The transducer is then angled rightward or leftward to visualize the adnexa. Again, the proximity of the ovaries to the iliac vessels can be used to locate them, and each structure should be imaged in two planes, coronal and sagittal.3,4 Typically, the ovaries are found lateral to the uterus, medial and anterior to the iliac vessels (Figure 43 E-6).
Major disorders encountered
Ectopic pregnancy
Ruling out ectopic pregnancy is the first step in evaluating a patient with a positive pregnancy test. The β–human chorionic gonadotropin (β-HCG) level at which an IUP can be visualized on TVS (termed the discriminatory zone) has been quoted to be between 1000 and 2000 mIU/mL. However, use of β-HCG has been shown to perform very poorly as a means of differentiating ectopic pregnancy from IUP, and ectopic pregnancy frequently occurs at very low levels.5,6
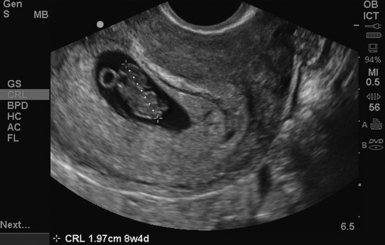
The stepwise appearance of the gestational sac (4-5 weeks), the yolk sac (5-6 weeks), the fetal pole (6–7 weeks), and, finally, a fetal heartbeat can be followed sonographically in a normal pregnancy (Figure 43-1). Determining the definitive presence of an IUP requires visualizing the yolk sac, which is a hyperechoic circular structure within the gestational sac. Although the earliest intrauterine sign of pregnancy is the gestational sac, this is not definitive evidence of an IUP. Another early sign of intrauterine pregnancy is the double decidual sign. This is characterized by two hyperechoic rings (the decidua capsularis and the decidua vera) separated by a hypoechoic ring that surrounds the gestational sac (Figure 43 E-7). Misinterpretation of this sign is common and may have serious consequences; therefore this is not considered a sign of definitive IUP in the setting of point-of-care ultrasound. Gestational age can be determined by adding 30 to the mean gestational sac diameter (length, width, and height of the gestational sac obtained in two orthogonal planes and divided by three). Between 6 to 12 weeks, crown rump length (CRL) is the most accurate sonographic measurement for determining gestational age. While measuring CRL, the longest measurement of an unflexed fetus should be obtained without including the yolk sac (Figure 43 E-8).
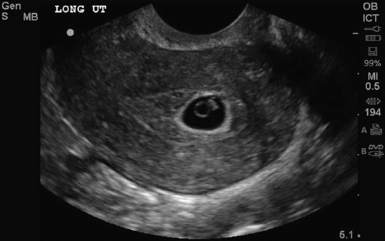
Figure 43-1 Longitudinal view of a pregnant uterus by transvaginal sonography (TVS) showing yolk sac and fetal pole within the gestational sac.
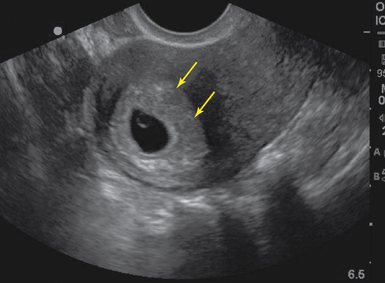
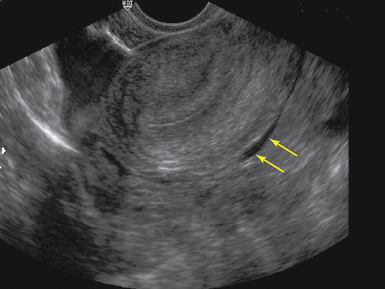
Figure 43 E-7 Sagittal view of a pregnant uterus with double decidual sign (arrows) on transvaginal sonography (TVS).
The prevalence of ectopic pregnancy is roughly 2% but has been noted to be significantly higher in patients presenting with symptoms in the acute care setting.5,7 Findings highly suggestive of ectopic pregnancy include an empty uterus, extrauterine empty gestational sac, extrauterine gestational sac with yolk sac or embryo with or without cardiac activity, complex adnexal mass, tubal ring, a large amount of free fluid or echogenic fluid in the pelvis (Figures 43-2, 43-3, and 43 E-9). A tubal ring is a hypoechoic circular structure with a hyperechoic border, clearly separate from the ovary, that is highly suggestive of ectopic pregnancy in the correct clinical setting.5 Adnexal masses adjacent to the ovary can be difficult to distinguish from a normal corpus luteum cyst. Applying pressure with the probe will help to separate an ectopic mass from the ovary. Identification of a live pregnancy in the adnexa is confirmatory and occurs in roughly 10% of cases.5 A pseudogestational sac (<10 mm ovoid endometrial fluid collection with poorly defined margins and no double decidual sign) is present in up to 20% of ectopic pregnancies. In a high-risk patient, heterotopic pregnancy must always be considered and ruled-out. The prevalence increases in patients with a history of pelvic inflammatory disease, fertility treatments, or other risk factors but is also known to occur in the absence of risk factors.
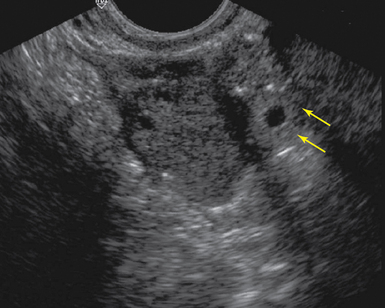
Figure 43-2 Tubal ring (arrows) representing an unruptured ectopic seen adjacent to the ovary on transvaginal sonography (TVS)
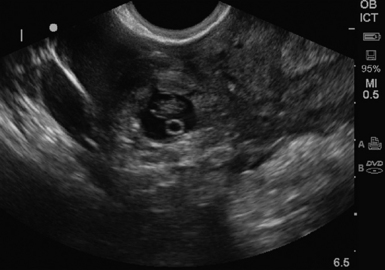
Figure 43-3 Ectopic pregnancy. An intact gestational sac with a yolk sac outside uterus in the left adnexa
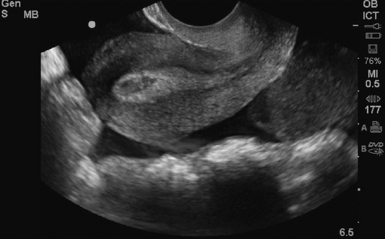
Figure 43 E-9 A ruptured ectopic sagittal view of an empty uterus, with large amount of free fluid on transvaginal sonography (TVS).
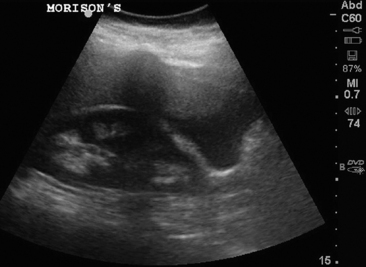
When considering a critically ill patient who is pregnant but does not have an established IUP, an evaluation of the abdomen for free fluid is necessary. An unstable patient, with a positive β-HCG, free fluid in the abdomen, and an empty uterus should proceed directly to the operating room for surgical management. Identification of free fluid in the hepatorenal space is highly predictive of need for surgical intervention.8 Patients with a large volume of pelvic fluid or echogenic fluid are at increased risk of ectopic pregnancy as well.9
Pregnancy loss
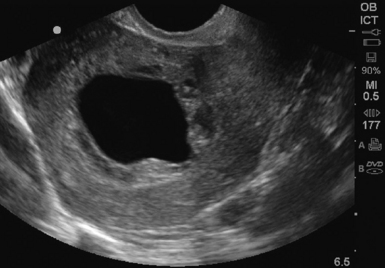
There are several sonographic signs of an abnormal pregnancy or impending miscarriage. First, a large gestational sac without a visualized yolk sac should alert the sonographer to a possible anembryonic pregnancy. A mean gestational sac diameter (MSD) of greater than 10 mm on TVS or greater than 20 mm on TAS, without a yolk sac visualized, is abnormal. In addition, a MSD of greater than 18 mm on TVS or 25 mm on TAS without a fetal pole, or a gestational sac with an irregular, ragged border or a collapsed gestational sac are considered abnormal (Figure 43 E-10).10 Once an embryo is visualized, the fetal heart tones should be assessed. M-mode is the preferred method, given the theoretic risk of thermal injury with the use of Doppler. Absent heart tones in an embryo of 5 mm in length on TVS is a good indicator of embryonic demise.2 Fetal heart rates less than 90/min when the CRL is greater than 5 mm are also associated with fetal demise. A patient with an IUP and vaginal bleeding or abdominal pain may be experiencing miscarriage. One quarter of pregnancies will experience vaginal bleeding, and one half of those will go on to have a miscarriage.11 In patients with an identified yolk sac, miscarriage occurred in 17% of those with pain, 41% with bleeding, and 35% with both pain and bleeding.12 In cases in which a live IUP was identified, 9.3% of first trimester patients with bleeding went on to have a miscarriage. However, if the patient presented with pain only, 2.5% miscarried.13 After a complete spontaneous abortion, an empty uterus with a clear midline echo is visualized on ultrasound. With an incomplete abortion, retained products of conception are visualized as a thickened midline stripe (>10 mm wide) or irregular echogenic material within the uterine cavity (Figure 43 E-11).
Subchorionic hemorrhage
Subchorionic hemorrhage appears sonographically as a hypoechoic or anechoic area between the placenta and the uterine wall. The risk of miscarriage, stillbirth, placental abruption, and preterm labor are all increased if a subchorionic hemorrhage is identified on ultrasound.14 The spontaneous abortion rate is about 9.3% when subchorionic hemorrhage is identified in the first trimester. The rate is further increased with advanced maternal age and gestational age less than 8 weeks.15 The prognosis is also associated with the size of the bleed. The appearance of acute hemorrhage is hyperechoic or isoechoic. Within 1 to 2 weeks, the hemorrhage becomes hypoechoic or anechoic with liquefaction of the clot (Figure 43 E-12).
Ovarian cyst rupture
Differentiation of benign versus malignant masses is not within the scope of bedside ultrasound; however, detecting the presence of these masses and the sequelae is within the scope of the bedside clinician. Any ovarian mass encountered should be evaluated for its size, it echogenicity (cystic, solid, mixed, etc), presence of septations, and thickness of the walls.4 Normal functional ovarian cysts will be 6 cm or less.3 When hemorrhagic, fibrin strands with a fishnet weave or fine reticular appearance and a retracting clot are generally seen within an enlarged ovary (Figure 43-4). Diagnosis of cyst rupture can be difficult sometimes because the cyst itself is no longer visible sonographically after rupture. Therefore the diagnosis is made in the correct clinical setting of sudden onset pelvic pain and free fluid in the pelvis.16 With simple ovarian cyst rupture, anechoic fluid is visualized in the pelvis. However, hemorrhagic ovarian cyst rupture results in echogenic fluid in the pelvis or massive hemoperitoneum. This can occasionally precipitate life-threatening hemodynamic instability. The presence of a large amount of abdominal free fluid should point to the diagnosis with the correct clinical picture.
Ovarian torsion
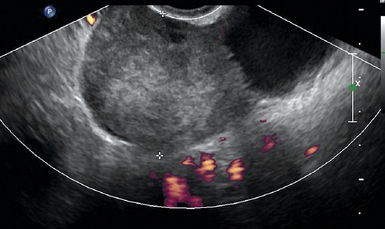
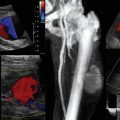
Ovarian torsion is another ovarian emergency for which ultrasound is the first-line diagnostic tool. Rotation of the ovary about its pedicle can impede and totally occlude its blood supply through the uterine artery. There is a progression from venous stasis, ovarian edema, and congestion to complete ischemia. This is often associated with an adnexal mass, but can be idiopathic. Up to 20% of adnexal torsion occurs in the setting of pregnancy.3 Ultrasonography has a diagnostic accuracy of 74.6% for ovarian torsion and is highly dependent on operator accuracy.17 The sonographic findings are quite variable and are dependent upon the duration of symptoms, degree of torsion, and whether the torsion is intermittent. The ovary is typically enlarged, with prominent heterogeneous central stroma and peripherally displaced small follicles (Figure 43 E-13). An underlying mass, such as a dermoid cyst or hemorrhagic cyst, may be visualized. Other findings include free fluid in the pelvis, an abnormal ovarian position, and a whirlpool sign, which is an enlarged fallopian tube with alternating hypoechoic and echogenic bands representing the twisted vessels within the adnexal pedicle.2 The absence of blood flow on Doppler in a painful, morphologically abnormal ovary is highly suspicious of ovarian torsion. However, normal arterial and venous pulse Doppler waveforms do not exclude ovarian torsion because of dual blood supply to the ovary from both the ovarian and uterine arteries. In addition, with partial torsion (obstruction to lymphatic and venous drainage before arterial occlusion) and intermittent torsion, normal arterial waveforms are noted.
Tubo-ovarian abscess
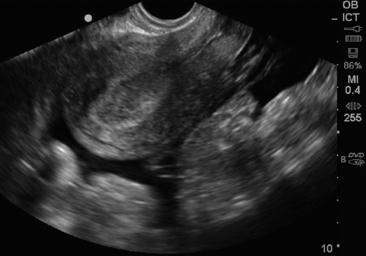
The sonographic findings of pelvic inflammatory disease (PID) without abscess are varied and are not sensitive for the diagnosis. For the bedside clinician, the findings of thickened and dilatated fallopian tubes with internal echoes or even air are the most striking and can lead to the diagnosis.18 Other sonographic signs may include an enlarged uterus or thickened heterogeneous endometrium and poor mobility of the adnexal structures. Along the spectrum of PID, tubo-ovarian abscess (TOA) is a severe consequence and can be confirmed sonographically. Findings typically include loss of the normal boundaries between the fallopian tube and the ovary (because of the pus-filled, edematous inflamed tissue), complex adnexal mass of varying echogenicity with irregular margins, debris, septations, and loculated or speckled or echogenic free fluid in the cul-de-sac (Figure 43 E-14).19 Any adnexal mass identified on pelvic ultrasound in the correct clinical setting should raise suspicion for TOA. Pelvic ultrasound has been reported to have a sensitivity of 56% to 94%, with a specificity of 86% to 98% in the detection of TOA.20 The wide range of reported sensitivities is likely secondary to variation modalities, study populations, and ultrasound interpreters in each of the studies that have been done. Larger TOAs may be difficult to diagnose on TVS because of the limited field of view with this probe and may be more easily imaged on TAS.
Pearls and highlights
• Pelvic ultrasound has become an invaluable adjunct to the clinical evaluation of patients with abdominal pain, pelvic pain, and vaginal bleeding.
• Transabdominal and transvaginal ultrasonography provide complimentary information.
• An intrauterine gestational sac is not definitive evidence of an intrauterine pregnancy until a yolk sac or fetal pole are visualized.
• The spectrum of ultrasound findings of ectopic pregnancy includes empty uterus, pseudogestational sac, complex adnexal mass, extrauterine empty gestational sac, extrauterine gestational sac with yolk sac or embryo with or without cardiac activity, tubal ring, and free fluid in cul-de-sac.
• Ruptured hemorrhagic ovarian cyst rupture can result in echogenic fluid in the pelvis or massive hemoperitoneum.
• Normal ovarian arterial and venous flow on Doppler does not exclude ovarian torsion.
References
1. Cosby, KS, Kendall, JL. Practical guide to emergency ultrasound. Philadelphia: Lippincott, Williams & Wilkins; 2006.
2. Ma OJ, Mateer JR, Blaivas M, eds. Emergency ultrasound, ed 2, New York: McGraw-Hill, 2008.
3. Goldstein, SR, Timor-Tritsch, IE. Ultrasound in gynecology. New York: Churchill Livingstone; 1995.
4. Sabbagha, RE. Diagnostic ultrasound applied to obstetrics and gynecology, ed 3. Philadelphia: JB Lippincott; 1994.
5. Adhikari, S, Blaivas, M, Lyon, M, Diagnosis and management of ectopic pregnancy using bedside transvaginal ultrasonography in the ED: a 2-year experience. Am J Emerg Med. 2007;25(6):591–596.
6. Wang, R, Reynolds, TA, West, HH, et al. Use of a beta-hCG discriminatory zone with bedside pelvic ultrasonography. Ann Emerg Med. 2011; 58(1):12–20.
7. Hahn, SA, Lavonas, EJ, Mace, SE, et al, Clinical policy: critical issues in the initial evaluation and management of patients presenting to the emergency department in early pregnancy. Ann Emerg Med. 2012;60(3):381–390.e28.
8. Moore, C, Todd, WM, O’Brien, E, Lin, H. Free fluid in Morison’s pouch on bedside ultrasound predicts need for operative intervention in suspected ectopic pregnancy. Acad Emerg Med. 2007; 14(8):755–758.
9. Dart, R, McLean, SA, Dart, L, Isolated fluid in the cul-de-sac: how well does it predict ectopic pregnancy?. Am J Emerg Med. 2002;20(1):1–4.
10. Dart, RG. Role of pelvic ultrasonography in evaluation of symptomatic first-trimester pregnancy. Ann Emerg Med. 1999; 33(3):310–320.
11. Eyvazzadeh, AD, Levine, D. Imaging of pelvic pain in the first trimester of pregnancy. Radiol Clin North Am. 2006; 44(6):863–877.
12. Hessert, MJ, Juliano, M. Fetal loss in symptomatic first-trimester pregnancy with documented yolk sac intrauterine pregnancy. Am J Emerg Med. 2012; 30(3):399–404.
13. Juliano, ML, Sauter, BM. Fetal outcomes in first trimester pregnancies with an indeterminate ultrasound. J Emerg Med. 2012; 43(3):417–422.
14. Ball, RH, Ade, CM, Schoenborn, JA, Crane, JP. The clinical significance of ultransonographically detected subchorionic hemorrhages. Am J Obstetr Gynecol. 1996; 174(3):996–1002.
15. Bennett, GL, Bromley, B, Lieberman, E, Benacerraf, BR, Subchorionic hemorrhage in first-trimester pregnancies: prediction of pregnancy outcome with sonography. Radiology. 1996;200(3):803–806.
16. Bottomley, C, Bourne, T. Diagnosis and management of ovarian cyst accidents. Best Pract Res Clin Obstetr Gynaecol. 2009; 23(5):711–724.
17. Mashiach, R, Melamed, N, Gilad, N, et al, Sonographic diagnosis of ovarian torsion: accuracy and predictive factors. J Utrasound Med. 2011;30(9):1205–1210.
18. Horrow, MM. Ultrasound of pelvic inflammatory disease. Ultrasound Q. 2004; 20(4):171–179.
19. Lambert, MJ, Villa, M. Gynecologic ultrasound in emergency medicine. Emerg Med Clin North Am. 2004; 22(3):683–696.
20. Lee, DC, Swaminathan, AK, Sensitivity of ultrasound for the diagnosis of tubo-ovarian abscess: a case report and literature review. J Emerg Med. 2011;40(2):170–175.