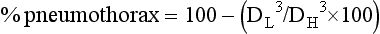Pneumothorax and Barotrauma
Definition and History
Pneumothorax is defined as air in the pleural space because of a break in the visceral or parietal pleura. The term pneumothorax was used for the first time in 1803.1 Laennec2 gave the first clinical description of pneumothorax in 1819; however, the first chest radiograph demonstrating this entity was not published until 1901.3 Definitive therapy became available with the advent of tube thoracostomy in 1876.4 It was thought to be always associated with tuberculosis until 1932 when “spontaneous pneumothorax in the apparently healthy” was first described.5
Spontaneous pneumothorax can be classified as primary or secondary. Primary spontaneous pneumothorax (PSP) occurs in patients with no apparent underlying lung disease. Secondary spontaneous pneumothorax (SSP) occurs in association with a known underlying lung disease such as chronic obstructive pulmonary disease (COPD).6 Nonspontaneous causes of pneumothorax include traumatic and iatrogenic (Table 47.1).
Table 47.1
Classification of Pneumothorax
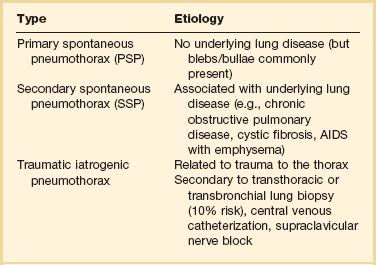
From Grundy S, Bentley A, Tschopp JM: Primary spontaneous pneumothorax: A diffuse disease of the pleura. Respiration 2012;83:185-189.
Incidence
The incidence of PSP in men varies geographically, from 7.4 per 100,000 population per year in the United States to 37 per 100,000 population per year in the United Kingdom. In women, the incidence is substantially less, ranging from 1.2 per 100,000 population per year in the United States to 15.4 per 100,000 population per year in the United Kingdom.7 The reason for these differences is not known. In a study that evaluated 1199 patients with pneumothorax that included 865 male patients and 334 female patients, 60.3% of the pneumothoraces were spontaneous, 33.6% were traumatic, and 6.1% were iatrogenic.8
The estimates of the incidence of recurrent PSP range from 20% to more than 50% with most recurrences occurring within the first year.9
Pathophysiology
Emphysema-like Changes
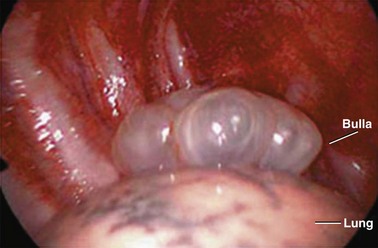
PSP occurs in patients with no previously known lung disease. It is important to note, however, that this does not mean there is no underlying pathologic process. A finding of abnormal pleura is very common in PSP if looked for carefully.10 Abnormalities seen in PSP are summarized in Table 47.2 and include blebs and bullae, which are otherwise known as emphysema-like changes (ELCs). These areas of weakness of the visceral pleura are prone to rupture, allowing air to leak into the pleural space. Abnormalities can be visualized radiographically with high-resolution computed tomography (CT) scans and macroscopically at thoracoscopy.10,11 High-resolution CT imaging reveals these defects in approximately 80% of PSP patients. The literature is mixed as to whether the presence of or extent of ELCs is directly related to the risk of recurrence. Some case series suggest that there is no association,12,13 although others suggest the presence of contralateral blebs/bullae is a risk factor for future pneumothorax.14,15 The only clear conclusion that can be drawn is that there is a direct association between the presence of ELCs and the occurrence (but not necessarily recurrence) of a pneumothorax (Table 47.2 and Fig. 47.1).
Table 47.2
Pathologic Changes Associated with Primary Spontaneous Pneumothorax (PSP)
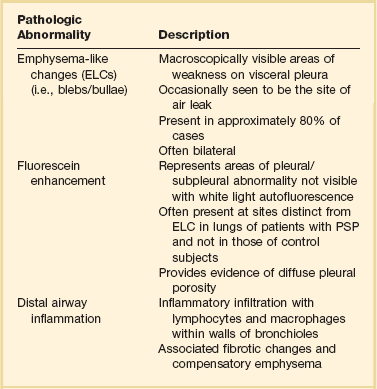
From Grundy S, Bentley A, Tschopp JM: Primary spontaneous pneumothorax: A diffuse disease of the pleura. Respiration 2012;83:185-189.
Pleural Porosity
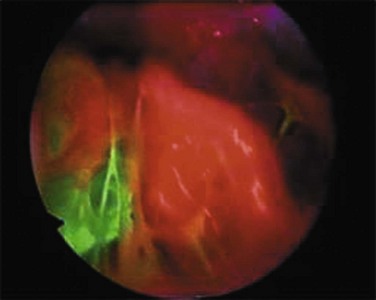
ELCs are not the sole cause of PSP. Air leak has been described in areas where no ELCs are seen, leading to the concept known as “pleural porosity.”16,17 When evaluated with fluorescein-enhanced autofluorescence, areas of high-grade abnormality in the visceral pleura were frequently visualized separate from any area of abnormality seen with white light at thoracoscopy in patients with PSP. High-grade abnormalities were not seen in control patients.18 Areas of fluorescein leak (i.e., areas of potential air leak) were visualized in only a small proportion of PSP, but these areas were noted to be distinct from areas of ELC. When studied with electron microscopy, the linings of some resected areas of ELC have been shown to be almost completely absent of mesothelial cells and have abnormal pores present19 (Fig. 47.2).
Distal Airway Inflammation
Pathologic findings suggest an inflammatory cause to the formation of ELCs. Chronic distal airway inflammation with lymphocyte and macrophage infiltration alongside fibrotic changes and compensatory emphysema can be seen microscopically in areas of lung tissue from patients with PSP.20 In a different study, the presence of respiratory bronchiolitis (RB) was seen in close to 90% of patients with PSP who underwent surgical resection in a different study.21 However, all these patients in this study were smokers, and smoking is a recognized cause of RB. It has been proposed that distal airway inflammation associated with PSP leads to obstructive gas trapping and consequent increases in distal airway pressure, which possibly causes air leak into the pleural space.22
Smoking
Cigarette smoking is a significant risk factor for PSP. It is thought to be due to the consequences of airway inflammation leading to airway obstruction with a check valve phenomenon, causing air trapping and development of pneumothorax.22 The lifetime risk is 12% in smokers compared to 0.1% in nonsmokers. Risk is also directly related to the amount of cigarette smoking. Compared to nonsmokers, the relative risk of PSP in men was seven times higher in light smokers (1-12 cigarettes per day), 21 times higher in moderate smokers (13-22 cigarettes per day), and 102 times higher in heavy smokers (>22 cigarettes per day). For women, the relative risk was 4, 14, and 68 times higher in light, moderate, and heavy smokers, respectively.23 Cessation of smoking appears to reduce the risk of recurrence,9 and continued smoking increases the risk of recurrence.24
RB, a form of airway inflammation associated with cigarette smoking, may contribute to the development and recurrence of PSP. In a study with 115 patients with PSP who underwent video-assisted thoracoscopic surgery (VATS), pneumothorax recurrence rates were higher in patients with extensive rather than nonextensive RB for both nonoperative and postoperative pneumothorax.25
Genetics
Familial inheritance of pneumothorax describing the clustering of PSP in certain families has been published. Autosomal dominant, autosomal recessive, polygenic, and X-linked recessive inheritance mechanisms have been proposed.26–28 Birt-Hogg-Dubé (BHD) is an autosomal dominant cancer disorder that predisposes patients to benign skin tumors and renal cancer. It is associated with pleuropulmonary blebs and cysts that lead to PSP.29 In one study of 198 patients with this syndrome, 48 patients (24%) had a history of pneumothorax.30 The gene responsible for this familial cancer syndrome (FLCN) has been mapped to chromosome 17p11.2.31,32 Other mutations of FLCN have been associated with spontaneous pneumothorax and bullous lung disease in the absence of Birt-Hogg-Dubé syndrome.33
Patients with Marfan syndrome are tall, and pneumothorax is a common pulmonary complication. Marfan syndrome is caused by the mutation in FBN1 gene on chromosome 15. This gene is responsible for the formation of 10- to 12-nm microfibrils in the extracellular matrix of connective tissue. It is hypothesized that familial spontaneous pneumothorax is caused by a connective tissue disorder that exhibits mendelian inheritance and FBN1 has been postulated as the causative gene.34
Classification
Spontaneous Pneumothorax
Primary Spontaneous Pneumothorax
PSP is classically seen in previously healthy young men with an asthenic body habitus. The incidence of PSP rises with increasing height among adults of both sexes, more so in men. For those 76 inches or taller, the rate was 200 per 100,000 person-years.35 It is hypothesized that individuals with tall stature and low body mass index combined with smoking are predisposed to develop ELCs owing to the pressure gradient between the lung base and the apex, resulting in increased alveolar distending pressures at the apex.36 Smoking, as previously described, greatly increases the risk of PSP. Smoking increases the relative risk of developing spontaneous pneumothorax about ninefold in women and 22-fold in men, and there is a statistically significant dose-response relationship between smoking and spontaneous pneumothorax.37
Secondary Spontaneous Pneumothorax
SSP has been described in a large variety of diseases including COPD with emphysema, cystic fibrosis (CF), tuberculosis, lung cancer, human immunodeficiency virus (HIV)-associated Pneumocystis jiroveci pneumonia, followed by more rare but “typical” disorders such as lymphangioleiomyomatosis (LAM) and histiocytosis X (Box 47.1). Because lung function in these patients is already compromised, SSP often presents as a potentially life-threatening disease requiring immediate action, as opposed to PSP, which is more of a nuisance than a dangerous condition. The general incidence is almost similar to that of PSP.38
Chronic Obstructive Pulmonary Disease
COPD is the most common cause of SSP, with nearly 70% of SSP attributed to COPD.39 The peak incidence of SSP from COPD typically occurs later in life averaging 60 to 65 years of age.40 The clinical presentation of pneumothorax in COPD is often atypical—pain may be absent, anxiety and breathlessness may predominate and be out of proportion to the collapsed lung, and the classic sign of hyperresonance may not be helpful because of the underlying emphysema. The air leak in these patients is usually large, and the tissues are slow to heal, so it is weeks before the tubes can be taken out.41
Pneumothorax in Drug Abusers
When the peripheral veins of chronic abusers of drugs become obliterated because of a sclerotic or infectious process, the individual may attempt to use larger veins in the groin or neck. Attempted subclavian or supraclavicular (“pocket shot”) injection of drugs in the street setting has led to unilateral or bilateral pneumothoraces.42–44 Douglas and Levison45 found that the incidence of pneumothoraces is equal in both sexes and that it is less of a problem in teenagers and in addicts older than 40 years of age. It was also noted that although most drug users describe using small (21- or 22-gauge) needles, a large, complete, or tension pneumothorax usually develops.
Pneumothorax in HIV-Infected Patients
Pneumothorax is an uncommon but potentially fatal complication of HIV infection. The first report of spontaneous pneumothorax in patients with acquired immunodeficiency syndrome (AIDS) was in 1984.46 With the diagnosis of AIDS, a patient’s risk of sustaining a nontraumatic pneumothorax increases to 450 times that of the general population.47 It has since been described in a generalized HIV-infected population.48,49 Pneumothorax complicated 1.2% of all hospital admissions in a cohort of 599 HIV-infected patients followed over 3 years in a prospective observational study. There was also an associated increase in in-hospital mortality rate (31% versus 6%) for patients without pneumothorax.48
A high incidence (2-9%) of pneumothorax has been reported in patients with AIDS and Pneumocystis carinii pneumonia (PCP).50–52 Pneumocystis carinii, which was thought to be a protozoan, has been renamed as Pneumocystis jiroveci and is now classified as an archiascomycetous fungus.53 Causes of pneumothorax in HIV-infected individuals include P. jiroveci54–57 along with other infectious agents such as Mycobacterium tuberculosis, M. avium intracellulare, pulmonary cytomegalovirus, Pneumococcus organisms,54 or pulmonary toxoplasmosis.54 Pneumothorax has also been described in HIV-infected individuals from Kaposi sarcoma.58
The cause of pneumothorax in patients with PCP is unclear. Several investigators believe that extensive tissue invasion within the alveolar interstitium in severe PCP is an important factor in causing necrosis and subsequent pneumothorax. Several observations highlight this point. The most common sites of tissue invasion with PCP are the alveolar septa, pleurae, and vasculature.59 Tissue invasion could cause necrosis as a result of direct tissue injury by toxins from Pneumocystis,59 infarction from vascular compromise,60,61 or as a result of the host inflammatory response.62
The administration of aerosolized pentamidine has been implicated in the pathogenesis of cavitation, cyst formation, and pneumothorax,50,63,64 but the biologic basis for this relationship is unknown. No direct toxic action of pentamidine on the lungs has been described, so an indirect effect may be present. Cavitation due to PCP may occur primarily in the upper lobes and periphery because aerosolized pentamidine is preferentially delivered to the proximal parenchyma of the lower lobes. Inadequate deposition of pentamidine in the periphery of the lung could allow a chronic, low-grade infection with Pneumocystis to persist, leading to peripheral lung destruction and pneumatocele formation. Increased survival time of AIDS patients due to prophylaxis could allow for development of these lesions.50
Several other risk factors for AIDS-related pneumothorax have been identified. In addition to previous or active infection of P. jiroveci and aerosolized pentamidine, cigarette smoking and the presence of pneumatoceles on chest radiograph are risk factors.65 The association between cigarette smoking and AIDS-related pneumothorax could be explained by subclinical obstructive disease preventing adequate deposition of aerosolized pentamidine in the lung periphery, resulting in subpleural Pneumocystis infection.65 Pulmonary tuberculosis also appears to increase the risk of pneumothorax in AIDS.66
Catamenial Pneumothorax
In most cases, catamenial pneumothorax is related to pelvic or thoracic endometriosis.67,68 Catamenial pneumothorax occurs typically within 24 to 72 hours after onset of menstruation. It is often recurrent and more common than previously thought. Two mechanisms have been described for pneumothorax related to endometriosis. The most common is the movement of endometrial implants to the diaphragm, preferentially to the right side because of the recognized peritoneal circulation up from the pelvis to the right side. These implants then create channels or “holes” through the diaphragm that allow the implants or air to move into the chest. The second and much less frequent cause of endometrial implants causing pneumothorax in the chest is through the venous implants that lodge into the lung itself.69 Clinical manifestations of thoracic endometriosis include chest pain, dyspnea, and hemoptysis. Treatment for the prevention of recurrence is indicated after a first episode of catamenial pneumothorax because recurrences are frequent.38
Cystic Fibrosis
SSP occurs in approximately 6% of all patients with CF and this number increases to 16% to 20% among those who survive to age 18.70,71 SSP from CF is usually due to rupture of apical subpleural cysts. The risk of pneumothorax is inversely proportional to the forced expiratory volume in 1 second (FEV1). Other factors associated with an increased risk of pneumothorax include infection with Pseudomonas aeruginosa, Burkholderia cepacia complex, or Aspergillus species. A previous history of massive hemoptysis also increases risk.
Nonspontaneous Pneumothorax
Traumatic Pneumothorax
Pneumothorax ranks second to rib fractures as the most common sign of chest trauma. It occurs in up to 50% of chest trauma victims.72 Most are caused by a penetrating injury, but closed chest trauma causing alveolar rupture from thoracic compression, fracture of a bronchus, and esophageal rupture have also been reported.73,74 Traumatic pneumothorax can be classified as open, closed, tension, or hemopneumothorax. A tension pneumothorax should be managed immediately by decompression with a large-bore needle usually in the second anterior interspace in the midclavicular line. Open pneumothorax should have a moist sterile gauze pack placed over the open wound, followed by a chest tube. Hemopneumothorax (20% of trauma patients) requires insertion of a large-bore (28-36F) chest tube.38
Occult pneumothorax may be present in half of blunt abdominal trauma patients, many of which are undetected by chest radiograph.75–79 CT of the chest should therefore always be performed in these patients. Most surgeons and emergency physicians will place a chest tube in occult and nonoccult pneumothoraces. Studies suggest, however, that clinically stable patients and those who do not have an enlarging pneumothorax may be treated conservatively, ultimately requiring chest tube placement in about 10% of cases.80
Traumatic Iatrogenic Pneumothorax
Iatrogenic pneumothorax occurs most often following transthoracic needle biopsy (24%), subclavian vein catheterization (22%), thoracentesis (20%), transbronchial lung biopsy (10%), pleural biopsy (8%), and positive-pressure ventilation (7%).81 Diagnosis of iatrogenic pneumothorax is often delayed. Small and asymptomatic iatrogenic pneumothorax, however, often do not need any treatment and resolve spontaneously. In larger or symptomatic pneumothoraces, simple manual aspiration or placement of a small catheter or chest tube attached to a Heimlich valve is usually sufficient.82 Larger tubes may be necessary in patients with emphysema or when the patient is placed on a mechanical ventilator.
Pulmonary Barotrauma During Mechanical Ventilation
Pulmonary barotrauma (PBT) refers to alveolar rupture due to elevated transalveolar pressure (the alveolar pressure minus the pressure in the adjacent interstitial space). PBT was previously estimated to range between 3.8% and 41.7% of patients undergoing mechanical ventilation.83 The rate in actuality may be lower because low tidal volume ventilation is becoming more common. Consequences of barotrauma include pneumothorax, pneumomediastinum, pneumoperitoneum, and subcutaneous emphysema.
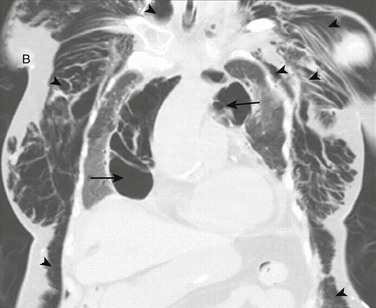
Positive-pressure ventilation increases transalveolar pressure, which can cause alveolar rupture.84 Alveolar rupture allows air from the alveolus to enter the pulmonary interstitium where it can dissect along the perivascular sheaths toward the mediastinum. This can lead to pneumothorax, pneumomediastinum, pneumoperitoneum, or subcutaneous emphysema85,86 (Fig. 47.3). Bronchopleural fistula, tension pneumothorax, tension lung cyst, and subpleural air cyst have also been reported but are less common.87
In a multicenter prospective cohort study of 5183 mechanically ventilated patients, the incidence of PBT was 3%.88 Asthma, chronic interstitial lung disease, and acute respiratory distress syndrome (ARDS) were identified as independent risk factors for barotrauma. Other studies have also demonstrated that acute lung injury (ALI) and ARDS are independent risk factors for PBT.89,90 Elevated peak and plateau pressures have been identified as risk factors.91,92
Neither open lung strategies using high levels of positive end-expiratory pressure (PEEP) nor recruitment maneuvers have been shown to increase the risk of barotrauma.93,94
Clinical Presentation
The clinical presentation of PBT can vary. With pneumothorax, patients may complain of dyspnea or chest pain. Physical findings can include tachycardia, tachypnea, hypertension, or oxyhemoglobin saturation accompanied by unilateral reduction of breath sounds. If a tension pneumothorax develops, there may be hypotension and tracheal deviation. Patients with pneumomediastinum may complain of dyspnea and chest or neck pain. Other findings include tachycardia, tachypnea, and hypertension. A crunching sound may be heard during auscultation. Rarely, hypotension from decreased venous return and cardiac output may occur if tension pneumomediastinum develops.95 Pneumoperitoneum may manifest itself as abdominal pain. Other physical findings include abdominal distention, tenderness, and tympany. Rarely, abdominal compartment syndrome may develop if the pneumoperitoneum progresses to a tension pneumoperitoneum.96 Subcutaneous emphysema generally presents as painless soft tissue swelling. It typically appears in the upper chest, neck, and face. Compression of the affected areas can reveal crepitus. A rare consequence of severe subcutaneous emphysema is compartment syndrome.97
Diagnosis
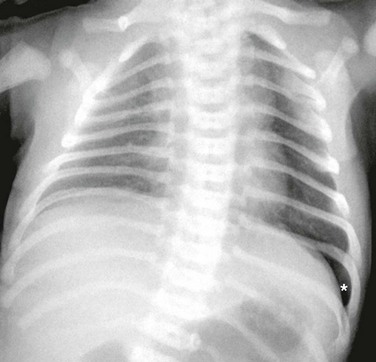
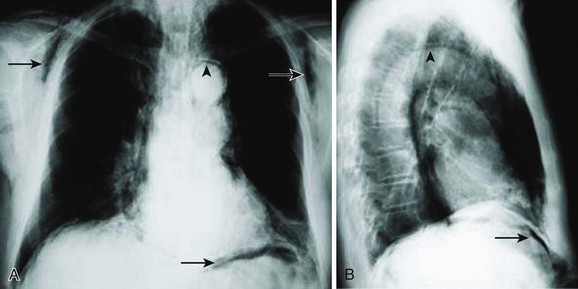
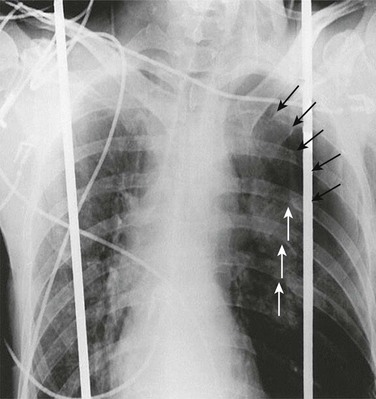
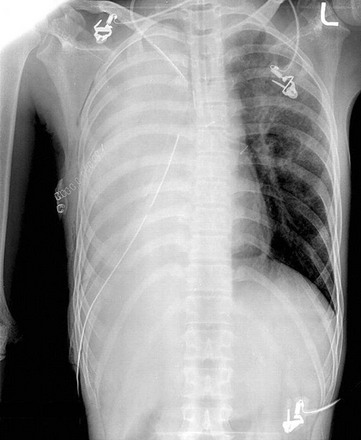
The diagnosis of pneumothorax is suspected when a patient presents with the symptoms and signs described earlier, then confirmed with a portable chest radiograph. An upright chest radiograph has the highest diagnostic yield for pneumothorax, although diagnosis in the intensive care unit (ICU) may be difficult as most patients are semirecumbent or supine.98 In a fully upright chest radiograph, a pneumothorax appears as a radiolucent collection between the visceral and parietal pleurae in the superior portion of the chest. In contrast, when a patient is supine, free air collects in the anterior chest, displacing the costophrenic angle inferiorly, often creating a “deep sulcus” sign. The deep sulcus sign refers to a unilateral increase in the apparent size of the costophrenic angle (Fig. 47.4).
Bedside ultrasound is being used more readily in the ICU to rapidly diagnose pneumothorax. Utilizing the M-mode, the absence of “lung sliding” is indicative of the presence of a pneumothorax99 (Video 47.1).
Pneumomediastinum frequently coexists with pneumothorax. It is usually diagnosed with a portable chest radiograph. It typically appears as radiolucent streaks in the mediastinum (Fig. 47.5).
Pneumoperitoneum is diagnosed with a chest radiograph less than one third of the time. A suspected pneumoperitoneum is best evaluated by chest CT.100 The patient should remain in position for 5 to 10 minutes before the radiograph is taken. This allows time for air to collect in a sufficient volume to be detected radiographically. Pneumoperitoneum may be identified on a supine abdominal radiograph (see Fig. 47.5). Free air accumulates anteriorly when the patient is supine and on a chest or abdominal radiograph may present in several ways. Gas appearing on both sides of the bowel wall is referred to as Rigler’s sign. Gas outlining the peritoneal cavity is known as the football sign. Gas outlining the medial umbilical folds is called an inverted V sign. Gas may also outline the falciform ligament or localize in the right upper quadrant.101
Subcutaneous emphysema is often found by identifying crepitus during physical examination. On chest radiograph of areas of tissue swelling, it can appear as radiolucent streaks throughout the subcutaneous tissue and muscle (see Fig. 47.5).
Prevention
To prevent barotrauma, it is generally recommended that plateau airway pressure be maintained at or below 35 cm H2O. Plateau pressure is the most indicative of the alveolar pressure and therefore is the measure of greatest concern for the prevention of PBT. Lower plateau airway pressures have been associated with a lower incidence of PBT. A threshold pressure appears to exist at 35 cm H2O, above which there is a higher incidence of barotrauma. A meta-analysis of 14 clinical trials demonstrated a strong relationship between PBT and a plateau airway pressure greater than 35 cm H2O or a static compliance less than 30 mL per cm H2O.102 There have not been direct comparisons between management targeting a plateau airway pressure or peak airway pressure. Peak airway pressure is likely a less reliable predictor of PBT given the conflicting data.103–109
Management
The best treatment for PBT is early recognition, and immediate attempts should be made to reduce plateau airway pressure.83 This may require lowering the tidal volume or PEEP, as well as increasing sedation, administering neuromuscular blockade, or advancing treatment of the underlying condition. In cases of pneumothorax while on a mechanical ventilator, there is no high-quality evidence that supports routine insertion of chest tubes for all patients. However, more than 30% of pneumothoraces in mechanically ventilated patients progress to tension pneumothoraces, indicating that these patients must be monitored closely. Treatment for mechanically ventilated patients who develop a pneumomediastinum, pneumoperitoneum, or subcutaneous emphysema is generally supportive unless there is evidence of tension pneumomediastinum or compartment syndrome from pneumoperitoneum or subcutaneous emphysema.95
Prognosis
PBT appears to be associated with increased mortality rate, even though barotrauma is not a direct cause of death in most patients. In a multicenter prospective cohort study, patients with barotrauma had a significantly higher mortality rate (51% versus 39%), a longer length of ICU stay (median 9 versus 7 days), and a longer duration of mechanical ventilation (median 6 versus 4 days) than patients without barotrauma.88 Mortality rate may be related to the severity of the PBT. In one retrospective cohort study of 1700 mechanically ventilated patients, the mortality rate approached 100% when PBT caused a large (>500 mL per breath) bronchopleural fistula.109 High-frequency jet ventilation is FDA (Food and Drug Administration) approved for the management of large bronchopleural fistulas, but this may be outweighed in some patients by increased plateau airway pressure (alveolar pressure), decreased oxygenation, or worse hypercapnia.110
Pneumothorax After Fiberoptic Bronchoscopy and Needle Biopsy of the Lung
Multiple literature reviews have documented the relative safety of fiberoptic bronchoscopy (FOB) with transbronchial biopsy. One review of more than 9000 such procedures found that the rate of pneumothorax was 1.9%.111 An immediate postbronchoscopic chest radiograph rarely provides clinically useful information, and in FOB without transbronchial biopsy an immediate postbronchoscopy radiograph is not necessary.112,113 Another study in 2006 concluded that in asymptomatic patients, routine radiograph after transbronchial biopsy is not necessary.114 It was determined that certain patient populations should have routine radiographs performed after FOB with transbronchial biopsy: comatose or mentally retarded patients, patients receiving positive-pressure ventilation, patients with severe respiratory compromise as a result of disease or surgery, patients with bullous disease, patients who complain of chest pain, and outpatients. Pneumothorax after bronchoalveolar lavage without biopsy is extremely rare. The complication of pneumothorax after transbronchial needle aspiration is also low.115
Pneumothorax is the most common complication of needle aspiration or biopsy of the lung. It has been reported to occur in 17% to 26.6% of patients.116–119 The chest tube insertion rate is much lower, ranging from 1% to 14.2%.116–119 Risk factors for the development of biopsy-related pneumothorax include the presence of COPD, the absence of a history of ipsilateral surgery, small lesion size, a long needle path, and repeated pleural puncture.116–121 Enlarging or symptomatic pneumothorax can be managed by manual aspiration or placement of a small-caliber chest tube.120
Delayed pneumothorax after percutaneous fine-needle aspiration has been reported. A study by Choi and colleagues122 reported on their series of 458 patients who had undergone transthoracic needle biopsy. A follow-up chest radiograph was obtained immediately and at 3, 8, and 24 hours after the biopsy procedure. A pneumothorax that developed after 3 hours was defined as delayed pneumothorax. Pneumothorax developed in 100 of the 458 patients (21.8%), and delayed pneumothorax developed in 15 patients (3.3%). Female gender and absence of emphysematous changes correlated with an increased rate of delayed pneumothorax.
Pneumothorax After Thoracentesis
According to a 1998 National Center for Health Statistics study,123 physicians perform an estimated 173,000 thoracenteses annually in the United States. Iatrogenic pneumothoraces resulting from thoracentesis increase morbidity rate, mortality rate, and length of hospitalization. Previous reports indicated chest tube insertion may be required in up to 50% of cases with a mean duration of placement of approximately 4 days.124,125 Gordon and colleagues126 performed a systematic review and meta-analysis of 24 studies reporting pneumothorax rates after thoracentesis involving 6605 thoracenteses. The overall pneumothorax rate was 6%. In cases in which pneumothorax developed, 34.1% required chest tube placement. Statistically significant risk factors for developing thoracentesis included performing thoracentesis as a therapeutic procedure as opposed to as a diagnostic procedure; the presence of cough, dyspnea, or chest pain during the procedure; and witnessing the aspiration of air during the procedure.126 Although not statistically significant, other possible predictors included the need for two or more needle insertions and concurrent mechanical ventilation.126 Ultrasonography guidance,127–129 more experienced operators,130 and fewer needle passes conferred lower complication rates,131 which paralleled findings from central venous catheter insertion studies. Various mechanisms may explain the pneumothoraces that occur after thoracentesis: the lung may be punctured at the time of needle entry or after the fluid has been withdrawn, or a small amount of air may be drawn into the chest during aspiration or along the needle track if high negative intrapleural pressure develops.132
Pneumothorax Resulting from Nasogastric Feeding Tubes
Small-bore Silastic feeding tubes are being used with increasing frequency for short- and long-term enteral hyperalimentation. The first reported case of pneumothorax as a complication of passing a narrow-bore feeding tube was in 1978.133 This once rare complication has now become more common.134–136 Narrow-bore feeding tubes are particularly likely to give rise to pneumothorax because of the tube’s small diameter (2.7 mm), self-lubricating properties, and wire stylet. These factors allow undetected entry of the tube into the tracheobronchial tree, perforation of pulmonary tissue, and lodging in the pleural cavity.137 Other factors that increase the risk of a misplaced feeding tube include the presence of an endotracheal or tracheostomy tube (these may increase pulmonary passage of the tube by preventing glottis closure and perhaps by inhibiting swallowing), altered mental status, denervation of airways, esophageal stricture, enlargement of the heart, and neuromuscular weakness.138 The clinical signs commonly used to determine correct placement of the feeding tube may be misleading. Normally, to confirm the correct placement of a feeding tube in the stomach, a small amount of air is injected. This produces a characteristic gurgle in the left upper quadrant of the abdomen, but a “pseudoconfirmatory gurgle” with a feeding tube in the chest has been reported.139 Aspiration of large amounts of fluid through the tube is also taken to be a test of correct placement into the stomach, but delayed aspiration of a large quantity of undigested feeding solution from the pleural space, mistaken for gastric contents, has been reported.140
Pneumothorax After Percutaneous Dilational Tracheostomy
Percutaneous dilational tracheostomy (PDT) was first described in 1985 by Ciaglia and colleagues.141 A case series described subcutaneous emphysema and pneumothorax as complications after percutaneous tracheostomy in a series of 326 cases.142 Their review of the literature showed that the incidence of subcutaneous emphysema was 1.4% and that of pneumothorax was 0.8%. Findings associated with pneumothorax included difficult PDT and the use of a fenestrated cannula.
Special Situations
Pneumothorax ex Vacuo
Pneumothorax after partial resolution of total bronchial obstruction,143 as a complication of lobar collapse,144 and after therapeutic thoracentesis for malignant effusions145 has been described. Acute lobar collapse results in a sudden increase in negative pleural pressure surrounding the collapsed lobe. Although the parietal and visceral pleural surfaces remain intact, the gas originating from the ambient tissues and blood is drawn into the pleural space, producing a pneumothorax called pneumothorax ex vacuo. Recognition of this type of pneumothorax is crucial because managing it requires relieving the bronchial obstruction rather than inserting a chest tube. The diagnosis of trapped lung requires documentation of chronicity and absence of pleural inflammation, pleural malignancy, or endobroncial lesion. The pathognomonic radiographic sign of a trapped lung is the pneumothorax ex vacuo, characterized as a small to moderate-sized air collection after evacuation of effusion.146
Sport-Related Pneumothorax
Pneumothorax as a result of blunt trauma from contact sports is a recognized but underreported event. Several cases of pneumothorax or pneumomediastinum sustained during a contact sport have been described in the literature.147,148 In a large case series, Kizer and MacQuarrie149 identified 20 patients who had sustained a spontaneous or traumatic air leak while engaged in an outdoor sport.
Barotrauma Unrelated to Mechanical Ventilation
Although the term barotrauma has traditionally been used to describe the development of extra-alveolar air while on mechanical ventilation, in other instances it may be due to increased intra-alveolar pressure, causing air to leak out of the alveoli. PBT of ascent is a well-known complication of compressed air diving. Pulmonary edema and hemorrhage occur when lung volume decreases below residual volume. As a diver ascends and transalveolar pressure exceeds 20 to 80 mm Hg, overexpansion injury in the form of alveolar rupture can occur.150–152 Divers who hold their breath as they ascend and those with obstructive airway diseases, such as asthma or COPD, are at increased risk.153
Pneumothorax is a relatively uncommon complication in divers, developing in only approximately 10% of those with evidence of barotrauma. Patients with a history of spontaneous pneumothorax, bullae, or cystic lung disease are at increased risk of pneumothorax and should be cautioned against diving.154
Tension Pneumothorax
The classic signs of a tension pneumothorax are deviation of the trachea away from the side with the tension, an increased percussion note, and a hyperexpanded chest that moves little with respiration. Radiographically, tension pneumothorax shows a distinct shift of the mediastinum to the contralateral side and flattening or inversion of the ipsilateral hemidiaphragm (Fig. 47.6). Clinically unstable patients should undergo immediate needle decompression followed by chest tube insertion. Decompression is performed by advancing a standard 14- or 16-gauge intravenous catheter into the pleural space at the junction of the midclavicular line and the second or third intercostal space. The needle is advanced until air can be aspirated into a syringe connected to the needle. The needle is withdrawn and the cannula is left open to air. An immediate rush of air out of the chest indicates the presence of a tension pneumothorax. The maneuver essentially converts a tension pneumothorax into a simple pneumothorax. A chest tube can then be placed.
Clinical Features
PSP usually occurs when the patient is at rest.155 Patients are typically in their early 20s when presenting with PSP, which is rare after age 40. Chest pain and dyspnea are the two main symptoms associated with the development of pneumothorax. One series evaluated 39 patients who presented with one of the two symptoms and 64% of them had both.156 The pain is generally reported as ipsilateral, which usually resolves spontaneously within 24 hours.40 The degree of dyspnea depends on the size of the pneumothorax and the condition of the underlying lung. Cough, malaise, orthopnea, and hemoptysis may also be presenting symptoms.
Hypoxemia is common because collapsed and poorly ventilated portions of lung continue to receive significant perfusion. However, hypercapnia is unusual because underlying lung function is relatively normal and adequate alveolar ventilation can be maintained by the contralateral lung.157 Acute respiratory alkalosis may be present if pain, anxiety, or hypoxia is substantial.
In certain situations, the symptoms of pneumothorax may have an atypical presentation and therefore require a high index of suspicion. During a transbronchial biopsy, a patient may complain of pleuritic chest pain followed by dyspnea. A pneumothorax after a subclavian vein catheterization may present with progressive dyspnea and an alteration of vital signs. In a mechanically ventilated patient, the initial presentation may include hypotension, new-onset respiratory distress, unilateral decrease in breath sounds, a decrease in static and dynamic compliance, and worsening oxygenation.41,158,159
Simultaneous bilateral pneumothoraces is a rare condition because, in humans, the left and right pleural spaces are completely separated. Patients can develop a persistent pleuro-pleuro channel after undergoing a median sternotomy, mediastinal surgery, or heart or heart-lung transplant surgery. This condition has been dubbed “iatrogenic buffalo chest” because the North American buffalo is one of few mammals that have communicating pleural spaces.160 A unilateral thoracic procedure in this situation has been described to cause bilateral pneumothoraces161,162 and “shifting pneumothorax.”163 Simultaneous bilateral spontaneous pneumothorax (SBSP) has been described in a case series with 12 patients.164 Of the 12 patients, 5 had no underlying lung disease. In 7 patients, SBSP was secondary to pulmonary metastases, histiocytosis, undefined interstitial pulmonary disease, tuberculosis, pneumonia, and COPD.
Electrocardiographic Features
The presence of a pneumothorax may lead to distinct electrocardiographic changes, which can be mistaken for myocardial ischemia or infarction. Most findings have been described for left-sided pneumothorax. Poor R wave progression in the anterior precordial leads with a decrease in R wave from V4 to V5, rightward shift of frontal axis, diminution of precordial R voltage, decrease in QRS amplitude, and precordial T-wave inversion have all been described.165–167 The absence of ST-segment elevation and a significant Q wave and reversal of electrocardiographic changes in the sitting position suggest pneumothorax. In right-sided pneumothorax, there is a loss of S wave in lead V2 and prominent R-wave voltage, which may mimic posterior wall myocardial infarction.168
Diagnostic Imaging Modalities
Radiographic Signs
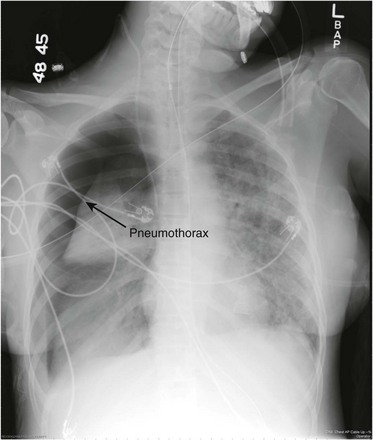
Chest radiography and CT are the first-line imaging modalities used to identify a pneumothorax. The main feature of a pneumothorax on a chest radiograph is a white visceral pleural line. This line separates the visceral pleura from the parietal pleura by a collection of gas (Fig. 47.7). A pneumothorax may be identified using an upright, supine, or lateral decubitus chest radiograph. The lateral decubitus view is the most sensitive, and the supine view is the least sensitive.
Upright Chest Radiograph
In an upright patient with a pneumothorax, most pleural gas accumulates in an apicolateral location. The visceral pleural line appears either straight or convex toward the chest wall. As little as 50 mL of pleural gas may be seen on a chest radiograph.169 Although there is generally a loss of lung volume with a pneumothorax, the collapsed lung preserves its translucency because hypoxic vasoconstriction diminishes the blood flow to the collapsed lung. The value of obtaining an expiratory chest radiograph has been overstated. Inspiratory and expiratory upright chest radiographs detected pneumothorax with equal sensitivity.170
Supine Chest Radiograph
In a supine patient with a pneumothorax, most pleural gas accumulates in a subpulmonary location. A “deep sulcus” sign occurs when gas outlines the anterior pleural reflection, the costophrenic sulcus, and the anterolateral border of the mediastinum (see Fig. 47.4). Rarely, pleural gas can accumulate in the phrenicovertebral sulcus. The visceral pleural line may be seen at the lung base and has a concave contour. Around 500 mL of pleural gas is needed in order to definitively diagnose pneumothorax on a supine chest radiograph.169
Lateral Decubitus Chest Radiograph
A pneumothorax is most easily detected with a lateral decubitus view. Most pleural gas in this position accumulates in the nondependent lateral location. The visceral pleural line appears as a straight or convex line toward the chest wall. As little as 5 mL of pleural gas may be visible on a lateral decubitus view.169
Computed Tomography
CT scanning is the most accurate imaging modality for the detection of pneumothorax although it is generally not the initial option. This method can identify small amounts of intrapleural gas, atypical collections of pleural gas, and loculated pneumothoraces. Complex pleural disease such as pleural effusion and cystic lung disease is optimally displayed by CT scanning.171
Pulmonary Ultrasonography
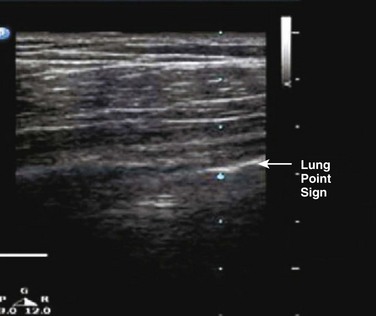
Ultrasound was first used to detect pneumothorax in a horse in 1986 and then in humans shortly thereafter.172 In a normal lung, the visceral and parietal pleurae are adjacent, and ultrasound shows shimmering or sliding at the pleural interface during respiration.173 The absence of “lung sliding” indicates a pneumothorax. Comet tails are an ultrasound artifact that arises when ultrasound encounters a small air-fluid interface (Fig. 47.8). The presence of “sliding lung” and “comet tail” artifacts appear to reliably rule out pneumothorax (Video 47.1). The presence of a “lung point” sign is nearly 100% specific for the detection of pneumothorax. Here the visceral pleura is seen to be intermittently coming into contact with the chest wall during inspiration. The lung point sign may also be helpful in determining the actual size of the pneumothorax (Fig. 47.9). A review of four prospective studies found the sensitivity and specificity of ultrasound for pneumothorax to range from 86% to 98%, which was superior to supine chest radiography (sensitivity 28-75%).174 A small pneumothorax may be missed with ultrasound, and patients with blebs or scarring may have a false-positive finding.175
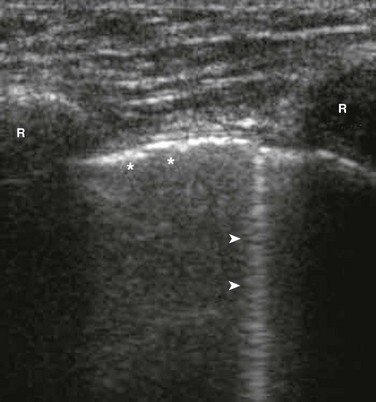
Figure 47.8 Rib shadows (R) are visible as bright reflectors with distal shadow. The pleura (* *) is a bright echogenic line beneath the ribs. Comet tail artifacts (arrows) arise from normal pleura reflecting sound waves. (From Mt. Sinai Emergency Ultrasound Division Tutorials. Accessed at http://sinaiem.us/tutorials/pneumothorax.)
Differential Diagnosis: Conditions Mimicking Pneumothorax
Large subpleural bullae can mimic a loculated pneumothorax. Bullae can be distinguished from pneumothorax due to the fact that only bullae typically have a medial border that is concave to the chest wall.176 Exceptions to this distinction occur with subpulmonary collections of gas, loculated collections of gas, and pleural adhesions. In trauma cases, the stomach can herniate into the chest following rupture of the left hemidiaphragm, and a gas-filled stomach may be mistaken for a loculated pneumothorax. This can be disastrous if drainage with a thoracostomy tube is attempted. A skinfold can generally be distinguished from a pneumothorax by careful evaluation of the radiograph. Skinfolds generally extend beyond the rib cage, stop short of the ribs, and gradually increase in opacity with an abrupt dropoff at the edge of the image. Blood vessels often extend beyond the skinfold.177
Management
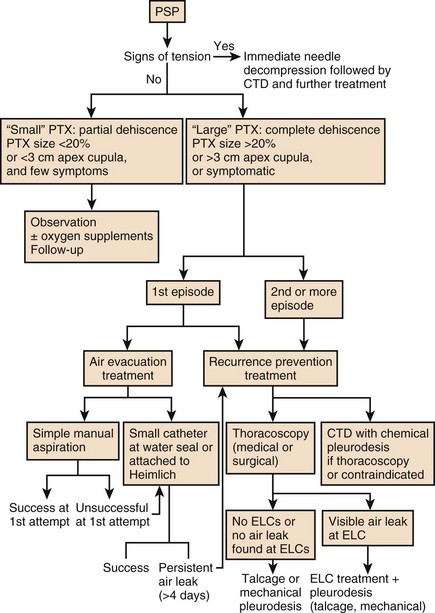
Figure 47.10 represents an algorithmic approach to the management of pneumothorax.
Management of the First Episode of Pneumothorax
Initial management is directed at removing air from the pleural space followed by preventing recurrence. Approaches for the management of the initial episode include observation, supplemental oxygen, simple aspiration of the pneumothorax, and tube thoracostomy. The choice of therapy in a given patient depends on various factors such as size of the pneumothorax, whether the pneumothorax is primary or secondary, the condition of the lungs, the clinical stability of the patient, the outcome of the patient, and whether the pneumothorax has occurred in a special setting. Various guidelines for managing pneumothorax have been published.178,179
Estimating the Size of a Pneumothorax
DL is diameter of the collapsed lung cubed.
DH is diameter of hemithorax on collapsed side cubed.
Both methods express the size of the pneumothorax as a percentage, although the Light method better correlates with the amount of pneumothorax gas removed by suction.180,181
These methods are difficult to apply and tend to underestimate the size of a pneumothorax. As a result, some clinicians tend to describe a pneumothorax as large or small rather than utilize percentages. The American College of Chest Physician guidelines defines a small pneumothorax as less than 3 cm in apex-to-cupola distance.178 British Thoracic Society guidelines define a pneumothorax as small if the distance from chest wall to visceral pleural line is less than 2 cm. They define a large pneumothorax if the distance from the chest wall to the visceral pleural line is 2 cm or greater.179
Treatment Options
Supplemental Oxygen
The choice of treatment depends on patient characteristics and clinical circumstances. Patients who are clinically stable and are having their first PSP can be observed with administration of supplemental oxygen if their pneumothorax is small (≤2 to 3 cm between the lung and chest wall on a chest radiograph).182 Supplemental oxygen is used to facilitate reabsorption of pleural air, and its importance should not be underestimated. The absorption of gas depends in part on the gradient between the partial pressure in the capillaries and that in the pleural space. On room air, the net gradient is only 54 mm Hg, whereas it exceeds 550 mm Hg when the patient is on 100% oxygen.180 A normal rate of reabsorption is 1.25% of the volume of the hemithorax per 24 hours.7 The rate of reabsorption increases sixfold if humidified 100% oxygen is administered.183 Therefore, hospitalized patients with any type of pneumothorax who are not subjected to aspiration of air or tube thoracostomy should be treated with supplemental oxygen at high concentrations.184
Aspiration
Once no further air can be aspirated, one of two methods may be approached.185 A closed stopcock can be attached and the indwelling catheter secured to the chest wall. After 4 hours, a chest radiograph should be obtained and if there is adequate lung expansion, the catheter can be removed. Following another 2 hours of observation, another chest radiograph should be performed. If the lung remains expanded on this chest radiograph, the patient can be discharged.186 Alternatively, the catheter can be left in place and attached to a Heimlich (i.e., one-way) valve. The patient can then be discharged with follow-up within 2 days.178,187 One advantage of aspiration over tube thoracostomy is that the patient need not be hospitalized whether the catheter is removed after aspiration or left attached to the Heimlich valve. There is a lower morbidity rate compared to tube thoracostomy and the procedure is better tolerated. Outcomes have been found to be similar between thoracostomy and aspiration. In a meta-analysis of three randomized, controlled trials (194 patients) that compared aspiration versus tube thoracostomy, aspiration resulted in shorter hospitalization stays and similar clinical outcomes.188 In another randomized trial, 137 patients who had a first episode of PSP were assigned to receive manual aspiration versus tube thoracostomy. The groups had similar rates of immediate (62 versus 68%) and 1-week success (89 versus 88%). Aspiration was associated with a shorter hospital stay (1.8 versus 4 days).189
Tube Thoracostomy
If necessary, most patients with PSP can be managed with a small chest tube (≤22F) or chest catheter (≤14F).190,191 In the absence of trauma and with good aseptic technique, prophylactic antibiotics are not recommended.192 The preferred location for insertion of the chest tube is via an incision at the fourth or fifth intercostal space in the anterior or midaxillary line.193–195 In men, this corresponds to the nipple line and in women, to the inframammary crease. It is important to direct the tube anteriorly because the tube tends to track between the lobes in patients who have complete fissures. If this happens, the tube may get walled off by the lung and cease functioning. The second intercostal space in the midclavicular line has been suggested as an alternative site for tubes, but this requires placement through the pectoralis muscle. This chest tube site is more painful for the patient and the tube is more difficult to dress and manage. Once an insertion site is identified, the tube is inserted using blunt dissection and secured in place.
Percutaneous Pneumothorax Catheters and Thoracic Vents
Alternatives to tube thoracotomy involve using small-lumen catheters or thoracic vents (one-way valve feature). Small catheter tubes have the advantages of ease of insertion, good response, and low incidence of complications. Liu and colleagues reported that in their study using pigtail catheters in 50 patients versus traditional chest tubes in 52 patients that the pigtail drainage was no less effective than the traditional chest tube.196 Complications can include catheter failure from kinking, malposition, inadvertent removal by the patient, and occlusion of the tube or valve by pleural fluid. A thoracic vent can also be used to manage a simple pneumothorax.197,198 It is inserted in the second intercostal space in the midclavicular line. This device has the advantages of a urethane tube that does not kink, a self-contained one-way valve, and a unique signal diaphragm that reflects pleural pressure. This device is not suitable for use in patients who are expected to have large-volume or protracted air leaks.
Persistent Air Leak and Bronchopleural Fistula
If a lung is at least 90% inflated but an air leak is present after 3 days, more aggressive treatment may be warranted. The simplest approach is to attach the chest tube to a unidirectional flutter valve such as a Heimlich valve. This allows rapid discharge of the patient with subsequent outpatient follow-up. An alternative approach is to perform an autologous blood patch.206,207 This involves withdrawing blood from the patient’s peripheral vein and aseptically infusing the blood into the pleural space through the chest tube. The ideal amount of blood to infuse is not known. The range in different series has been 24 to 200 mL. After infusion of the blood, the tubing from the chest tube is draped over a hook approximately 60 cm above the patient’s head and then down to a water seal device on the floor. The chest tube is then removed 24 hours after cessation of the air leak. The most serious side effect is empyema, which occurred in 9% of patients in one series.208 Lastly, if the lung is less than 90% expanded and the patient has a persistent air leak, the preferred intervention is VATS. An air leak persisting for more than 7 days is termed a bronchopleural fistula and can occur in up to a third of pneumothorax cases.209 After 7 days of a persistent air leak, tube thoracostomy is deemed to have failed and more definitive treatment such as surgery or pleurodesis can be planned.
Recurrence Prevention
Once the initial episode of pneumothorax has resolved, the decision to prevent future pneumothoraces must be made. Different recurrence rates have been reported in the literature; from 20% to 52% after the first PSP.210–212 In the following groups of patients, further management against recurrence is recommended: recurrent pneumothorax, patients with chronic air leak, patients with large bullae, patients who live in remote areas, and patients in which a recurrence could be a hazard (e.g., airline personnel or divers). The following are established risk factors for recurrence: more than one previous episode, COPD, air leak for more than 48 hours after first episode, and large cysts seen on radiograph. The following are possible risk factors for recurrence: nonoperative management of first episode (versus tube drainage) and tube drainage for only 24 hours during first episode (versus 3 to 4 days). Further management in these high-risk groups is aimed at preventing recurrence. Therapies to prevent recurrence include chemical pleurodesis, VATS, or surgical thoracotomy.
Chemical Pleurodesis
In patients who are unable or unwilling to undergo VATS, pleurodesis (adhesion of visceral and parietal pleura) can be done by introducing the sclerotic agent via the chest tube. Tetracycline had been used for pleurodesis,213 but sterile tetracycline is no longer available. As a result, intrapleural instillation of doxycycline has been used as an alternative for pleurodesis.214 Intrapleural doxycycline can be painful, so it is recommended that patients be premedicated with analgesics or anxiolytics. Talc slurry, which is composed of finely powdered magnesium silicate, can also be used for pleurodesis.215–217 Recurrence rates after treatment vary between 5% and 8%. Controversy exists whether talc should be used as a sclerosant in young, otherwise healthy individuals due to safety concerns and fear of long-term complications. Intrapleural injection of talc for malignant pleural effusions has been associated with development of ARDS in 1% to 2% of patients.218 Extensive pleural thickening and calcifications were reported in another patient years after treatment.219 However, several studies support the safety of talc pleurodesis for prevention of recurrent pneumothorax.215,220,221
Video-Assisted Thoracoscopic Surgery Pleurodesis
VATS is effective not only in the treatment of spontaneous pneumothorax but also in the prevention of recurrent pneumothorax.222,223 The rate of recurrence is less than 5% after VATS with bleb/bullae resection and pleurodesis. Mechanical pleurodesis with dry gauze, chemical pleurodesis with talc, and laser ablation of the parietal pleura are among the techniques used.
Surgical Thoracotomy
The indications for open thoracotomy are the same as those for VATS. Thoracoscopy has virtually replaced open thoracotomy in the management of spontaneous pneumothorax due to shorter hospitalizations and less postoperative pain.224,225 Thoracotomy is recommended only if thoracoscopy is unavailable or has failed.
Management Under Special Circumstances
Secondary Spontaneous Pneumothorax
Patients with SSP should be hospitalized owing to their diminished pulmonary reserve from their underlying lung disease. Patients with a small pneumothorax (≤2 cm between the lung and chest wall on a chest radiograph) who are clinically stable may be observed. Patients with a large pneumothorax or who are clinically unstable should have a chest tube placed. Tube thoracotomy is generally preferred over needle aspiration because it is more likely to be successful. In one trial, tube thoracostomy was more likely to have the pleural air completely evacuated than with needle aspiration (93% versus 67%).226 About 80% of patients with SSP will have lung expansion and cessation of their air leak within 7 days after tube thoracostomy.227,228 Patients who are on mechanical ventilation or who are at risk for a large air leak should be managed with a 24 to 28F chest tube. A smaller chest tube (16 to 22F) is preferred for most other patients. The chest tube should be connected to a water seal device with or without suction. In general, the chest tube should remain in place until a procedure is performed to prevent a recurrent pneumothorax. Patients who decline preventive interventions can have their chest tube clamped 12 hours after the last evidence of an air leak. A chest radiograph should be done 24 hours after the last evidence of an air leak, and if the pneumothorax has not recurred, the chest tube can be removed.
Pneumothorax in HIV-Infected Patients
Because the majority of pneumothoraces in HIV-infected patients occurs in association with P. jiroveci, all HIV-infected patients who present with a pneumothorax should undergo a diagnostic evaluation for P. jiroveci infection. P. jiroveci pneumonia–related pneumothorax is complicated by a virulent form of necrotizing subpleural lesions, which result in diffuse air leaks that are refractory to standard treatment.229 Asymptomatic patients with a small pneumothorax (less than 15-20%) may be observed. Symptomatic patients and those with a larger pneumothorax will need chest tube thoracostomy and those with a persistent air leak will likely need additional therapy with video-assisted thoracoscopy for stapling and pleurodesis. Patients who are poor operative candidates may benefit from bedside pleurodesis.
Pneumothorax in Cystic Fibrosis
Pleurodesis as an initial step in the management of pneumothorax in CF is considered contraindicated because it results in extensive pleural adhesions that jeopardize subsequent lung transplantation.230 If initial tube thoracostomy does not bring resolution of air leak within 5 days, blebectomy should be performed. If blebectomy is unsuccessful, a definitive pleural ablative procedure should be considered.
Catamenial Pneumothorax and Pneumothorax Complicating Pregnancy
The initial episode is managed in the usual manner. Recurrences, which occur 72 hours before or after menstrual flow, are managed by pleurodesis or hormonal treatment.231 Therapeutic options include oral contraceptive pills, danazol, progestational agents, and gonadotropin-releasing hormone (GnRH) analogs.69 Thoracotomy should be considered if the patient is unable to take ovulation-suppressing drugs, has a recurrent pneumothorax while on drugs, or wants to become pregnant. Pneumothorax complicating pregnancy is managed in the usual way, but due to the fact that there is a high rate of recurrence during parturition, thoracotomy with resection of blebs if present should be considered.232
Pneumothorax in Air Travelers
The volume of gas is inversely proportional to the pressure at which it is exposed. As barometric pressure falls in the aircraft cabin during ascent, trapped air in any noncommunicating body cavity, such as in a lung bleb or bulla, will expand. Regulatory government agencies, such as the Federal Aviation Administration, have requirements specifying that commercial aircraft cabins be pressurized to simulate an altitude (so-called cabin altitude) of approximately 8000 ft (2438 m). It is estimated that the volume of air in a noncommunicating body cavity will increase by approximately 38% upon ascent from sea level to the maximum “cabin altitude” of 8000 feet (2438 m).233 For patients who develop signs and symptoms of a pneumothorax in-flight, administration of supplemental oxygen is the most important intervention. For patients in respiratory distress, emergency landing at the nearest airport will allow prompt evaluation and insertion of a chest tube, if needed. The optimal length of time to wait after resolution of a pneumothorax is unknown.234 For patients with a prior pneumothorax, the decision regarding air travel must be made on an individual basis. One must take into consideration the likelihood of recurrence and how well the patient would tolerate a subsequent pneumothorax. A patient with relatively normal lung parenchyma could be permitted to fly 2 weeks after resolution of an iatrogenic pneumothorax. In a patient with severe bullous emphysema, limited cardiopulmonary reserve, and a prior spontaneous pneumothorax, air travel may be contraindicated.
Pneumothorax in Lymphangioleiomyomatosis
Lymphangioleiomyomatosis (LAM) is a rare and often fatal disease that affects predominantly women of childbearing age. The normal architecture of the lung is distorted by multiple small cysts, ranging from 0.1 cm to several centimeters in diameter, with progressive decline in lung function. Spontaneous pneumothorax occurs in 50% of cases. It is often recurrent, can be bilateral, and may necessitate pleurodesis.235,236 Because of the morbidity and cost associated with multiple recurrences, recommendations include definitive intervention at the time of the initial pneumothorax. Pleurodesis in these cases does not preclude successful transplantation.237
Complications Related to Management
Reexpansion pulmonary edema may occur after rapid reexpansion of a collapsed lung in patients with a pneumothorax. It is typically unilateral238 (Fig. 47.11). The pathophysiologic mechanism is unknown. The incidence of reexpansion pulmonary edema initially appeared to be related to the rapidity of lung reexpansion and to the severity and duration of lung collapse. However, a study examining development of reexpansion pulmonary edema following thoracentesis found that it was independent of the volume of fluid removed and pleural pressures, and recommended that even large pleural effusions be drained completely as long as chest pain or end-expiratory pleural pressure less than −20 cm H2O does not develop.239 Patients typically present soon after the inciting event, although presentation can be delayed for up to 24 hours in some cases. The clinical course varies from isolated radiographic changes to complete cardiopulmonary collapse. Mortality rates as high as 20% have been described.240 Treatment is generally supportive. Supplemental oxygen is administered and, if necessary, mechanical ventilation is used. The disease is usually self-limited.
References
1. Getz, SB, Beasley, WE. Spontaneous pneumothorax. Am J Surg. 1983; 145:823.
2. Laennec, RT. De l’Auscultation Mediate. Paris, JA Brosson and JS Chaude, 1819, cited by Ransdell HT Jr, McPherson RC: Management of spontaneous pneumothorax. Arch Surg. 1963; 87:1023.
3. Joseph, M, Goulston, K, Grant, AF, Little, JM. Spontaneous pneumothorax. Med J Aust. 1964; 1:1.
4. Hewett, FC. Thoracentesis: The plan of continuous aspiration. BMJ. 1876; 1:317.
5. Kjaergaard, H. Spontaneous pneumothorax in the apparently healthy. Acta Med Scand. 1932; 43(Suppl 1):159.
6. Grundy, S, Bentley, A, Tschopp, JM. Primary spontaneous pneumothorax: A diffuse disease of the pleura. Respiration. 2012; 83:185–189.
7. Light, RW. Pleural Diseases, 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2001.
8. Chen, K, Jerng, J, Liao, W, et al. Pneumothorax in the ICU. Patient outcomes and prognostic factors. Chest. 2002; 122:678.
9. Sadikot, RT, Greene, T, Meadows, K, et al. Recurrence of primary spontaneous pneumothorax. Thorax. 1997; 52:805.
10. Noppen, M. Spontaneous pneumothorax: Epidemiology, pathophysiology and cause. Eur Respir Rev. 2010; 19:217–219.
11. Noppen, M, De Keukeliere, T. Pneumothorax. Respiration. 2008; 76:121–127.
12. Martinez-Ramos, D, Angel-Yepes, V, Escrig-Sos, J, et al. Usefulness of computed tomography in determining risk of recurrence after a first episode of primary spontaneous pneumothorax: Therapeutic implication. Arch Bronconeumol. 2007; 43:304–308.
13. Ouanes-Besbes, L, Golli, M, Knani, J, et al. Prediction of recurrent spontaneous pneumothorax: CT scan findings versus management features. Respir Med. 2007; 101:230–236.
14. Chou, SH, Li, HP, Lee, JY, et al. Is prophylactic treatment of contralateral blebs in patients with primary spontaneous pneumothorax indicated? J Thorac Cardiovasc Surg. 2010; 139:1241–1245.
15. Huang, TW, Lee, SC, Cheng, YL, et al. Contralateral recurrence of primary spontaneous pneumothorax. Chest. 2007; 132:1146–1150.
16. Noppen, M, Stratakos, G, Verbanck, S, et al. Fluorescein-enhanced autofluorescence thoracoscopy in primary spontaneous pneumothorax. Am J Respir Crit Care Med. 2004; 170:680–682.
17. Baumann, MH. Management of spontaneous pneumothorax. Clin Chest Med. 2006; 27:369.
18. Noppen, M, Dekeukeleire, T, Hannon, S, et al. Fluorescein-enhanced autofluorescence thoracoscopy in patients with primary spontaneous pneumothorax and normal subjects. Am J Respir Crit Care Med. 2006; 174:26–30.
19. Ohata, M, Suzuki, H. Pathogenesis of spontaneous pneumothorax. With special reference to the ultrastructure of emphysematous bullae. Chest. 1980; 77:771–776.
20. Lichter, I, Gwynne, JF. Spontaneous pneumothorax in young subjects. A clinical and pathological study. Thorax. 1971; 26:409–417.
21. Cottin, V, Streichenberger, N, Gamondes, J-P, et al. Respiratory bronchiolitis in smokers with spontaneous pneumothorax. Eur Respir J. 1998; 12:702–704.
22. Smit, HJ, Golding, RP, Schramel, FMNH, et al. Lung density measurements in spontaneous pneumothorax demonstrate air trapping. Chest. 2004; 125:2083–2090.
23. Bense, L, Eklund, G, Wiman, L-G, et al. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest. 1987; 92:1009–1012.
24. Smit, H, Chatrou, M, Postmus, P. The impact of spontaneous pneumothorax, and its treatment, on the smoking behavior of young adult smokers. Respir Med. 1998; 92:1132.
25. Cheng, YL, Huang, TW, Lin, CK, et al. The impact of smoking in primary spontaneous pneumothorax. J Thorac Cardiovasc Surg. 2009; 138:192.
26. Koivisto, PA, Mustonen, A. Primary spontaneous pneumothorax in two siblings suggests autosomal recessive inheritance. Chest. 2001; 119:1610.
27. Abolnik, IZ, Lossos, IS, Zlotogora, J, Brauer, R. On the inheritance of primary spontaneous pneumothorax. Am J Med Genet. 1991; 40:155.
28. Gibson, GJ. Familial pneumothoraces and bullae. Thorax. 1977; 32:88.
29. Painter, JN, Tapanainen, H, Somer, M, et al. A 4-bp deletion in the Birt-Hogg-Dube gene (FLNC) causes dominantly inherited spontaneous pneumothorax. Am J Hum Genet. 2005; 76:522.
30. Toro, JR, Paulter, SE, Stewart, L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations with 89 families with Birt-Hogg-Dube syndrome. Am J Respir Crit Care Med. 2007; 175:1044.
31. Khoo, SK, Giraud, S, Kahnoski, K, et al. Clinical and genetic studies of Birt-Hogg-Dube syndrome. J Med Genet. 2002; 39:906.
32. Zbar, B, Alvord, WG, Glenn, G, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in Birt-Hogg-Dube syndrome. Cancer Epidemiol Biomarkers Prev. 2002; 11:393.
33. Graham, RB, Nolasco, M, Peterlin, B, Garcia, CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med. 2005; 172:39.
34. Cardy, CM, Maskell, NA, Handford, PA, et al. Familial spontaneous pneumothorax and FBN1 mutations. Am J Respir Crit Care Med. 2004; 169:1260.
35. Melton, LJ, III., Hepper, NGG, Offord, KP, et al. Influence of height on the risk of spontaneous pneumothorax. Mayo Clin Proc. 1981; 56:678.
36. Light, R. Pleural Disease, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007.
37. Bense, L, Eklund, G, Odont, D, et al. Smoking and increased risk of contracting spontaneous pneumothorax. Chest. 1987; 92:1009.
38. Noppen, M, De Keukelieire, T. Pneumothorax. Respiration. 2008; 76:121–127.
39. Guo, Y, Xie, C, Rodriguez, RM, Light, RW. Factors related to recurrence of spontaneous pneumothorax. Respirology. 2005; 10:378.
40. Noppen, M, Schramel, F. Pneumothorax. Eur Respir Monogr. 2002; 22:279–296.
41. Vukich, DJ. Diseases of the pleural space. Med Clin North Am. 1989; 7:309.
42. Lewis, JW, Jr., Groux, N, Elliott, JP, Jr., et al. Complications of attempted central venous injection performed by drug abusers. Chest. 1980; 78:613.
43. Cohen, HL, Cohen, SW. Spontaneous bilateral pneumothorax in drug addicts. Chest. 1984; 86:645.
44. Wisdom, K, Nowak, RM, Richardson, HH, et al. Alternate therapy for traumatic pneumothorax in “pocket-shooters. Ann Emerg Med. 1986; 15:428.
45. Douglas, RE, Levinson, MA. Pneumothorax in drug abusers: An urban epidemic? Am Surg. 1986; 52:377.
46. Wollschlager, CM, Khan, FA, Chitkara, RK, et al. Pulmonary manifestations of acquired immune deficiency syndrome (AIDS). Chest. 1984; 85:197.
47. Becker, CE, Reynolds, M, Roy, TM. Treatment of pneumothorax in the patients with AIDS. J Ky Med Assoc. 1996; 94:59.
48. Afessa, B. Pleural effusions and pneumothorax in hospitalized patients with HIV infection: The Pulmonary Complications, ICU support, and Prognostic Factors of Hospitalized Patients with HIV Study. Chest. 2000; 117:1031.
49. Radhi, S, Alexander, T, Ukwu, M, et al. Outcome of HIV-associated Pneumocystis pneumonia in hospitalized patients from 2000 through 2003. BMC Infect Dis. 2008; 8:118.
50. Sepkowitz, KA, Telzak, EE, Gold, JWM, et al. Pneumothorax in AIDS. Ann Intern Med. 1991; 114:455.
51. Truitt, T, Bagheri, K, Bagheri, K, Safirstein, BH, et al. Spontaneous pneumothorax in Pneumocystis carinii pneumonia: Common or uncommon? AJR Am J Roentgenol. 1992; 158:916.
52. Fleischer, AG, McElvaney, G, Lawson, L, et al. Surgical management of spontaneous pneumothorax in patients with acquired immunodeficiency syndrome. Ann Thorac Surg. 1988; 45:21.
53. Sidhu, GS, Cassai, ND, Pei, Z. Pneumocystis carinii: An update. Ultrastruct Pathol. 2003; 27:115.
54. Byrnes, TA, Brevig, JK, Yeoh, CB, et al. Pneumothorax in patients with acquired immunodeficiency syndrome. J Thorac Cardiovasc Surg. 1989; 98:546.
55. McClellan, MD, Miller, SB, Parsons, PE, et al. Pneumothorax with Pneumocystis carinii pneumonia in AIDS. Incidence and clinical characteristics. Chest. 1991; 100:1224.
56. Hoyt, TL, Tarver, RD. HIV, Pneumocystis carinii pneumonia, and pneumothoraces. Semin Respir Infect. 2002; 17:315.
57. Goodman, PC, Daley, C, Minagi, H. Spontaneous pneumothorax in AIDS patients with Pneumocystis carinii pneumonia. AJR Am J Roentgenol. 1986; 147:29.
58. Floris, C, Sullis, ML, Bernascani, M, et al. Pneumothorax in pleuropulmonary Kaposi’s sarcoma related to acquired immunodeficiency syndrome. Am J Med. 1989; 87:123.
59. Murray, CE, Schmidt, RA. Tissue invasion by Pneumocystis carinii: A possible cause of cavitary pneumonia and pneumothorax. Human Pathol. 1992; 23:1380.
60. Saldana, MJ, Mones, JM. Cavitation and other atypical manifestations of Pneumocystis carinii pneumonia. Semin Diagn Pathol. 1989; 6:273.
61. Liu, YC, Tomashefski, JF, Jr., Tomford, JW, Green, H. Necrotizing Pneumocystis carinii vasculitis associated with lung necrosis and cavitation in a patient with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1989; 113:494.
62. Eng, RH, Bishburg, E, Smith, SM. Evidence for destruction of lung tissues during Pneumocystis carinii infection. Arch Intern Med. 1987; 147:746.
63. Jules-Elysee, KM, Stover, DE, Zaman, MB, et al. Aerosolized pentamidine: Effect on diagnosis and presentation of Pneumocystis carinii pneumonia. Ann Intern Med. 1990; 112:750.
64. Gutierrez, Y. The biology of Pneumocystis carinii. Semin Diagn Pathol. 1989; 6:203.
65. Metersky, ML, Colt, HG, Olson, LK, Shanks, TG. AIDS-related spontaneous pneumothorax. Risk factors and treatment. Chest. 1995; 108:946.
66. Tumbarello, M, Tacconelli, E, Pirronti, T, et al. Pneumothorax in HIV-infected patients: Role of Pneumocystis carinii pneumonia and pulmonary tuberculosis. Eur Respir J. 1997; 10:1332.
67. Alifano, M, Roth, T, Broet, SC, et al. Catamenial pneumothorax: A prospective study. Chest. 2003; 124:1004–1008.
68. Augoulea, A, Lambrinoudaki, I, Christodoulakos, G. Thoracic endometriosis syndrome. Respiration. 2008; 75:113–119.
69. Hazelrigg, SR. Secondary spontaneous pneumothorax. Catamenial pneumothorax. Chest. 2003; 124:781.
70. Flume, PA, Strange, C, Ye, X, et al. Pneumothorax in cystic fibrosis. Chest. 2005; 128:720.
71. Flume, PA. Pneumothorax in cystic fibrosis. Chest. 2003; 123:217.
72. Bridges, KC, Welch, G, Silver, M, et al. CT detection of occult pneumothorax in multiple trauma patients. J Emerg Med. 1996; 13:173–174.
73. Guest, JL, Anderson, JN. Major airway injury in closed chest trauma. Chest. 1977; 72:63.
74. Amauchi, W, Birolini, D, Branco, PD, et al. Injuries to the tracheobronchial tree in closed trauma. Thorax. 1983; 38:923.
75. Garramone, RR, Jr., Jacobs, LM, Sahdev, P. An objective method to measure and manage occult pneumothorax. Surg Gynecol Obstet. 1991; 173:257.
76. Collins, JC, Levine, G, Waxman, K. Occult traumatic pneumothorax: Immediate tube thoracostomy versus expectant management. Am Surg. 1992; 58:743.
77. Wolfman, NT, Glipin, JW, Bechtold, RE, et al. Occult pneumothorax in patients with abdominal trauma: CT studies. J Comput Assist Tomogr. 1993; 17:56.
78. Enderson, BL, Abdalla, R, Frame, SB, et al. Tube thoracostomy for occult pneumothorax: A prospective randomized study of its use. J Trauma Inj Infect Crit Care. 1993; 35:726.
79. Collins, JA, Samra, GS. Failure of chest x-rays to diagnose pneumothoraces after blunt trauma. Anaesthesia. 1998; 53:69.
80. Johnson, G. Traumatic pneumothorax: Is a chest drain always necessary? J Accid Emerg Med. 1996; 13:173–174.
81. Baumann, MH, Noppen, M. Pneumothorax. Respirology. 2004; 9:157–164.
82. Brown, KT, Brody, LA, Getrajdmann, GI, Napp, TE. Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology. 1997; 205:249–252.
83. Burns, KE, Adhikari, NK, Slutsky, AS, et al. Pressure and volume limited ventilation for the ventilatory management of patients with acute lung injury: A systematic review and meta-analysis. PLoS One. 2011; 6(1):e14623.
84. Parker, JC, Hernandez, LA, Peevy, KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med. 1993; 21:131.
85. Pingleton, SK. Barotrauma in acute lung injury: Is it important? Crit Care Med. 1995; 23:223.
86. Maunder, RJ, Pierson, DJ, Hudson, LD. Subcutaneous and mediastinal emphysema. Pathophysiology, diagnosis, and management. Arch Intern Med. 1984; 144:1447.
87. Morris, WP, Butler, BD, Tonnesen, AS, Allen, SJ. Continuous venous air embolism in patients receiving positive end-expiratory pressure. Am Rev Respir Dis. 1993; 147:1034.
88. Anzueto, A, Frutos-Vivar, F, Esteban, A, et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004; 30:612.
89. Gammon, RB, Shin, MS, Groves, RH, Jr., et al. Clinical risk factors for barotrauma: A multivariate analysis. Am J Respir Crit Care Med. 1995; 152:1235.
90. Schnapp, LM, Chin, DP, Szaflarski, N, et al. Frequency and importance of barotrauma in 100 patients with acute lung injury. Crit Care Med. 1995; 23:272.
91. Petersen, GW, Baier, H. Incidence of pulmonary barotrauma in a medical ICU. Crit Care Med. 1983; 11:67.
92. International Consensus Conferences in Intensive Care Medicine. Ventilator-associated lung injury in ARDS, July 1999. Am J Respir Crit Care Med. 1999; 160:2118.
93. Hodgson, C, Keating, JL, Holland, AE, et al. Recruitment manoeuvers for adults with acute lung injury receiving mechanical ventilation. Cochrane Database Syst Rev. 2009.
94. Briel, M, Meade, M, Mercat, A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA. 2010; 303:865.
95. Shennib, HF, Barkun, AN, Matouk, E, Blundell, PE. Surgical decompression of tension pneumomediastinum. A ventilator complication of status asthmaticus. Chest. 1988; 93:1301.
96. Winer-Muram, HT, Rumbak, MJ, Bain, RS, Jr. Tension pneumoperitoneum as a complication of barotrauma. Crit Care Med. 1993; 21:941.
97. DeGorordo, A, Vallejo-Manzur, F, Chanin, K, Varon, J. Diving emergencies. Resuscitation. 2003; 59:171.
98. Rankine, JJ, Thomas, AN, Fluechter, D. Diagnosis of pneumothorax in critically ill adults. Postgrad Med J. 2000; 76:399.
99. Vezzani, A, Brusasco, C, Palermo, S, et al. Ultrasound localization of central vein catheter and detection of post procedural pneumothorax: An alternative to chest radiography. Crit Care Med. 2010; 38:533.
100. Caceres, M, Braud, RL, Maekawa, R, et al. Secondary mediastinum: A retrospective comparative analysis. Lung. 2009; 187:341.
101. Levine, MS, Scheiner, JD, Rubesin, SE, et al. Diagnosis of pneumoperitoneum on supine abdominal radiographs. AJR Am J Roentgenol. 1991; 156:731.
102. Boussarsar, M, Thierry, G, Jaber, S, et al. Relationship between ventilator settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002; 28:406.
103. Manning, HL. Peak airway pressure: Why the fuss? Chest. 1994; 105:242.
104. Marini, JJ, Ravenscraft, SA. Mean airway pressure: Physiologic determinants and clinical importance—Part 2: Clinical implications. Crit Care Med. 1992; 20:1604.
105. Williams, TJ, Tuxen, DV, Scheinkestel, CD, et al. Risk factors for morbidity in mechanically ventilated patients with acute severe asthma. Am Rev Respir Dis. 1992; 146:607.
106. Weg, JG, Anzueto, A, Balk, RA, et al. The relation of pneumothorax and other air leaks to mortality in the acute respiratory distress syndrome. N Engl J Med. 1998; 338:341.
107. Eisner, MD, Thompson, BT, Schoenfeld, D, et al. Airway pressures and early barotrauma in patients with acute lung injury and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002; 165:978.
108. Petersen, GW, Baier, H. Incidence of pulmonary barotrauma in a medical ICU. Crit Care Med. 1983; 11:67.
109. Pierson, DJ, Horton, CA, Bates, PW. Persistent bronchopleural fistula air leak during mechanical ventilation. A review of 39 cases. Chest. 1986; 90:321.
110. Bishop, MJ, Benson, MS, Sato, P, Pierson, DJ. Comparison of high-frequency jet ventilation with conventional mechanical ventilation for bronchopleural fistula. Anesth Analg. 1987; 66(9):833.
111. Milam, MG, Evins, AE, Sahn, SA, et al. Immediate chest roentgenography following fiberoptic bronchoscopy. Chest. 1989; 96:477.
112. Frazier, WD, Pope, TL, Jr., Findely, LJ, et al. Pneumothorax following transbronchial biopsy: Low diagnostic yield with routine chest roentgenograms. Chest. 1990; 97:539.
113. Blasko, LH, Hernandez, IMS, Grarrido, UV, et al. Safety of the transbronchial biopsy in outpatients. Chest. 1991; 99:562.
114. Izbicki, G, Shitrit, D, Yarmolovsky, A, et al. Is routine chest radiography after transbronchial biopsy necessary? A prospective study of 350 cases. Chest. 2006; 129:1561.
115. Reichenberger, F, Weber, J, Tamm, M, et al. The value of transbronchial needle aspiration in the diagnosis of peripheral pulmonary lesions. Chest. 1999; 116:704.
116. Covey, AM, Gandhi, R, Brody, LA, et al. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Intervent Radiol. 2004; 15:479–483.
117. Saji, H, Nakumura, H, Tsuchida, T, et al. The incidence and risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: The angle of the needle trajectory is a novel predictor. Chest. 2002; 121:1521–1526.
118. Yeow, KM, Su, IH, Pan, KT, et al. Risk factors of pneumothorax and bleeding: Multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004; 126:748–754.
119. Khan, MF, Straub, R, Moghaddam, SR, et al. Variable affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol. 2008; 18:1356–1363.
120. Wu, C, Maher, M, Shepard, J. Complications of CT-guided percutaneous needle biopsy of the chest: Prevention and management. AJR Am J Roentgenol. 2011; 196:678–682.
121. Fish, GD, Stanley, JH, Miller, KS, et al. Postbiopsy pneumothorax: Estimating the risk by chest radiography and pulmonary function tests. AJR Am J Roentgenol. 1988; 150:71–74.
122. Choi, C-M, Um, S-W, Yoo, C-G, et al. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest. 2004; 126:151.
123. Owings, MF. Ambulatory and inpatient procedures in the United States. Vital Health Stat 13. 1998; 139:1–119.
124. Despars, JA, Sassoon, CS, Light, RW. Significance of iatrogenic pneumothoraces. Chest. 1994; 105(4):1147–1150.
125. Sassoon, CS, Light, RW, O’Hara, VS, et al. Iatrogenic pneumothorax; etiology and morbidity: Results of a Department of Veterans Affairs Cooperative Study. Respiration. 1992; 59(4):215–220.
126. Gordon, CE, Feller-Kopman, D, Balk, EM, Smetana, GW. Pneumothorax following thoracentesis. Arch Intern Med. 2010; 170(4):332–339.
127. Raptopoulos, V, Davis, LM, Lee, G, et al. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991; 156:917.
128. Hind, D, Calvert, N, McWilliams, R, et al. Ultrasonic locating devices for central venous cannulation: Meta-analysis. BMJ. 2003; 327(7411):e361.
129. Randolph, AG, Cook, DJ, Gonzales, CA, Pribble, CG. Ultrasound guidance for placement of central venous catheters: A meta-analysis of the literature. Crit Care Med. 1996; 24(12):2053–2058.
130. Sznajder, JI, Zveibil, FR, Bitterman, H, et al. Central vein catheterization: Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986; 146(2):259–261.
131. Mansfield, PF, Hohn, DC, Fornage, BD, et al. Complications and failures of subclavian-vein catheterization. N Engl J Med. 1994; 331(26):1735–1738.
132. Swinburne, AJ, Bixby, K, Fedullo, AJ, et al. Pneumothorax after thoracentesis (letter). Arch Intern Med. 1991; 151:2095.
133. James, RH. An unusual complication of passing a narrow bore nasogastric tube. Anesthesia. 1978; 33:716.
134. Wendell, GD, Lenchner, GS, Promisloff, RA. Pneumothorax complicating small-bore feeding tube placement. Arch Intern Med. 1991; 151:599.
135. Scholten, DJ, Wood, TL, Thompson, DR. Pneumothorax from nasogastric feeding tube insertion: A report of five cases. Am Surg. 1986; 52:381.
136. Marderstein, EL, Simmons, RL, Ochoa, JB. Patient safety: Effect of institutional protocols on adverse events related to feeding tube placement in the critically ill. J Am Coll Surg. 2004; 199:39.
137. Hand, RW, Kempster, M, Levy, JH, et al. Inadvertent transbronchial insertion of narrow bore feeding tubes into the pleural space. JAMA. 1984; 251:2396.
138. Dobranowski, J, Fitzgerald, JM, Baxter, F, et al. Incorrect positioning of nasogastric feeding tubes and the development of pneumothorax. Can Assoc Radiol J. 1992; 43:35.
139. Torrington, KG, Bowman, MA. Fatal hydrothorax and empyema complicating a malpositioned nasogastric tube. Chest. 1981; 79:240.
140. Hollimon, PW, McFee, AS. Pneumothorax attributable to nasogastric tube. Arch Surg. 1981; 116:970.
141. Ciaglia, P, Firsching, R, Syniec, C. Elective percutaneous dilational tracheostomy: A new simple bedside procedure; preliminary report. Chest. 1985; 87:715.
142. Fikkers, BG, van Veen, JA, Kooloos, JG, et al. Emphysema and pneumothorax after percutaneous tracheostomy. Case reports and an anatomic study. Chest. 2004; 125:1805.
143. Nishioka, M, Fukoaka, M, Nakagawa, K, et al. Spontaneous pneumothorax following partial resolution of total bronchial obstruction. Chest. 1993; 104:160.
144. Woodring, JH, Baker, MD, Stark, P. Pneumothorax ex vacuo. Chest. 1996; 110:1102.
145. Boland, GW, Gazelle, GS, Girard, MJ, et al. Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusion. AJR Am J Roentgenol. 1998; 170:943.
146. Jantz, MA, Antony, VB. Pleural fibrosis. Clin Chest Med. 2006; 27:181.
147. Levy, AS, Bassett, F, Lintner, S, et al. Pulmonary barotrauma: Diagnosis in American football players. Three cases in three years. Am J Sports Med. 1996; 24:227.
148. Patridge, RA, Coley, A, Bowie, R, et al. Sports-related pneumothorax. Ann Emerg Med. 1997; 30:539.
149. Kizer, KW, MacQuarrie, MB. Pulmonary air leaks resulting from outdoor sports. A clinical series and literature review. Am J Sports Med. 1999; 27:517.
150. Hardy, KR. Diving related emergencies. Emerg Med Clin North Am. 1997; 15(1):223.
151. Balk, M, Goldman, JM. Alveolar hemorrhage as a manifestation of pulmonary barotrauma after scuba diving. Ann Emerg Med. 1990; 19(8):930.
152. Slade, JB, Jr., Hattori, T, Ray, CS, et al. Pulmonary edema associated with scuba diving: Case reports and review. Chest. 2001; 120(5):1686.
153. Colebatch, HJ, Smith, MM, Ng, CK. Increased elastic recoil as a determinant of pulmonary barotrauma in divers. Respir Physiol. 1976; 26(1):55.
154. British Thoracic Society Fitness to Dive Group (Subgroup of the British Thoracic Society Standards of Care Committee). British Thoracic Society guidelines on respiratory aspects of fitness for diving. Thorax. 2003; 58(1):3.
155. Bense, L, Wiman, LG, Hedenstierna, G. Onset of symptoms in spontaneous pneumothorax: Correlations to physical activity. Eur J Respir Dis. 1987; 71:181.
156. Vail, WJ, Always, AE, England, NJ. Spontaneous pneumothorax. Chest. 1960; 38:512.
157. Sahn, SA, Heffner, JE. Spontaneous pneumothorax. N Engl J Med. 2000; 342:868.
158. Wiedermann, HP, Matthay, MA, Matthay, RA. Cardiovascular-pulmonary monitoring in the intensive care unit (Part 2). Chest. 1984; 85:656.
159. Glauser, FL, Polatty, RC, Sessler, CN, et al. Worsening oxygenation in the mechanically ventilated patient: Causes, mechanisms, and early detection. Am Rev Respir Dis. 1988; 138:458.
160. Schorlemmer, GR, Khouri, RK, Murray, GF, et al. Bilateral pneumothoraces secondary to iatrogenic buffalo chest; an unusual complication of median sternotomy and subclavian catheterization. Ann Surg. 1984; 199:372.
161. Paranjipe, DV, Wittich, GR, Hamid, LW, et al. Frequency and management of pneumothoraces in heart-lung transplant patients. Radiology. 1994; 190:255.
162. Wittich, GR, Kusnick, CA, Starness, VA, et al. Communication between the two pleural cavities after major cardiothoracic surgery: Relevance to percutaneous intervention. Radiology. 1992; 184:461.
163. Engler, CE, Olson, PN, Engler, CM, et al. Shifting pneumothorax after heart-lung transplantation. Radiology. 1992; 185:715.
164. Sayar, A, Turna, A, Metin, M, et al. Simultaneous bilateral spontaneous pneumothorax: Report of 12 cases and review of literature. Acta Chir Belg. 2004; 104:572.
165. Copeland, RB, Omenn, GS. Electrocardiogram changes suggestive of coronary artery disease in pneumothorax: Their reversibility with upright posture. Arch Intern Med. 1970; 125:151.
166. Walston, A, Brewer, DL, Kitchens, CS, et al. The electrocardiographic manifestations of spontaneous left pneumothorax. Ann Intern Med. 1974; 80:375.
167. Ruo, W, Rupani, G. Left tension pneumothorax mimicking myocardial infarction after percutaneous central venous cannulation. Anesthesiology. 1992; 76:306.
168. Aikhan, M, Biddison, JH. Electrocardiographic changes with right-sided pneumothorax. South Med J. 1998; 91:677.
169. Carr, JJ, Reed, JC, Choplin, RH, et al. Plain and computed radiography for detecting experimentally induced pneumothorax in cadavers: Implications for detection in patients. Radiology. 1992; 183:193.
170. Seow, A, Kazerooni, EA, Cascade, PN, et al. Comparison of upright inspiratory and expiratory chest radiographs for detecting pneumothoraces. AJR Am J Roentgenol. 1996; 166:313.
171. Phillips, GD, Trotman-Dickenson, B, Hodson, ME, et al. Role of CT in management of pneumothorax in patients with complex cystic lung disease. Chest. 1997; 112:275.
172. Rantanem, NW. Diseases of the thorax. Vet Clin North Am Equine Pract. 1986; 2:49–66.
173. Moore, C, Copel, JA. Point-of-care ultrasonography. N Engl J Med. 2011; 364:749–775.
174. Wilkerson, RG, Stone, MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010; 17:11.
175. Dulchavsky, SA, Schwarz, KL, Kirkpatrick, AW, et al. Prospective evaluation of thoracic ultrasound in the detection of pneumothorax. J Trauma. 2001; 50:201–205.
176. Jacobsen, F, Stark, P. Pneumothorax or giant bullae? Clin Intensive Care. 1992; 3:188.
177. Stark, P, Eber, C. Pneumothorax or skin fold? Clin Intensive Care. 1993; 4:45.
178. Baumann, MH, Strange, C, Heffner, JE, et al. Management of spontaneous pneumothorax. An American College of Chest Physicians Delphi Consensus Statement. Chest. 2001; 119:590.
179. MacDuff, A, Arnold, A, Harvey, J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. British Thoracic Society Pleural Disease Guideline Group. Thorax. 2010; 65(Suppl 2):18.
180. Light, RW. Pneumothorax. In: Light RW, ed. Pleural Diseases. 3rd ed. Baltimore: Williams & Wilkins; 1990:242.
181. Noppen, M, Alexander, P, Driesen, P, et al. Quantification of the size of primary spontaneous pneumothorax: Accuracy of the Light index. Respiration. 2001; 68:396.
182. Kelly, AM, Kerr, D, Clooney, M. Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest. 2008; 134:1033.
183. Chernick, V, Amery, ME. Spontaneous alveolar rupture at birth. Pediatrics. 1963; 32:816.
184. Light, RW, Broaddus, VC. Pneumothorax, chylothorax, hemothorax, and fibrothorax. In Murray JF, Nadel JA, eds. : Textbook of Respiratory Medicine, 3rd ed, Philadelphia: WB Saunders, 2000.
185. Ho, KK, Ong, ME, Koh, MS, et al. A randomized controlled trial comparing mini chest tube and needle aspiration in outpatient management of primary pneumothorax. Am J Emerg Med. 2011; 29:1152.
186. Vallee, P, Sullivan, M, Richardson, H, et al. Sequential treatment of a simple pneumothorax. Ann Emerg Med. 1988; 17:936.
187. Hasani, B, Foote, J, Borgundvaag, B. Outpatient management of primary spontaneous pneumothorax in emergency department of a community hospital using a small-bore catheter and Heimlich valve. Acad Emerg Med. 2009; 16:513.
188. Devenand, A, Koh, MS, Ong, TH, et al. Simple aspiration versus chest tube insertion in the management of primary spontaneous pneumothorax: A systematic review. Respir Med. 2004; 98:579.
189. Ayed, AK, Chandrasekaran, C, Sukumar, M. Aspiration versus tube drainage in primary spontaneous pneumothorax: A randomized study. Eur Respir J. 2006; 27:477.
190. Benton, IJ, Benfield, GF. Comparison of a large and small-caliber tube drain for managing spontaneous pneumothoraces. Respir Med. 2009; 103:1436.
191. Minami, H, Saka, H, Senda, K, et al. Small catheter drainage for spontaneous pneumothorax. Am J Med Sci. 1992; 304:345.
192. Olgac, G, Aydogmus, U, Mulazimoglu, L, Kutlu, CA. Antibiotics are not needed during tube thoracostomy for spontaneous pneumothorax: An observational case study. J Cardiothorac Surg. 2006; 1:43.
193. Miller, KS, Sahn, SA. Chest tubes. Indications, technique, management and complications. Chest. 1987; 91:258.
194. Symbas, PN. Chest drainage tubes. Surg Clin North Am. 1989; 69:41.
195. Dalbec, DL, Krome, RL. Thoracostomy. Emerg Med Clin North Am. 1986; 4:441.
196. Liu, CM, Hang, LW, Chen, WK, et al. Pigtail tube drainage in the treatment of spontaneous pneumothorax. Am J Emerg Med. 2003; 21:241.
197. Samelson, SL, Goldberg, EM, Ferguson, MK, et al. The thoracic vent: Clinical experience with a new device for treating simple pneumothorax. Chest. 1991; 100:880.
198. Martin, T, Fontana, G, Olak, J, et al. Use of pleural catheter for the management of simple pneumothorax. Chest. 1996; 110:1169.
199. Sawada, S, Watanabe, Y, Moriyama, S. Video-assisted thoracoscopy surgery for primary spontaneous pneumothorax; evaluation of indications and long-term outcome compared with conservative treatment and open thoracotomy. Chest. 2005; 127:2226.
200. Ayed, AK, Al-Din, HJ. The results of thoracoscopic surgery for primary spontaneous pneumothorax. Chest. 2000; 118:235.
201. Freixinet, JL, Canalis, E, Juliá, G, et al. Axillary thoracotomy versus videothoracoscopy for the treatment of primary spontaneous pneumothorax. Ann Thorac Surg. 2004; 78:417.
202. Inderbitzi, RG, Leiser, A, Furrer, M, Althaus, U. Three years’ experience in video-assisted thoracic surgery (VATS) for spontaneous pneumothorax. J Thorac Cardiovasc Surg. 1994; 107:1410.
203. Cardillo, G, Facciolo, F, Giunti, R, et al. Videothoracoscopic treatment of primary spontaneous pneumothorax: A 6-year experience. Ann Thorac Surg. 2000; 69:357.
204. Hatz, RA, Kaps, MF, Meimarakis, G, et al. Long-term results after video-assisted thoracoscopic surgery for first-time and recurrent spontaneous pneumothorax. Ann Thorac Surg. 2000; 70:253.
205. Lang-Lazdunski, L, de Kerangal, X, Pons, F, Jancovici, R. Primary spontaneous pneumothorax: One-stage treatment by bilateral videothoracoscopy. Ann Thorac Surg. 2000; 70:412.
206. Oliveira, FH, Cataneo, DC, Ruiz, RL, Jr., Cataneo, AJ. Persistent pleuropulmonary air leak treated with autologous blood: Results from a university hospital and review of literature. Respiration. 2010; 79:302.
207. Ozpolat, B. Autologous blood patch pleurodesis in the management of prolonged air leak. Thorac Cardiovasc Surg. 2010; 58:52.
208. Cagirici, U, Sahin, B, Cakan, A, et al. Autologous blood patch pleurodesis in spontaneous pneumothorax with persistent air leak. Scand Cardiovasc J. 1998; 32:75.
209. Chee, CBE, Abisheganaden, J, Yeo, JKS, et al. Persistnet air leak in spontaneous pneumothorax—clinical course and outcome. Respir Med. 1998; 92:757.
210. Cannon, WB, Mark, JBD, Jamplis, RW. Pneumothorax: A therapeutic update. Am J Surg. 1981; 142:26.
211. Gobbel, WG, Jr., Rhea, WG, Jr., Nelson, IA, et al. Spontaneous pneumothorax. J Thorac Cardiovasc Surg. 1963; 46:331.
212. Hart, GJ, Stokes, TC, Couch, AHC, et al. Spontaneous pneumothorax in Norfolk. Br J Dis Chest. 1983; 77:164.
213. Light, RW, O’Hara, VS, Moritz, TE, et al. Intrapleural tetracycline for the prevention of recurrent spontaneous pneumothorax. Results of a Department of Veterans Affairs Cooperative Study. JAMA. 1990; 264:2224.
214. Heffner, JE, Unruh, LC. Tetracycline pleurodesis: Adios, farewell, adieu. Chest. 1992; 101:5.
215. Györik, S, Erni, S, Studler, U, et al. Long-term follow-up of thoracoscopic talc pleurodesis for primary spontaneous pneumothorax. Eur Respir J. 2007; 29:757.
216. Almind, M, Lange, P, Viskum, K. Spontaneous pneumothorax: Comparison of simple drainage, talc pleurodesis, and tetracycline pleurodesis. Thorax. 1989; 44:627.
217. Tschopp, JM, Boutin, C, Astoul, P, et al. Talcage by medical thoracoscopy for primary spontaneous pneumothorax is more cost-effective than drainage: A randomized study. Eur Respir J. 2002; 20:1003.
218. Light, RW. Talc should not be used for pleurodesis. Am J Respir Crit Care Med. 2000; 162:2024.
219. Lange, P, Mortensen, J, Groth, S, et al. Lung function 22-35 years after treatment of idiopathic spontaneous pneumothorax with talc poudrage or simple drainage. Thorax. 1988; 43:559.
220. Cardillo, G, Carleo, F, Carbone, L, et al. Long-term function following videothoracoscopic talc poudrage for primary spontaneous recurrent pneumothorax. Eur J Cardiothorac Surg. 2007; 6:117.
221. Hunt, I, Barber, B, Southon, R, Treasure, T. Is talc pleurodesis safe for young patients following primary spontaneous pneumothorax? Interact Cardiovasc Thorac Surg. 2007; 6:117.
222. Nathan, DP, Taylor, NE, Low, DW, et al. Thoracoscopic total parietal pleurectomy for primary spontaneous pneumothorax. Ann Thorac Surg. 2008; 85:1825.
223. Rena, O, Massera, F, Papalia, E, et al. Surgical pleurodesis for Vanderschueren’s stage III primary spontaneous pneumothorax. Eur Respir J. 2008; 31:837.
224. Hazelrigg, SR, Landreneau, RJ, Mack, M, et al. Thoracoscopic stapled resection for spontaneous pneumothorax. J Thorac Cardiovasc Surg. 1993; 105:389.
225. Urschel, JD. Thoracoscopic treatment of spontaneous pneumothorax. A review. J Cardiovasc Surg (Torino). 1993; 34:535.
226. Andrivet, P, Djedaini, K, Teboul, JL, et al. Spontaneous pneumothorax. Comparison of thoracic drainage vs immediate or delayed needle aspiration. Chest. 1995; 108:335.
227. Seaton, D, Yognathan, K, Coady, T, Barker, R. Spontaneous pneumothorax: Marker gas technique for predicting outcome of manual aspiration. BMJ. 1991; 302:262.
228. Ng, AW, Chan, KW, Lee, SK. Simple aspiration of pneumothorax. Singapore Med J. 1994; 35:50.
229. Afessa, B, Green, WR, Williams, WA, et al. Pneumocystis carinii pneumonia complicated by lymphadenopathy and pneumothorax. Arch Intern Med. 1988; 148:2651.
230. Griffith, BP, Hardesty, RL, Trento, A, et al. Heart-lung transplantation: Lessons learned and future hopes. Ann Thorac Surg. 1987; 43:6.
231. Slabbynck, H, Laureys, M, Impens, N, et al. Recurring catamenial pneumothorax treated with a Gn-RH analogue. Chest. 1991; 100:851.
232. Terndrup, TE, Bosco, SF, McLean, ER. Spontaneous pneumothorax complicating pregnancy—Case report and review of the literature. J Emerg Med. 1989; 7:245.
233. Mohr, LC. Hypoxia during air travel in adults with pulmonary disease. Am J Med Sci. 2008; 335:71.
234. Szymanski, TJ, Jaklitsch, MT, Jacobson, F, et al. Expansion of postoperative pneumothorax and pneumomediastinum: Determining when it is safe to fly. Aviat Space Environ Med. 2010; 81:423.
235. Johnson, SR, Tattersfield, AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax. 2000; 55(12):1052.
236. Berkman, N, Bloom, A, Cohen, P, et al. Bilateral spontaneous pneumothorax as the presenting feature in lymphangioleiomyomatosis. Respir Med. 1995; 89(5):381.
237. Almoosa, KF, Ryu, JH, Mendez, J, et al. Lymphangioleiomyomatosis. Management of pneumothorax in lymphangioleiomyomatosis. Effects on recurrence and lung transplantation complications. Chest. 2006; 129:1274.
238. Sohara, Y. Reexpansion pulmonary edema. Ann Thorac Cardiovasc Surg. 2008; 14(4):205.
239. Feller-Kopman, D, Berkowitz, D, Boiselle, P, Ernst, A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007; 84(5):1656.
240. Sherman, SC. Reexpansion pulmonary edema: A case report and review of the current literature. J Emerg Med. 2003; 24(1):23.