9 Pneumoconioses
Overview and General Considerations
The toxicity and corresponding fibrogenicity of inorganic particulates are related to both the nature of the dust and the nature of the host response.1 One important feature of particle toxicity is the aerodynamic diameter, with particles in the size range of 1 to 5 μm having the highest probability of deposition and retention within the respiratory tract. In addition, the total inhaled dose and intrinsic properties of the dust are important determinants of fibrosis. For example, crystalline silica is highly fibrogenic, whereas carbon is an innocuous “nuisance” dust. Host factors include efficiency of clearance mechanisms and individual susceptibility. Many of the dusts have a characteristic reaction pattern or appearance in histologic sections, which permits an accurate diagnosis. The silicotic nodule and the asbestos body are familiar examples. Others are associated with a reaction pattern that may suggest the diagnosis, but a careful occupational history or use of supplemental analytic techniques may be required to confirm the diagnosis, such as with berylliosis, in which the histologic findings closely resemble those in sarcoidosis.
Analytic electron microscopy provides a powerful tool for identification of dusts in lung tissue samples, and these methods are emphasized when appropriate.2 An analytic electron microscope consists of a scanning or transmission electron microscope equipped with an energy-dispersive spectrometer. Electron microscopic techniques may permit the detection of particles too small to be observed by light microscopy. Furthermore, energy-dispersive x-ray analysis (EDXA) identifies the elemental composition of individual particulates, which can be critical to the identification and, in some cases, the source of the inhaled dust. It must be emphasized, however, that the identification of a particular xenobiotic in lung tissue is in and of itself not proof of disease and must be correlated with the pathologic response (if any) to the dust in routine histologic sections.
Types of Pneumoconiosis
Silicosis
Silicosis results from the inhalation of particles of crystalline silica. It is characterized by circumscribed areas of nodular fibrosis that tend to have the greatest profusion in the upper lung zones. Occupations with exposure to crystalline silica are summarized in Box 9-1.3 In the past, very heavy exposures occurred from sandblasting. This type of exposure has been banned in most places. Soil in the extreme eastern and western portions of the United States is rich in alpha quartz (the most common form of crystalline silica). Silicotic nodules may be found in the thoracic lymph nodes and even within the lung parenchyma in farmers and agricultural laborers working in these regions.
Pathologic Findings
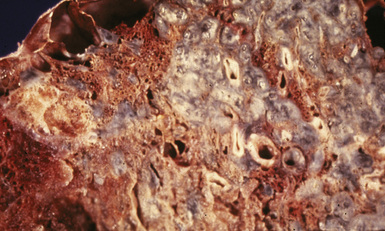
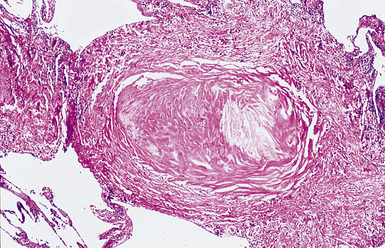
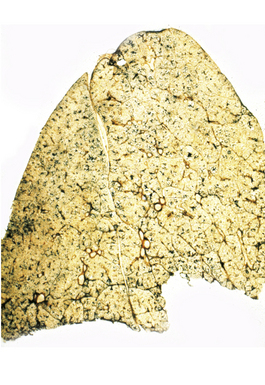
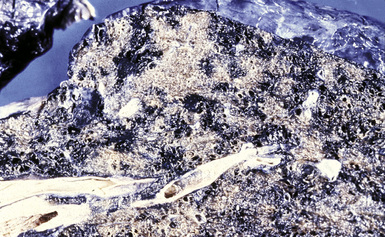
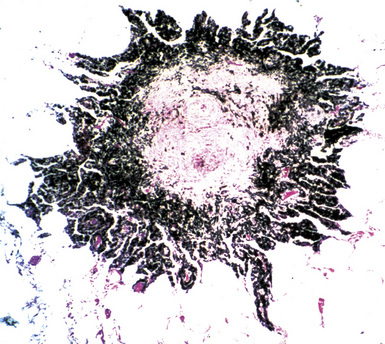
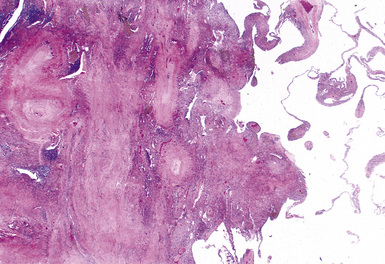
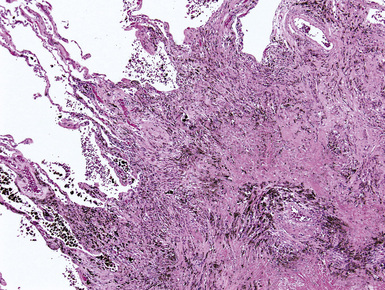
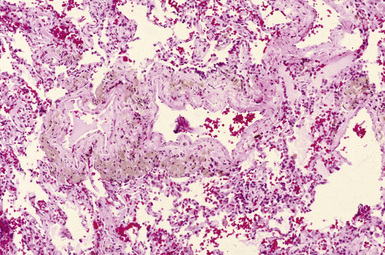
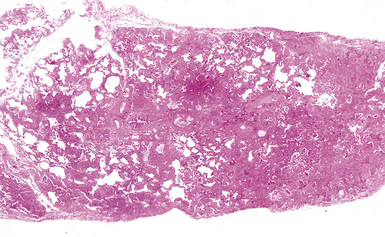
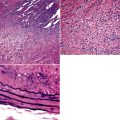
Silicotic nodules are of firm consistency and typically slate gray in appearance, measuring from a few millimeters to approximately 1 cm in diameter (Figs. 9-1 and 9-2). As the disease progresses in severity, the nodules may become confluent (Figs. 9-3 and 9-4). Areas of confluent fibrosis greater than 2 cm in maximum dimension are the defining feature of conglomerate silicosis. Cavitation may occur within areas of confluent fibrosis and, when present, suggests the possibility of superimposed tuberculosis.
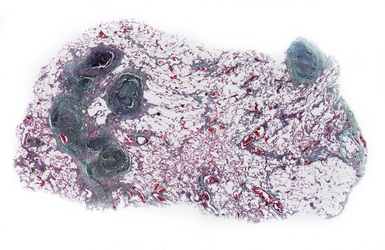
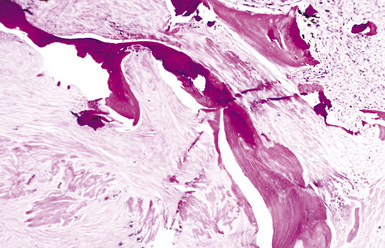
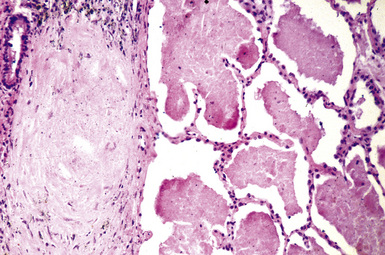
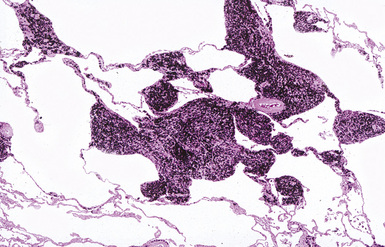
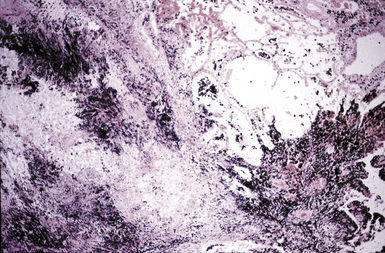
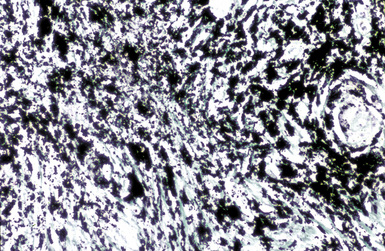
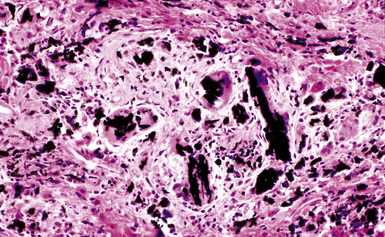
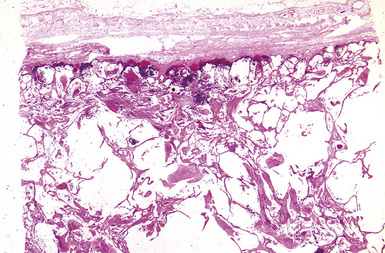
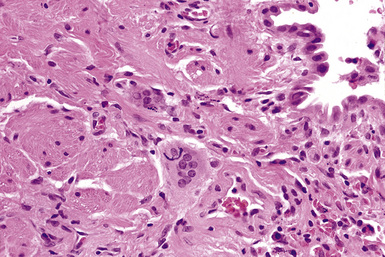
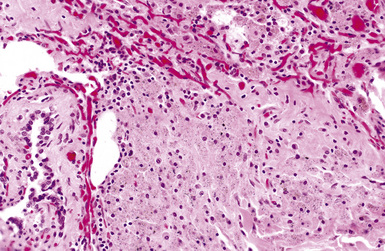
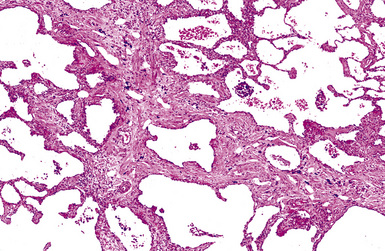
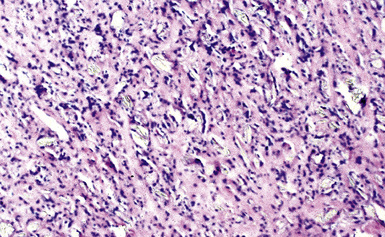
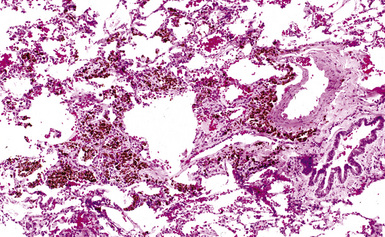
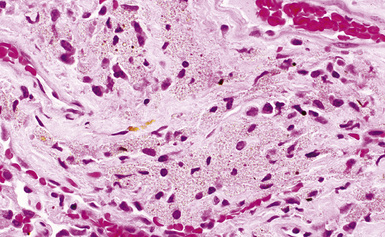
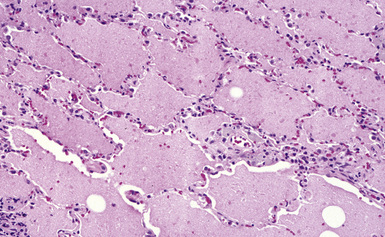
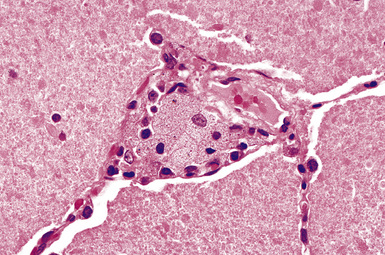
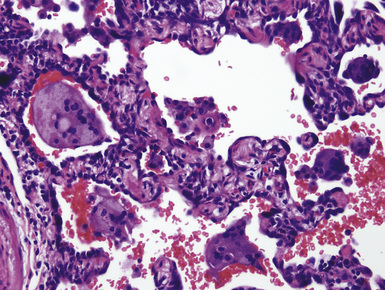
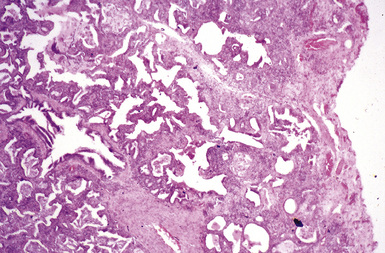
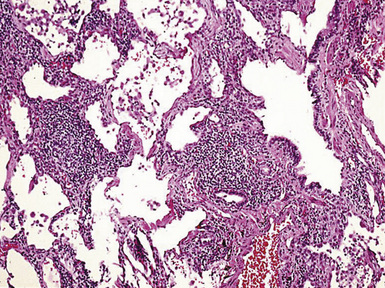
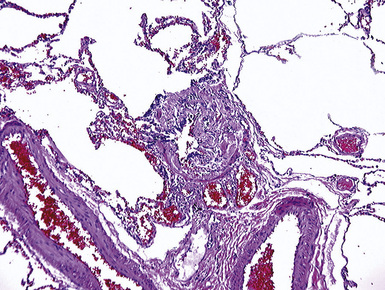
The histologic hallmark of silicosis is the silicotic nodule.4,5 This lesion is a sharply circumscribed nodule consisting of dense, whorled hyalinized collagen (Fig. 9-5). More loosely arranged collagen bundles are typically found at the periphery of the nodule. In recently formed lesions, macrophages form a mantle around the fibrotic center. Longstanding lesions may be calcified or even ossified (Fig. 9-6). The nodules may be present anywhere within the lung parenchyma but typically are most numerous in the upper lung zones. Not uncommonly, they are concentrated beneath the pleura (Fig. 9-7). There may also be extensive pleural fibrosis6 (Fig. 9-8). Nodules are frequently also present within hilar lymph nodes (Fig. 9-9). In patients with extremely high exposures to very fine silica particles, the pattern of resultant lung injury may closely resemble that in pulmonary alveolar proteinosis, characterized by the presence of granular eosinophilic material filling the alveoli, alveolar ducts, and bronchioles (Fig. 9-10). Cholesterol clefts may be prominent within the intra-alveolar material. The granular proteinaceous exudate typically stains strongly positive with periodic acid/Schiff with diastase (D-PAS).
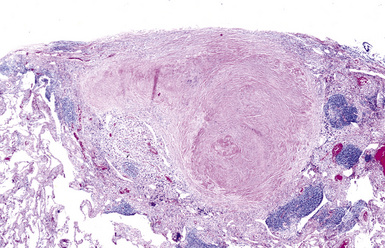
Figure 9-7 Silicosis. The lung parenchyma underlying the pleura is a common location for silicotic nodules.
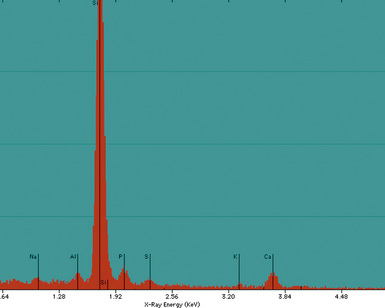
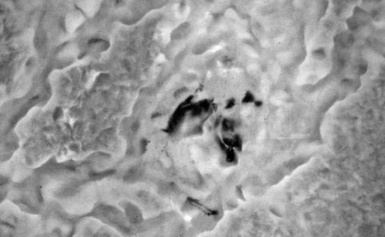
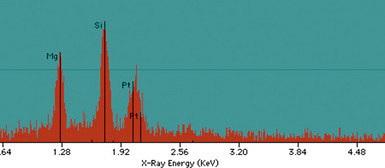
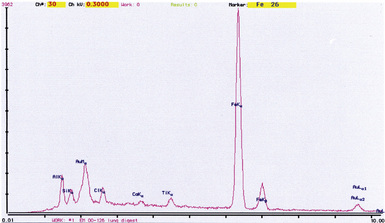
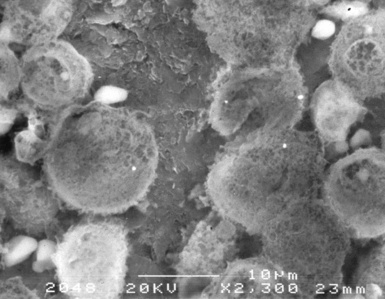
Examination with polarizing microscopy shows faintly birefringent particulates within the fibrotic nodules (Fig. 9-11). Larger, brightly birefringent particles, which represent silicates, may also be seen, but these should not predominate (see later section on silicatosis).7 On scanning electron microscopy the particles appear angulated (Fig. 9-12). Analytic electron microscopy with EDXA reveals peaks for silicon only (Fig. 9-13).
Differential Diagnosis
Silicotic nodules must be distinguished from the fibrotic nodules of healed or “burned-out” sarcoidosis, as well as healed mycobacterial or fungal infections, a classic example being the so-called histoplasmoma. The presence of multinucleate giant cells and the absence of significant dust deposits favor sarcoidosis. Sarcoid granulomas may contain fine needle-like or large, platy birefringent particles, which represent endogenous calcium carbonate or oxalate, respectively (Fig. 9-14). These particles should not be confused with the foreign material of pneumoconiosis. The presence of necrosis in association with giant cells favors an infectious etiology. Patients with silicosis are at increased risk for acquiring tuberculosis.8 Both processes may be present simultaneously. Concurrent tuberculosis infection is most likely to occur with conglomerate silicosis.
Extrathoracic location does not rule out a silicotic origin of a fibrous nodule, because silicotic nodules have been found in the liver, spleen, bone marrow, and abdominal lymph nodes.9 Extrathoracic involvement, when present, usually occurs in the setting of advanced pulmonary silicosis. The diagnostic yield of transbronchial biopsy in silicosis is low, probably because the firm circumscribed nodules are pushed aside by the biopsy forceps.
Coal Worker’s Pneumoconiosis
Clinical Presentation
Patients exhibit a range of clinical presentations, from asymptomatic with simple CWP to markedly dyspneic with PMF. The latter is associated with hypoxemia and cor pulmonale and may be fatal.10,11
Pathologic Findings
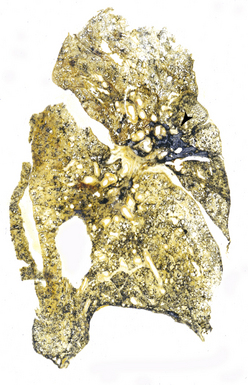
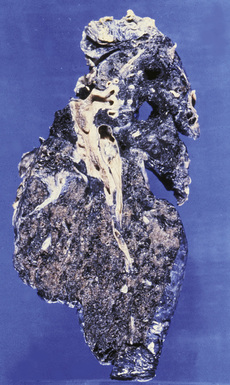
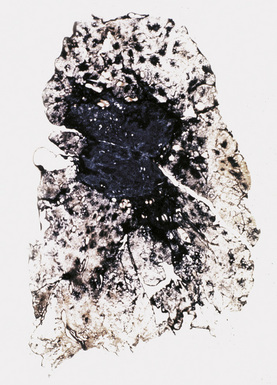
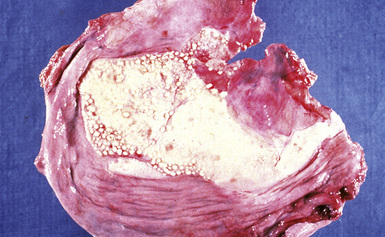
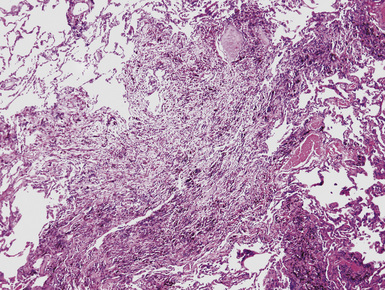
CWP is characterized by increased pigmentation in the lungs resulting from the deposition of coal dust (Figs. 9-15 to 9-17). Foci of accentuated pigmentation, superimposed on a background of diffusely increased pigment, are present on the pleura and within the lung parenchyma. In some cases, palpable nodules are present within the lung parenchyma and usually are most numerous in the upper lung zones. These nodules are grossly similar to silicotic nodules, except for being black rather than slate gray (Fig. 9-18). The most advanced cases of CWP feature greater than 2 cm in maximal dimension confluent areas of irregular fibrosis with the consistency of vulcanized rubber, typically in the upper to mid-lung zones (Fig. 9-19). These are the lesions of PMF. Cavitation may occur in areas of PMF and, when present, suggests superimposed tuberculosis.12
The histologic hallmark of CWP is the coal dust macule (Fig. 9-20). Coal dust macules consist of discrete collections of interstitial pigment deposition in the vicinity of respiratory bronchioles. Areas of emphysematous destruction, referred to as focal emphysema, are typically present at the periphery of macules. Pigment-laden macrophages may be present within alveolar spaces, and pigment deposits may occur anywhere along the lymphatic routes of the lung, including the secondary lobular septa, as well as in the pleura. Lymph nodes frequently contain numerous pigmented macrophages and may also demonstrate silicotic nodules. Silicotic nodules may also be present within the lung parenchyma, but unlike cases of pure silicosis, a collarette of pigmented macrophages often surrounds the nodules, imparting a “Medusa head” appearance (Fig. 9-21). Areas of massive fibrosis (Fig. 9-22) consist of collagen bundles that are arranged in a haphazard distribution and intermixed with abundant pigment (Figs. 9-23 and 9-24). Vascular obliteration is common within areas of PMF, and ischemia may be the cause of cavitation in some cases.
Examination with polarizing microscopy typically shows numerous faintly to brightly birefringent particulates within a background of black pigment (Fig. 9-25). This appearance reflects the mixed nature of coal dust, which is composed of amorphous carbon, silicates, and silica. The presence of silica in coal dust is responsible for the formation of silicotic nodules and is an important factor in the pathogenesis of PMF.13 Ferruginous bodies are sometimes seen in the lungs of coal workers, typically within the alveolar spaces (Fig. 9-26). These may be distinguished from true asbestos bodies by virtue of their black carbonaceous cores14 (Fig. 9-27). Tuberculosis may also complicate CWP (Fig. 9-28).
Differential Diagnosis
CWP must be distinguished from the anthracotic pigment deposition that occurs in urban dwellers and cigarette smokers, and from graphite worker’s pneumoconiosis. The extent of pigmentation in normal persons is a function of environmental exposure to carbon-containing dust and the natural ability of the lung to rid itself of particulates. Distinction from CWP is somewhat a matter of degree, but the presence of true coal dust macules, as described earlier, is indicative of CWP. The finding of anthracotic pigment-laden macrophages within alveoli is also a useful feature, but such macrophages may be absent in miners who have been retired for many years. Graphite worker’s pneumoconiosis appears similar to CWP, but graphite is crystalline, whereas the carbon present in coal is amorphous (Fig. 9-29). A giant cell reaction to the crystalline carbon of graphite assists in this distinction.3 Transbronchial biopsy may be useful in the diagnosis of CWP, showing the typical changes described earlier. However, areas of nodular fibrosis or PMF may be missed by such sampling. Therefore, transbronchial biopsy is not useful for assessing disease severity.
Asbestosis
Asbestosis is defined as pulmonary interstitial fibrosis caused by the inhalation of asbestos fibers.15,16 Substantial and significant exposures to asbestos can occur in a variety of occupational settings, including the mining and milling of asbestos, the manufacture of asbestos-containing products, and the use of products containing asbestos. Occupations that involve the use of asbestos-containing products include insulators, shipyard workers, railroad workers, power plant workers, U.S. Navy or Merchant Marine seamen, oil or chemical refinery workers, construction workers, steel and other molten metal workers, and paper mill workers (Box 9-2). A few cases of asbestosis have also occurred among household contacts of asbestos workers, apparently as a consequence of exposure to asbestos brought home on the workers’ clothing.
Pathologic Findings
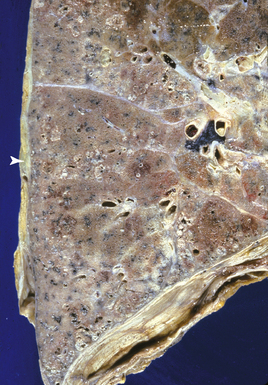
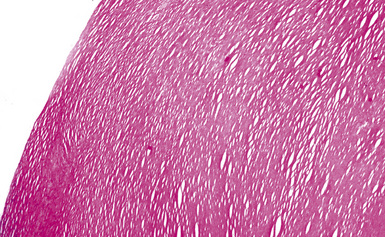
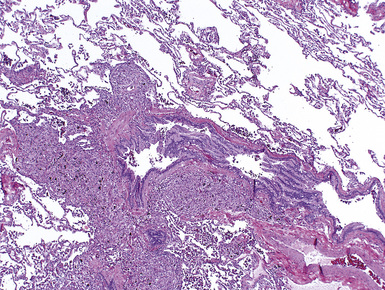
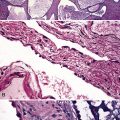
The fibrosis in asbestosis demonstrates a fine, reticular pattern, and the macroscopic appearance of the lungs ranges from normal to severely scarred and shrunken (Fig. 9-30) with evidence of honeycombing.17,18 Diffuse visceral pleural fibrosis is frequently present and is usually most severe over the lower lung zones. Parietal pleural plaques, which are frequently bilateral (Fig. 9-31), are present in the vast majority of cases and may be calcified. Although diffuse pleural fibrosis and plaques serve as a suggestive indicator of an asbestos etiology of pulmonary fibrosis, the term “asbestosis” should not be applied to these pleural abnormalities when they occur in the absence of parenchymal disease.
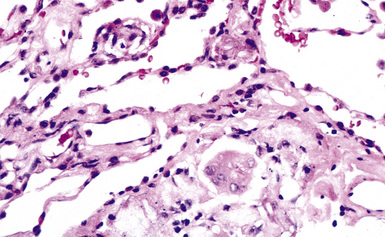
Histologically, asbestosis is characterized by discrete foci of fibrosis in the walls of respiratory bronchioles accompanied by asbestos bodies17 (Box 9-3 and Fig. 9-32). As the fibrotic process progresses, it extends distally to the alveolar ducts and proximally to the membranous (terminal) bronchioles. The fibrosis also extends radially to involve alveolar septa distant from the respiratory bronchiole (Fig. 9-33). In the most advanced cases, honeycomb fibrosis is present (Fig. 9-34), characterized by 0.5- to 1-cm–diameter fibrotic-walled cysts that are lined by bronchiolar epithelium and often contain pools of mucus. Alveolar macrophages are sometimes so prominent as to suggest a diagnosis of desquamative interstitial pneumonia (DIP). In some cases, multinucleate giant cells may be identified, within either the interstitium or the alveolar spaces (Fig. 9-35). Rarely, hyperplastic alveolar type II pneumocytes may contain cytoplasmic hyaline (Fig. 9-36) reminiscent of that found in the cytoplasm of hepatocytes in alcoholic liver disease.
Box 9-3 Histologic Grading of Severity of Fibrosis in Asbestosis*
From Roggli VL, Gibbs AR, Attanoos R, et al. Pathology of asbestosis—an update of the diagnostic criteria. Report of the Asbestosis Committee of the College of American Pathologists and Pulmonary Pathology Society. Arch Pathol Lab Med. 2010;134:462–480.
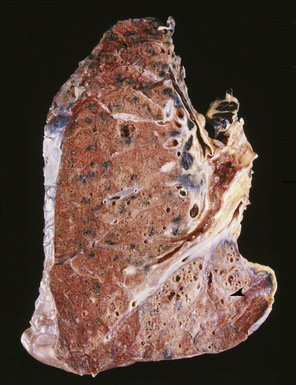
Figure 9-34 Asbestosis. In a more advanced case, honeycombing (arrowhead) is seen, in addition to lower lobe fibrosis.
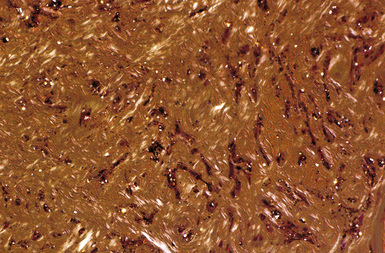
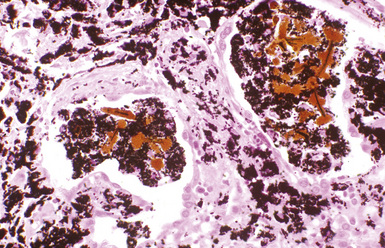
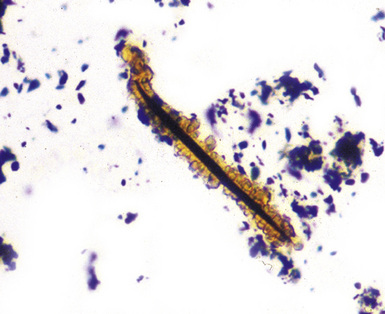
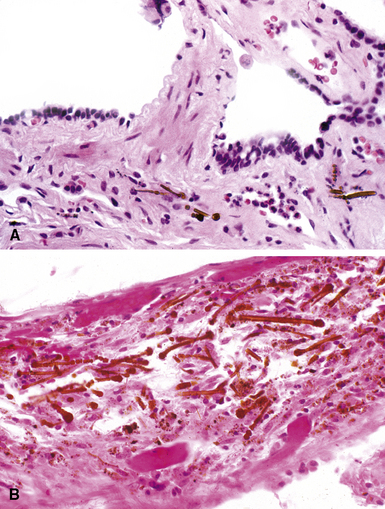
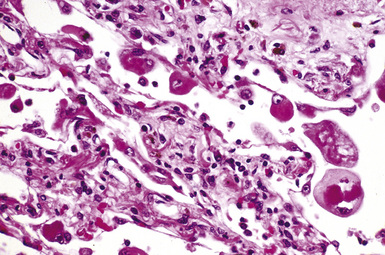
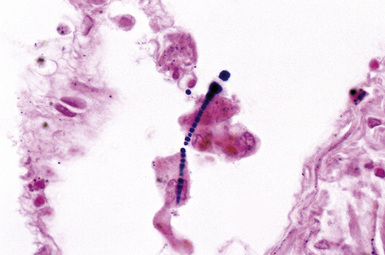
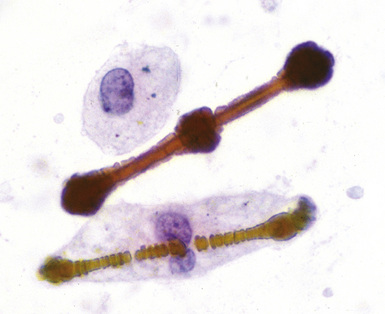
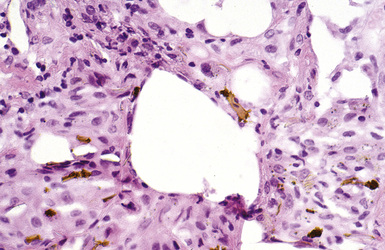
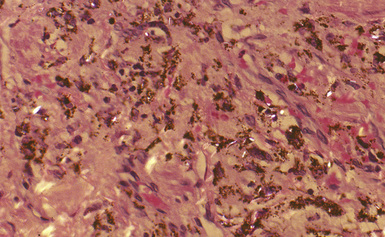
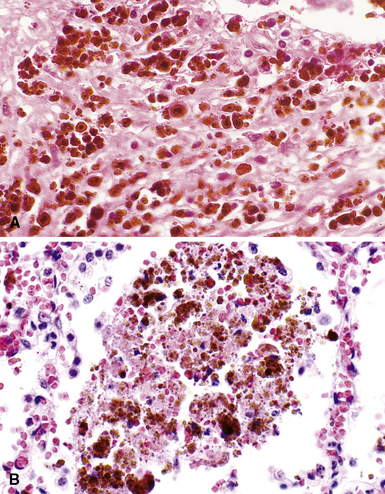
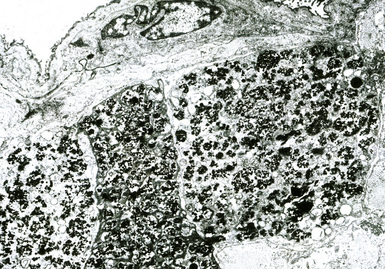
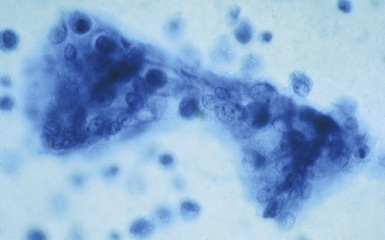
The hallmark of asbestos exposure is the asbestos body, a rod-like, beaded, or dumbbell-shaped structure with golden brown coating and a thin, translucent core.19 Asbestos bodies are typically found in the peribronchiolar interstitium (Fig. 9-37), but, with heavy exposure, may be seen in the alveolar spaces (Fig. 9-38). Detection of asbestos bodies may be facilitated by the use of iron stains, which impart a deep blue color (Fig. 9-39). Asbestos bodies may also be seen in sputum (Fig. 9-40) and in thoracic lymph nodes (Fig. 9-41) of patients with heavy exposure to asbestos. Pleural plaques consist of layers of acellular hyalinized collagen, arranged in a “basketweave” pattern (Fig. 9-42). Visceral pleural fibrosis may show this basketweave pattern or appear as compact layers of collagen. A mild lymphocytic infiltrate sometimes accompanies the fibrosis.
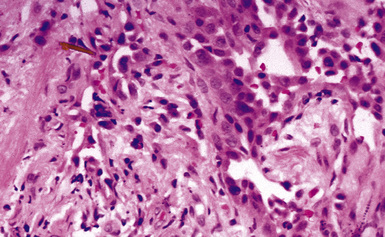
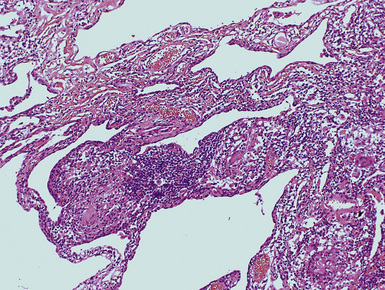
Figure 9-37 Asbestosis. In a transbronchial biopsy, interstitial fibrosis and an asbestos body (upper left) can be seen.
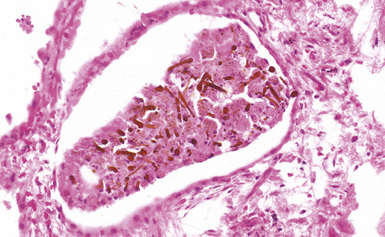
Figure 9-38 Asbestosis. Asbestos bodies are present within an alveolar space in this case of severe asbestosis.
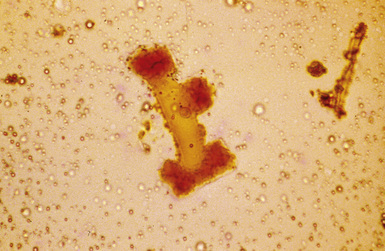
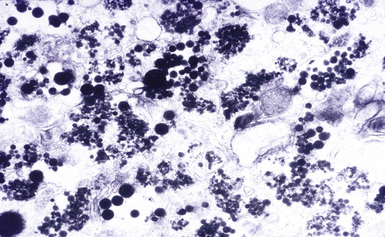
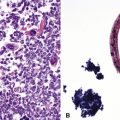
Digestion procedures have been developed for quantifying the content of asbestos in lung tissue (Fig. 9-43). Any of the commercial forms of asbestos (chrysotile and amphiboles) may be identified in lung tissue from patients with asbestosis by means of analytic electron microscopy20 (Figs. 9-44 and 9-45). In cases in which asbestos bodies are not identified in histologic sections, the fiber burden is typically always more than 2 standard deviations below the mean value in cases with bona fide (histologically confirmed) asbestosis.21 Polarizing microscopy is not useful for the detection of asbestos in histologic sections.
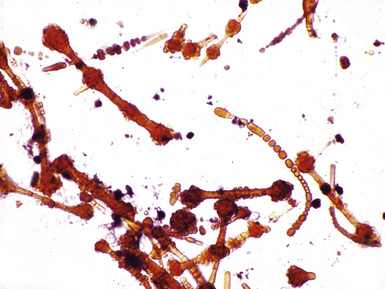
Figure 9-43 Asbestosis. Asbestos bodies from a lung tissue digest on a Nuclepore filter.
(From Roggli VL, Oury TD, Sporn TA, eds. Pathology of Asbestos-Associated Diseases, 2nd ed. New York: Springer; 2004, with permission.)

Figure 9-44 Asbestosis. Scanning electron microscopy image of an asbestos body. Note the thin, beaded appearance.
Differential Diagnosis
Asbestosis must be distinguished not only from usual interstitial pneumonia (UIP) and other forms of diffuse pulmonary fibrosis but also from peribronchiolar fibrosis associated with cigarette smoking. UIP is characterized by honeycomb changes, fibroblastic foci, and the absence of asbestos bodies in histologic sections (see Chapter 7). Honeycomb changes are a rarity in asbestosis, and, in our experience, fibroblastic foci are also uncommon. Pleural changes are much more common in individuals with asbestosis than in individuals with UIP. In contrast to asbestosis, the form of small-airway disease known as respiratory bronchiolitis that is seen in cigarette smokers and is characterized by peribronchiolar fibrosis and pigmented smokers’-type macrophages tends to involve membranous (terminal) bronchioles and is often accompanied by mucous plugging and goblet cell metaplasia. Asbestos bodies are absent in respiratory bronchiolitis.
Asbestos bodies must be distinguished from non-asbestos ferruginous bodies (“pseudoasbestos bodies”) that instead have broad yellow or black central cores (see Fig. 9-27) (also see “Silicatosis” section). The use of transbronchial biopsy in the diagnosis of asbestosis is controversial.16,20 In our experience, patients with evidence of diffuse pulmonary fibrosis on plain chest films or HRCT scans who have pulmonary fibrosis and asbestos bodies on transbronchial biopsy can be reliably diagnosed as having asbestosis. Conversely, the absence of asbestos bodies on transbronchial biopsy does not exclude the possibility that asbestosis is the cause of pulmonary fibrosis.
Silicatosis (Silicate Pneumoconiosis)
Silicatosis is caused by the inhalation of silicates. A variety of silicate minerals may be encountered in the workplace, usually in the setting of mining and quarry work. Silicates include talc (see section on talcosis), vermiculite, mica, feldspar, kaolinite, bentonite, and fuller’s earth.3,22–25 Mixed dust pneumoconiosis occurs among patients exposed to a mixture of silica and non-fibrous silicates. Silicate pneumoconiosis has also been described in farm workers in areas with silica-rich soil.26
Pathologic Findings
Silicatosis is characterized by irregular deposits of collagen, predominantly in a peribronchiolar and perivascular distribution, associated with numerous birefringent particulates.5,27 The lungs may be macroscopically normal in mild disease or may be firm and fibrotic (Fig. 9-46). In patients with significant exposure to silica in addition to silicates, silicotic nodules and even massive fibrosis may also be present (Fig. 9-47). Paracicatricial emphysema may sometimes be seen adjacent to areas of fibrosis (Fig. 9-48).
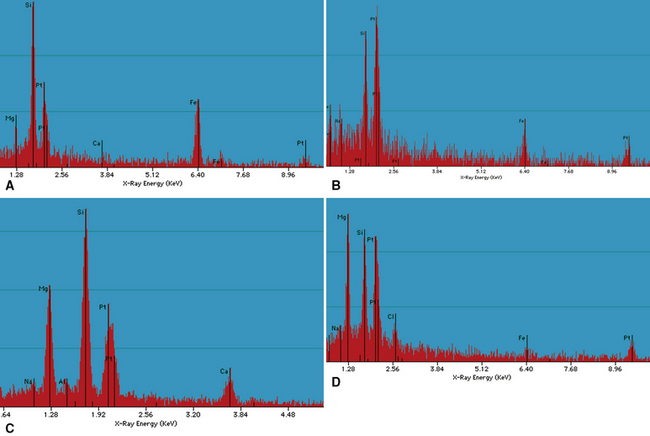
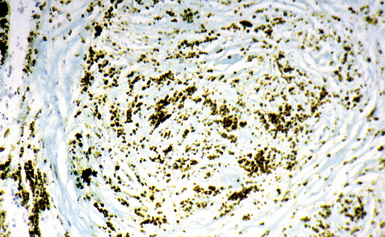
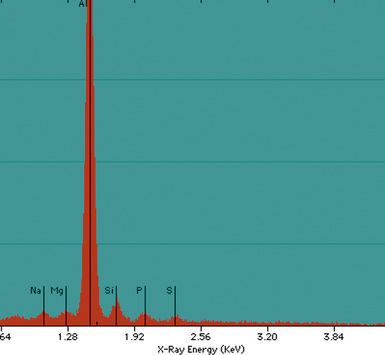
The histologic findings in silicatosis include perivascular and peribronchiolar deposits of dust-laden macrophages (dust macules) (Fig. 9-49). Interstitial fibrosis may also be present, characterized by irregularly contoured, stellate lesions with variable collagenization. Non-asbestos ferruginous bodies with broad yellow sheet silicate-type cores (Figs. 9-50 and 9-51) may be observed in some cases.14,27 Examination with polarizing microscopy typically demonstrates numerous brightly birefringent particulates (Fig. 9-52) associated with macrophages or within stellate lesions. Analytic electron microscopy shows numerous particulates, most of which consist of silicon combined with other elements, such as magnesium, aluminum, potassium, calcium, or iron.28
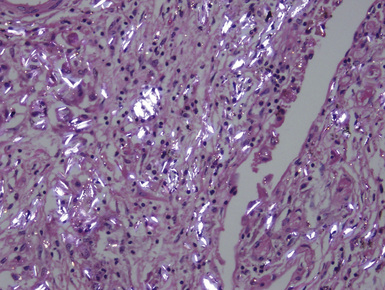
Figure 9-52 Silicatosis. Partial polarization of a fibrotic area demonstrates brightly birefringent silicate particles.
(Courtesy of Dr. Thomas V. Colby, Mayo Clinic, Scottsdale, AZ.)
A special variant of silicatosis is mixed dust pneumoconiosis (MDP), defined as the occurrence of dust macules and stellate lesions producing so-called “mixed dust fibrotic nodules” (Figs. 9-53 and 9-54), with or without accompanying silicotic nodules.29 For a diagnosis of MDP, the macules and mixed dust fibrotic nodules should outnumber silicotic nodules. If silicotic nodules predominate, the preferred diagnosis is silicosis. MDP can occur in individuals who have worked in the coal mining industry, most typically those involved in the installation of roof bolts in coal mine shafts. Analytic electron microscopy in MDP shows aluminum silicates with varying numbers of silica (SiO2) particles.
Differential Diagnosis
Silicatosis must be distinguished from silicosis, UIP, and nonspecific interstitial pneumonia (NSIP). In cases with dust macules, mixed dust fibrotic lesions, and silicotic nodules, a diagnosis of silicosis should be made when silicotic nodules predominate. UIP has well-defined features that are not seen in silicate pneumoconiosis (see Chapter 7). It is important to keep in mind that a few scattered birefringent particulates may be found in the lungs of members of the general population, including those with UIP and NSIP. Such findings should not be confused with those in silicate pneumoconiosis, in which numerous brightly birefringent silicate particles are present within dust macules or mixed dust fibrotic lesions (Fig. 9-55).
Talcosis (Talc Pneumoconiosis)
Clinical Presentation
Patients are often asymptomatic, but fatal pulmonary fibrosis has been reported among talc miners and millers.30 Intravenous drug abusers may develop pulmonary hypertension, massive fibrosis, or paracicatricial emphysema with spontaneous pneumothorax as a complication of massive intravascular deposition of talc within the lungs.31,32
Pathologic Findings
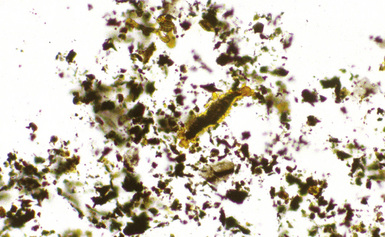
Macroscopically, the lungs in talcosis may be normal or firm in consistency.33,34 Histologically, patchy peribronchiolar and perivascular fibrosis is associated with abundant dust deposits (Fig. 9-56). The particles within these deposits are needle-like and have a bluish-gray color. Examination by polarizing microscopy shows numerous brightly birefringent, needle-like particles within giant cells (Fig. 9-57), granulomas, or foci of interstitial fibrosis. Multinucleate giant cells are variably present in talcosis, and in some cases, a granulomatous reaction resembling sarcoidosis is seen. Ferruginous bodies with broad yellow sheet silicate-type cores may also be seen.14 True asbestos bodies may also be observed in cases in which talc is contaminated with substantial amounts of asbestos (anthophyllite or tremolite). Similarly, silicotic nodules may be seen when there is substantial contamination with quartz.
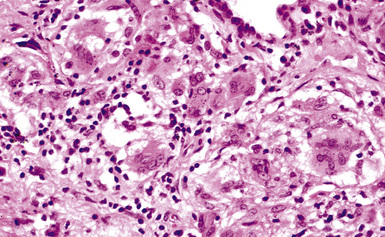
Figure 9-56 Talcosis. In this example, needle-like talc particles are associated with an exuberant giant cell response.
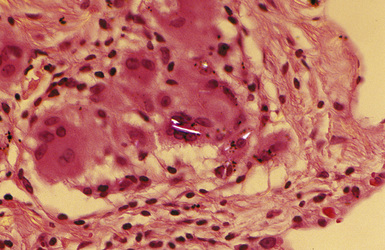
Figure 9-57 Talcosis. Partially polarized view of talc, exhibiting a characteristic needle-like morphology.
Intravenous drug abuse talcosis is characterized by the accumulation of numerous talc granulomas within the pulmonary vasculature and alveolar septal walls (Figs. 9-58 and 9-59). Progressive massive fibrosis has been reported in some cases.31 Concomitant paracicatricial emphysema may be pronounced.32 Accumulations of talc can also be seen in talc pleurodesis, which is characterized by deposits of talc (Figs. 9-60 and 9-61) within the pleura that are associated with macrophages and are also accompanied by a giant cell reaction.35
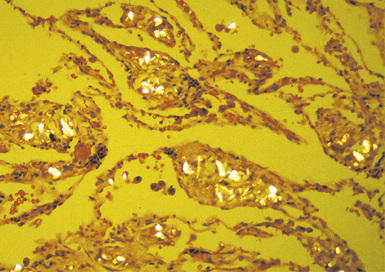
Figure 9-59 Intravenous talcosis. Talc particles appear brightly birefringent under polarized light.
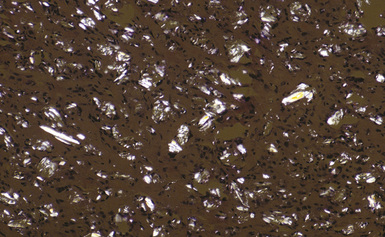
Figure 9-61 Talc pleurodesis. Partial polarization of the case in Figure 9-60 shows numerous platy and needle-shaped birefringent talc particles.
Analytic electron microscopy in talcosis demonstrates platy particles composed of magnesium and silicon36 (Figs. 9-62 and 9-63).
Siderosis
Clinical Presentation
Iron is minimally fibrogenic, so even patients with heavy exposures are typically asymptomatic. Chest x-rays may suggest interstitial fibrosis owing to shadows cast by the deposits of iron pigment.37 Patients with significant exposures to silica or asbestos in addition to iron may demonstrate clinical features related to the inhalation of such dusts.38
Pathologic Findings
Iron pigment imparts a reddish-brown color to the lung parenchyma.3,39 Because iron is minimally fibrogenic, there is no increase in firmness in pure siderosis. However, in cases in which there is concomitant exposure to significant amounts of silica or asbestos, excess collagen may be deposited (Fig. 9-64).
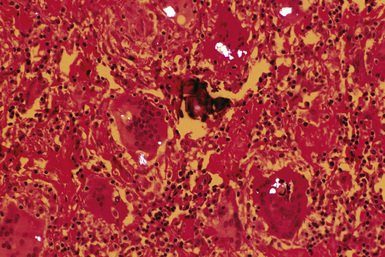
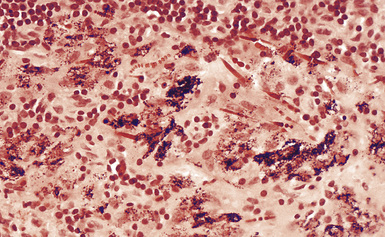
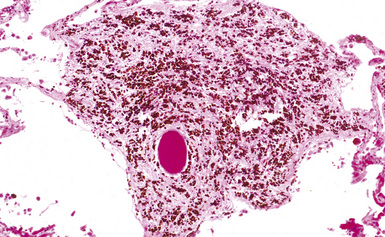
The histologic hallmark of siderosis is perivascular and peribronchiolar deposition of iron pigment (Fig. 9-65).40 The pigment, which consists predominantly of iron oxide, typically is dark brown to black, often with a distinctive golden brown halo (Fig. 9-66). The pigment may be found in macrophages or the interstitium, or both, with very little fibrous response. As mentioned earlier, the finding of significant amounts of fibrosis should prompt a search for evidence of exposure to asbestos or silica (Figs. 9-67 and 9-68). Ferruginous bodies may be observed in some cases14 (Figs. 9-69 and 9-70). These may have black iron oxide cores, particularly in iron foundry workers (Fig. 9-71), or broad yellow sheet silicate cores in welders. True asbestos bodies may also be observed if there has been significant exposure to asbestos, as with shipyard welders.
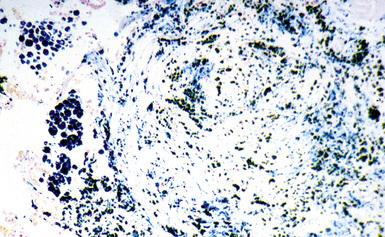
Figure 9-68 Siderosilicosis. The iron pigment appears deep blue in this iron-stained section of the silicotic nodule depicted in Figure 9-67.
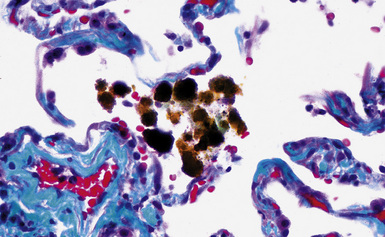
Figure 9-69 Siderosis. Pseudoasbestos bodies with broad yellow sheet silicate cores are seen in this case from a welder.
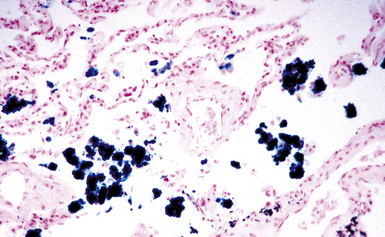
Figure 9-70 Siderosis. Iron-stained section of lung from the same patient as in Figure 9-69 demonstrates numerous pseudoasbestos bodies.
Iron oxide pigment is typically nonrefringent when viewed with polarizing microscopy. Analytic electron microscopy demonstrates spherical particles with prominent peaks for iron (Figs. 9-72 and 9-73).
Aluminosis
Aluminosis is a pneumoconiosis caused by the inhalation of aluminum-containing dusts. Although aluminum is relatively ubiquitous within the environment, aluminosis is a rare disease. Hypersensitivity to aluminum is believed to play a role in the pathogenesis of aluminosis. Substantial exposure to aluminum-containing dust may occur in the setting of aluminum smelting, manufacture of aluminum oxide (corundum) abrasives, aluminum polishing, and aluminum arc-welding.41–43
Clinical Presentation
Aluminosis in which interstitial fibrosis is the dominant tissue reaction may manifest as dyspnea on exertion and restrictive changes on pulmonary function testing. Fatal cases with severe interstitial fibrosis have been reported.43
Pathologic Findings
Macroscopically, the lung parenchyma in aluminosis ranges from essentially normal to heavy and grayish black with dense fibrotic areas scattered throughout3 (Fig. 9-74). A metallic sheen, resembling tarnished aluminum, has been described in some cases.
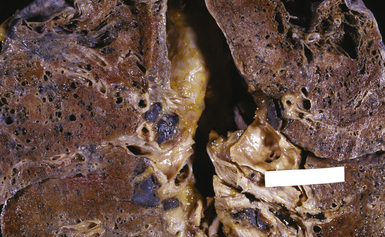
Figure 9-74 Aluminosis. Scattered areas of fibrosis are present in the lungs of this aluminum arc welder.
Histologic examination discloses perivascular and peribronchiolar accumulations of dust-laden macrophages (Fig. 9-75). The dust is refractile and gray to brown (Fig. 9-76). Tissue reaction to aluminum ranges in degree from nil to interstitial fibrosis to granulomatous inflammation.43–46 Cases with a prominent granulomatous response may mimick sarcoidosis. Areas resembling DIP may also be observed.47 Rare cases have been described with an alveolar proteinosis–like pattern (Figs. 9-77 and 9-78) similar to that seen in acute silicoproteinosis.40
Aluminum dust is nonrefringent when examined by polarizing microscopy. Analytic electron microscopy shows electron-dense spherical particles (Figs. 9-79 and 9-80) composed of aluminum (Fig. 9-81).
Hard Metal Lung Disease
Tungsten carbide is used in the manufacture of cutting tools, drilling equipment, armaments, alloys, and ceramics (Box 9-4). Cobalt is used as a binder and may constitute up to 25% of the final product by weight. Hard metal lung disease occurs as a consequence of the inhalation of hard metal dust. Exposure may occur during the manufacturing process of hard metal–containing products or during their use.48 Cobalt exposure has also been reported in diamond polishers who had no exposure to hard metal dust.49,50
Clinical Presentation
Workers with hard metal lung disease present with dyspnea of insidious onset and restrictive changes with small lung volumes on pulmonary function testing. Diffusely increased interstitial markings are observed on plain chest films and CT scans. Disease develops in less than 1% of those exposed, suggesting that hypersensitivity to cobalt is the underlying pathogenic mechanism. Workers may also present with asthma that predates interstitial lung disease by months to years. Hard metal lung disease has been reported to recur after lung transplantation without additional exposure.51
Pathologic Findings
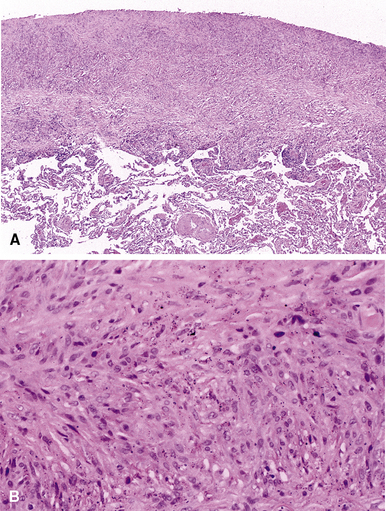
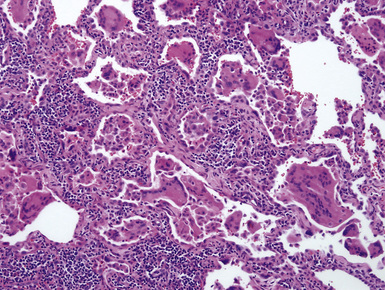
Macroscopically, the lungs in hard metal lung disease are small and fibrotic. Microscopically, hard metal lung disease is synonymous with giant cell interstitial pneumonia (GIP),52 once considered to be one of the “idiopathic” interstitial pneumonias. In this disorder, the alveolar septa are thickened and fibrotic and lined by hyperplastic type II pneumocytes (Figs. 9-82 and 9-83). A moderate chronic inflammatory infiltrate is present. Multinucleate giant cells are a conspicuous feature (Fig. 9-84) and are found both within the alveolar spaces and lining the alveolar septa. Alveolar macrophages are present in increased numbers, and in some cases a pattern reminiscent of DIP is observed (Fig. 9-85). Occasionally, the overall pattern mimics that of UIP, with areas of microscopic honeycombing (Figs. 9-86 and 9-87). The fibrotic and inflammatory reaction may be accentuated around bronchioles.
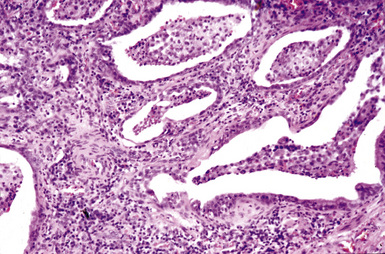
Figure 9-87 Hard metal pneumoconiosis. Detail of the case shown in Figure 9-86, demonstrating honeycomb cysts filled with macrophages.
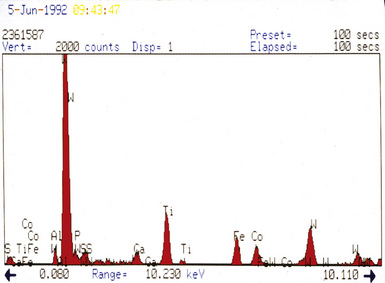
Dust deposits are not readily identified by either routine or polarizing light microscopy. The individual metal particles can be observed by analytic electron microscopy (Figs. 9-88 and 9-89).53 Tungsten particles are most common, followed by titanium and tantalum (Fig. 9-90). Cobalt, the suspected causative agent of the disease, may or may not be identified, because its water solubility makes it susceptible to removal from tissue during fixation and processing.
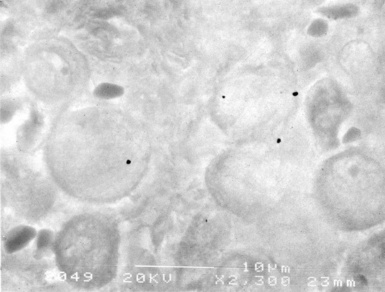
Figure 9-89 Hard metal pneumoconiosis. The metal particles shown in Figure 9-88 appear as dark dots in this backscatter electron microscopic image.
(Courtesy of Dr. Frank Johnson and Dr. Jose Centano, Armed Forces Institute of Pathology, Washington, DC.)
Berylliosis
Berylliosis is a granulomatous lung disease caused by the inhalation of beryllium-containing dust.55,56 Beryllium is used in the aerospace industry in the manufacture of structural materials, guidance systems, optical devices, rocket motor parts, and heat shields. It is also used in the manufacture of ceramic parts, thermal couplings, and crucibles and as a controller in nuclear reactors (Box 9-5). Exposure may occur in any of these industries, as well as in the mining or extraction of beryllium ores.57–59 Historically, beryllium was used in the manufacture of fluorescent lightbulbs, which accounted for most of the initial reports of berylliosis.
Clinical Presentation
Patients with berylliosis present with insidiously progressive dyspnea. Pulmonary function testing shows restriction with diminished diffusing capacity. Plain films of the chest show a fibronodular process. Only about 1% of patients at risk develop berylliosis. Beryllium hypersensitivity has therefore been postulated as the likely pathogenic mechanism, as in the diseases caused by exposure to aluminum and hard metal. In vitro reactivity of peripheral blood or bronchoalveolar lavage lymphocytes to beryllium salts has been used as part of the diagnostic workup.60
Pathologic Findings
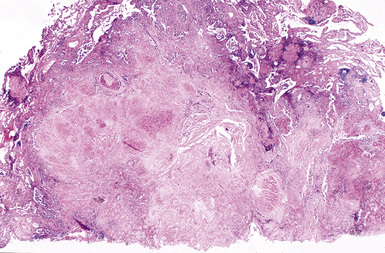
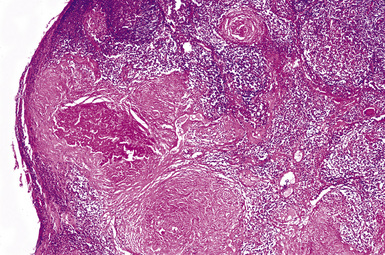
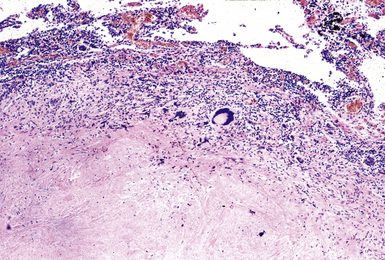
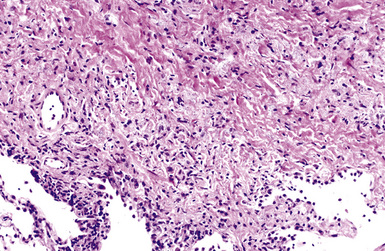
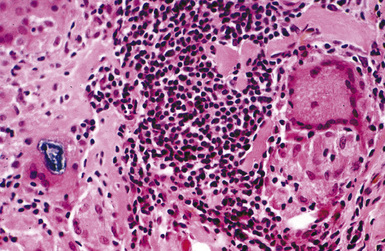
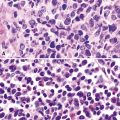
Macroscopically, the lungs in chronic berylliosis are small and fibrotic and may show honeycomb changes.3 Bilateral hilar lymphadenopathy may be present. Microscopically, there are well-formed non-necrotizing granulomas (Figs. 9-91 and 9-92). A chronic interstitial inflammatory infiltrate typically is present (Fig. 9-93). Granulomas may also be found in hilar lymph nodes. Schaumann bodies (Fig. 9-94) and asteroid bodies (Fig. 9-95) within multinucleate giant cells are observed in some cases.39,40
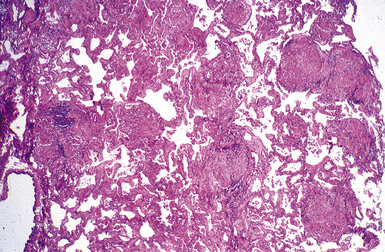
Figure 9-91 Berylliosis. The presence of numerous granulomas is characteristic of berylliosis.
(Courtesy of Dr. Fred Askin, Johns Hopkins University, Baltimore, MD.)
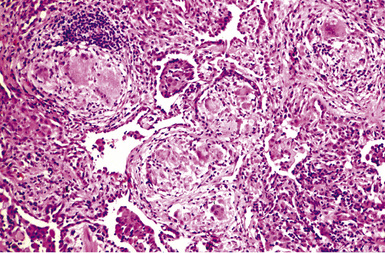
Figure 9-92 Berylliosis. The granulomas in berylliosis are compact and lack necrosis.
(From Roggli VL, Shelburne JD. Pneumoconioses, mineral and vegetable. In: Dail DH, Hammar SP, eds. Pulmonary Pathology, 2nd ed. New York: Springer-Verlag; 1994: 867–900, with permission.)
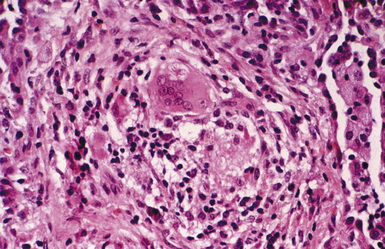
Figure 9-95 Berylliosis. This granuloma features a giant cell containing an asteroid body (upper center).
(From Roggli VL. Rare pneumoconioses: metalloconioses. In: Saldana MJ, ed. Pathology of Pulmonary Disease. Philadelphia: Lippincott; 1994: 411–422, with permission.)
Beryllium is a lightweight metal that has recently become detectable by analytic electron microscopy.61 Other techniques, such as wet chemical analysis, electron energy loss spectrometry, or ion or laser microprobe mass spectrometry, are also used for detection.2 Polarizing microscopy is not useful in the diagnosis of berylliosis.
Rare Earth Pneumoconiosis
Rare earth (or cerium oxide) pneumoconiosis is an uncommon disease caused by the inhalation of rare earth metals, primarily cerium oxide. Only about 20 cases have been reported, and descriptions of the pathologic findings are sparse. Most patients with rare earth pneumoconiosis have been employed in settings in which they were exposed to dust from carbon arc lamps. Two patients were exposed to cerium oxide in an extraction plant, two patients used cerium oxide rouge to polish lenses, and one patient was a producer of glass rubbing polish.62–65
Pathologic Findings
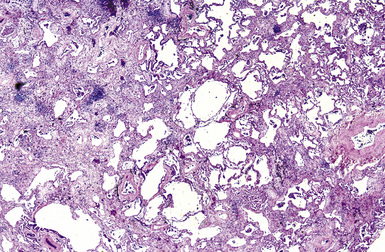
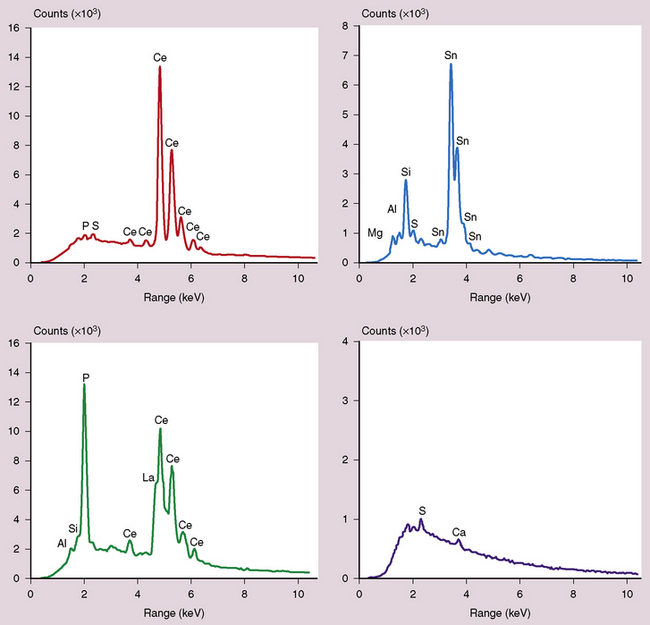
The spectrum of histopathologic features includes granulomatous disease and interstitial fibrosis.62 The fibrosis is similar to that observed with UIP or NSIP (Fig. 9-96). Pigmented dust deposits may be observed with light microscopy, although these may be sparse. Cerium oxide is birefringent on polarizing microscopy. Analytic electron microscopy demonstrates rare earth metals, primarily cerium and to a lesser degree lanthanum, samarium, and neodymium (Figs. 9-97 and 9-98).
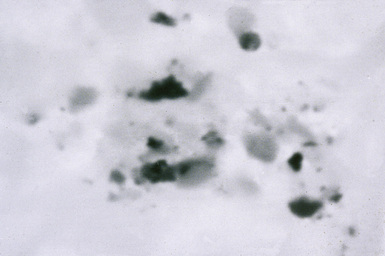
Figure 9-97 Rare earth pneumoconiosis. Backscatter electron microscopy image of electron-dense cerium oxide particles.
Other Pneumoconioses
Acute high-intensity exposure to cadmium results in acute respiratory distress syndrome, whereas chronic exposure is purported to cause emphysema.66,67 Most reports on the pulmonary effects of chronic cadmium exposure appear not to have taken smoking as a confounding factor into consideration.68,69 In addition to being a major cause of emphysema, cigarette smoking is itself a source, albeit small, of cadmium exposure (approximately 2 μg per cigarette).70–72 One study that reported an increased rate of emphysema in workers exposed to cadmium did control for smoking but unfortunately included only clinicoradiographic data and no histopathologic descriptions.73
Granulomatous interstitial inflammation and nodular fibrosis reminiscent of silicosis have been reported in some vineyard workers.74,75 Vineyard sprayer’s lung is believed to be caused by chronic exposure to copper sulfate, a main constituent of fungicidal solutions commonly used in viticulture. Histochemical stains for copper reportedly highlight the dust within macrophages and fibrotic nodules.74
Exposure to silicon carbide (carborundum), a synthetic abrasive, has been associated with nodular and diffuse interstitial fibrosis resembling silicosis or mixed dust pneumoconiosis.76–78 Abundant dust and ferruginous bodies with black silicon carbide cores have been described.
A variety of pathologic features, which in some cases appear to resemble those of silicosis or mixed dust pneumoconiosis, have been reported under the rubric of “dental technician’s pneumoconiosis.”77,79–83 The heterogeneity of reported findings is not surprising, in view of the plethora of substances that have been used in dental prostheses, including silica, beryllium, chromium, cobalt, and molybdenum.
Exposure to oil mists or fine sprays in certain machining and engineering applications, particularly oils low in viscosity or high in mineral oil content, has been reported to cause exogenous lipoid pneumonia. The histologic features are similar to those of mineral oil aspiration.84–87
Metal-working fluids (MWFs) are used extensively in automotive parts manufacturing and other metal-working industries as coolants, cleaning agents, and anti-corrosives that are sprayed onto the fabrication surfaces during the machining process.88 Composed of pure petroleum or a mixture of petroleum or synthetic oils and water, MWFs provide a lipid-rich substrate for the growth of microorganisms. Fungal and bacterial antigens in contaminated MWFs have been implicated in outbreaks of hypersensitivity pneumonia in MWF-exposed workers, with recent outbreaks attributed to non-tuberculous mycobacterial antigens89–91 (Fig. 9-99).
Flock worker’s lung derives its name from an interstitial lung disease that has been reported in some individuals employed in the flocking industry. Flocking involves the application of short synthetic fibers, frequently nylon, onto an adhesive backing, resulting in a plush material. Respired shards generated in the process of cutting fibers to length with a rotary cutter are believed to cause a restrictive process characterized histologically by lymphoid hyperplasia, lymphocytic bronchiolitis, and peribronchiolar interstitial inflammation92–95 (Fig. 9-100). A spectrum of other histologic features and patterns have been reported, including diffuse lymphocytic interstitial inflammation, interstitial fibrosis, fibroblastic foci, bronchiolitis obliterans organizing pneumonia (BOOP), and NSIP.93
Initially recognized in former workers at a microwave popcorn plant and thus dubbed popcorn worker’s lung, a form of lung disease featuring bronchiolitis obliterans (BO) and occasionally peribronchiolar granulomas has also been reported in workers at food flavorings production plants96–100 (Fig. 9-101). The broader term flavorings-related lung disease has been invoked as a more accurate designation for these recently recognized exposures. Although it is possible that other flavoring agents to which workers at these plants have been exposed contribute to the development of this condition, exposure data and animal inhalational studies suggest that diacetyl (2,3-butanedione), a principal component of butter flavoring, plays a causal role.101
Pulmonary disease caused by polluted indoor air is a vastly underrecognized problem.102–110 Cooking indoors with coal or biomass fuels such as wood, peat, crop residues, or dung in open pit fires or poorly ventilated stoves is commonplace in developing countries. This practice releases numerous particulates, including silicates, into the air. Not surprisingly, most reported cases of this pneumoconiosis, which has been referred to as “hut lung,” or domestically acquired particulate lung disease (DAPLD), have been in women. The rare cases that have come to biopsy exhibit dust macules, nodular fibrosis, or occasionally, progressive massive fibrosis (Fig. 9-102).
1 Sporn T.A., Roggli V.L. Pneumoconioses, mineral and vegetable. In: Tomashefski J.F., editor. Dail and Hammar’s Pulmonary Pathology. vol 1, 3rd ed. New York: Springer-Verlag; 2008:911-949.
2 Ingram P., Shelburne J.D., Roggli V.L., LeFurgey E.A. Biomedical Applications of Microprobe Analysis. San Diego: Academic Press, 1999.
3 Spencer H. The pneumoconioses and other occupational lung diseases, 4th ed. Spencer H., editor. Pathology of the Lung, vol. 1,. Pergamon Press, Oxford, 1985;413-510.
4 Churg A., Green F.H.Y. Pathology of Occupational Lung Disease, 2nd ed. Baltimore: Williams & Wilkins, 1998.
5 Craighead J.E., Kleinerman J., Abraham J.L., et al. Diseases associated with exposure to silica and nonfibrous silicate minerals. Arch Pathol Lab Med. 1988;112:673-720.
6 Zeren E.H., Colby T.V., Roggli V.L. Silica-induced pleural disease: an unusual case mimicking mesothelioma. Chest. 1997;112:1436-1438.
7 McDonald J.W., Roggli V.L. Detection of silica particles in lung tissue by polarizing light microscopy. Arch Pathol Lab Med. 1995;119:242-246.
8 Cowie R.L. The epidemiology of tuberculosis in gold miners with silicosis. Am J Respir Crit Care Med. 1994;150:1460-1462.
9 Slavin R.E., Swedo J.L., Brandes D., et al. Extrapulmonary silicosis: a clinical, morphologic, and ultrastructural study. Hum Pathol. 1985;16:393-412.
10 Vallyathan V., Brower P.S., Green F.H.Y., Attfield M.D. Radiographic and pathologic correlation of coal workers’ pneumoconiosis. Am J Respir Crit Care Med. 1996;154:741-748.
11 Marine W.M., Gurr D., Jacobsen M. Clinically important respiratory effects of dust exposure and smoking in British coal miners. Am Rev Respir Dis. 1988;137:106-112.
12 Kleinerman J., Green F.H.Y., Laquer W., et al. Pathology standards for coal workers pneumoconiosis. Arch Pathol Lab Med. 1979;103:375-432.
13 Pratt P.C. Role of silica in progressive massive fibrosis in coal workers’ pneumoconiosis. Arch Environ Health. 1968;16:734-737.
14 Roggli V.L. Asbestos bodies and non-asbestos ferruginous bodies. In: Roggli V.L., Oury T.D., Sporn T.A., editors. Pathology of Asbestos-Associated Diseases. 2nd ed. New York: Springer; 2004:34-70.
15 Hammar S.P., Dodson R.F. Asbestos. In: Dail D.H., Hammar S.P., editors. Pulmonary Pathology. 2nd ed. New York: Springer-Verlag; 1994:901-983.
16 Roggli V.L., Oury T.D., Sporn T.A., editors. Pathology of Asbestos-Associated Diseases, 2nd ed., New York: Springer, 2004.
17 Roggli V.L., Gibbs A.R., Attanoos R., et al. Pathology of asbestosis–an update of the diagnostic criteria. Report of the Asbestosis Committee of the College of American Pathologists and Pulmonary Pathology Society. Arch Pathol Lab Med. 2010;134:462-480.
18 Roggli V.L. Pathology of human asbestosis: a critical review. Fenoglio-Preiser C., editor. Advances in Pathology, vol 2. Year Book, Chicago, 1989;31-60.
19 Roggli V.L. The pneumoconioses: asbestosis. In: Saldana M.J., editor. Pathology of Pulmonary Disease. Philadelphia: Lippincott; 1994:395-410.
20 Churg A. Nonneoplastic disease caused by asbestos. In: Churg A., Green F.H.Y., editors. Pathology of Occupational Lung Disease. 2nd ed. Baltimore: Williams & Wilkins; 1998:277-338.
21 Schneider F., Sporn T.A., Roggli V.L. Asbestos fiber content of lungs with diffuse interstitial fibrosis: an analytical scanning electron microscopic analysis of 249 cases. Arch Pathol Lab Med. 2010;134:457-461.
22 Morgan W.K.C., Donner A., Higgins I.T.T., et al. The effects of kaolin on the lung. Am Rev Respir Dis. 1988;138:813-820.
23 Lapenas D., Gale P., Kennedy T., et al. Kaolin pneumoconiosis: radiologic, pathologic, and mineralogic findings. Am Rev Respir Dis. 1984;130:282-288.
24 Wagner J.C., Pooley F.D., Gibbs A., et al. Inhalation of china stone and china clay dusts: relationship between the mineralogy of dust retained in the lungs and pathologic changes. Thorax. 1986;41:190-196.
25 Landas S.K., Schwartz D.A. Mica-associated pulmonary interstitial fibrosis. Am Rev Respir Dis. 1991;144:718-721.
26 Sherwin R.P., Barman M.L., Abraham J.L. Silicate pneumoconiosis of farm workers. Lab Invest. 1979;40:576-582.
27 Green F.H.Y., Churg A. Diseases due to nonasbestos silicates. In: Churg A., Green F.H.Y., editors. Pathology of Occupational Lung Disease,. 2nd ed. Baltimore: Williams & Wilkins; 1998:235-276.
28 Roub L.W., Dekker A., Wagenblast H.W., Reece G.J. Pulmonary silicatosis: a case diagnosed by needle-aspiration biopsy and energy-dispersive x-ray analysis. Am J Clin Pathol. 1979;72:871-875.
29 Honma K., Abraham J.L., Chiyotani K., et al. Proposed criteria for mixed dust pneumoconiosis: definition, descriptions, and guidelines for pathological diagnosis and clinical correlation. Hum Pathol. 2004;35:1515-1523.
30 Vallyathan N.V., Craighead J.E. Pulmonary pathology in workers exposed to nonasbestiform talc. Hum Pathol. 1981;12:28-35.
31 Crouch E., Churg A. Progressive massive fibrosis of the lung secondary to intravenous injection of talc: a pathologic and mineralogic analysis. Am J Clin Pathol. 1983;80:520-526.
32 Pare J.P., Cote G., Fraser R.S. Long-term follow-up of drug abusers with intravenous talcosis. Am Rev Respir Dis. 1989;139:233-241.
33 Bemer A., Gylseth B., Levy F. Talc dust pneumoconiosis. Acta Pathol Microbiol Scand [A]. 1981;89:17-21.
34 Vallyathan N.V. Talc pneumoconiosis. Respir Ther. 1980;10:34-39.
35 Kennedy L., Sahn S.A. Talc pleurodesis for the treatment of pneumothorax and pleural effusion. Chest. 1994;106:1215-1222.
36 Miller A., Teirstein A.S., Bader M.E., et al. Talc pneumoconiosis: significance of sublight microscopic mineral particles. Am J Med. 1971;50:395-402.
37 Sferlazza S.J., Beckett W.S. The respiratory health of welders. Am Rev Respir Dis. 1991;143:1134-1148.
38 Stern R.M. The assessment of risk: application to the welding industry. Lung Cancer. The Danish Welding Institute Report. 1983;83:13.
39 Churg A., Colby T.V. Diseases caused by metals and related compounds. In: Churg A., Green F.H.Y., editors. Pathology of Occupational Lung Disease,. 2nd ed. Baltimore: Williams & Wilkins; 1998:77-128.
40 Roggli V.L. Rare pneumoconioses: metalloconioses. In: Saldana M.J., editor. Pathology of Pulmonary Disease. Philadelphia: Lippincott; 1994:411-422.
41 Abramson M.J., Wlodarczyk J.H., Saunders N.A., Hensley M.J. Does aluminum smelting cause lung disease? Am Rev Respir Dis. 1989;139:1042-1057.
42 Vallyathan V., Bergeron W.N., Robichaux P.A., Craighead J.E. Pulmonary fibrosis in an aluminum arc welder. Chest. 1982;81:372-374.
43 Jederlinic P.J., Abraham J.L., Churg A., et al. Pulmonary fibrosis in aluminum oxide workers: investigation of nine workers, with pathologic examination and microanalysis in three of them. Am Rev Respir Dis. 1990;142:1179-1184.
44 Gilks B., Churg A. Aluminum-induced pulmonary fibrosis: do fibers play a role? Am Rev Respir Dis. 1987;136:176-179.
45 Chen W.-J., Monnat R.J., Chen M., Moffet N.K. Aluminum induced pulmonary granulomatosis. Hum Pathol. 1978;9:705-711.
46 De Vuyst P., Dumortier P., Schandene L., et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
47 Herbert A., Sterling G., Abraham J., Corrin B. Desquamative interstitial pneumonia in an aluminum welder. Hum Pathol. 1982;13:694-699.
48 Sprince N.L., Oliver L.C., Eisen E.A., et al. Cobalt exposure and lung disease in tungsten carbide production: a cross-sectional study of current workers. Am Rev Respir Dis. 1988;138:1220-1226.
49 Nemery B., Nagels J., Verbeken E., et al. Rapidly fatal progression of cobalt lung in a diamond polisher. Am Rev Respir Dis. 1990;141:1373-1378.
50 Nemery B., Casier P., Roosels D., et al. Survey of cobalt exposure and respiratory health in diamond polishers. Am Rev Respir Dis. 1992;145:610-616.
51 Frost A.E., Keller C.A., Brown R.W., et al. Giant cell interstitial pneumonitis: disease recurrence in the transplanted lung. Am Rev Respir Dis. 1993;148:1401-1404.
52 Ohori N.P., Sciurba F.C., Owens G.R., et al. Giant-cell interstitial pneumonia and hard-metal pneumoconiosis: a clinicopathologic study of four cases and review of the literature. Am J Surg Pathol. 1989;13:581-587.
53 Stettler L.E., Groth D.H., Platek S.F. Automated characterization of particles extracted from human lungs: three cases of tungsten carbide exposure. Scan Electron Microsc. 1983;I:439-448.
54 Tabatowski K., Roggli V.L., Fulkerson W.J., et al. Giant cell interstitial pneumonia in a hard-metal worker: cytologic, histologic and analytical electron microscopic investigation. Acta Cytol. 1988;32:240-246.
55 Meyer K.C. Beryllium and lung disease. Chest. 1994;106:942-946.
56 Kriebel D., Brain J.D., Sprince N.L., Kazemi H. The pulmonary toxicity of beryllium. Am Rev Respir Dis. 1988;137:464-473.
57 Cullen M.R., Kominsky J.R., Rossman M.D., et al. Chronic beryllium disease in a precious metal refinery. Am Rev Respir Dis. 1987;135:201-208.
58 Newman L.S., Kreiss K., King T.E., Seay S., Campbell P.A. Pathologic and immunologic alterations in early stages of beryllium disease: re-examination of disease definition and natural history. Am Rev Respir Dis. 1989;139:1479-1486.
59 Kotloff R.M., Richman P.S., Greenacre J.K., Rossman M.D. Chronic beryllium disease in a dental laboratory technician. Am Rev Respir Dis. 1993;147:205-207.
60 Newman L.S., Kreiss K. Nonoccupational beryllium disease masquerading as sarcoidosis: identification by blood lymphocyte proliferative response to beryllium. Am Rev Respir Dis. 1992;145:1212-1214.
61 Butnor K.J., Sporn T.A., Ingram P., et al. Beryllium detection in human lung tissue using electron probe X-ray microanalysis. Mod Pathol. 2003;16:1171-1177.
62 McDonald J.W., Ghio A.J., Sheehan C.E., Bernhardt P.F., Roggli V.L. Rare earth (cerium oxide) pneumoconiosis: analytical scanning electron microscopy and literature review. Mod Pathol. 1995;8:859-865.
63 Waring P.M., Waring R.J. Rare earth deposits in a deceased movie projectionist: a new case of rare earth pneumoconiosis? Med J Aust. 1990;153:726-730.
64 Sulotto F., Romano C., Berra A., et al. Rare earth pneumoconiosis: a new case. Am J Ind Med. 1986;9:567-575.
65 Husain M.H., Dick J.A., Kaplan Y.S. Rare earth pneumoconiosis. J Soc Occup Med. 1980;30:15-19.
66 Beton D.C., Andrews G.S., Davies H.J., et al. Acute cadmium fume poisoning. Five cases with one death from renal necrosis. Br J Ind Med. 1966;23:292-301.
67 Yamamoto K., Ueda M., Kikuchi H., et al. An acute fatal occupational cadmium poisoning by inhalation. Z Rechtsmed. 1983;91:139-143.
68 Lane R.E., Campbell A.C. Fatal emphysema in two men making a copper cadmium alloy. Br J Ind Med. 1954;11:118-122.
69 Bonnell J.A. Emphysema and proteinuria in men casting copper-cadmium alloys. Br J Ind Med. 1955;12:181-195.
70 Kelleher P., Pacheco K., Newman L.S. Inorganic dust pneumonias: the metal-related parenchymal disorders. Environ Health Perspect. 2000;108(suppl 4):685-696.
71 Elinder C.G., Kjellstrom T., Lind B., et al. Cadmium exposure from smoking cigarettes: variations with time and country where purchased. Environ Res. 1983;32:220-227.
72 Hirst R.N.Jr, Perry H.M.Jr, Cruz M.G., et al. Elevated cadmium concentration in emphysematous lungs. Am Rev Respir Dis. 1973;108:30-39.
73 Davison A.G., Fayers P.M., Taylor A.J., et al. Cadmium fume inhalation and emphysema. Lancet. 1988;1:663-667.
74 Pimentel J.C., Marques F. “Vineyard sprayer’s lung”: a new occupational disease. Thorax. 1969;24:678-688.
75 Villar T.G. Vineyard sprayer’s lung. Clinical aspects. Am Rev Respir Dis. 1974;110:545-555.
76 Funahashi A., Schlueter D.P., Pintar K., et al. Pneumoconiosis in workers exposed to silicon carbide. Am Rev Respir Dis. 1984;129:635-640.
77 Loewen G.M., Weiner D., McMahan J. Pneumoconiosis in an elderly dentist. Chest. 1988;93:1312-1313.
78 Masse S., Begin R., Cantin A. Pathology of silicon carbide pneumoconiosis. Mod Pathol. 1988;1:104-108.
79 Silicosis in dental laboratory technicians—five states, 1994–2000. MMWR Morb Mortal Wkly Rep. 2004;53:195-197.
80 De Vuyst P., Vande Weyer R., De Coster A., et al. Dental technician’s pneumoconiosis. A report of two cases. Am Rev Respir Dis. 1986;133:316-320.
81 Morgenroth K., Kronenberger H., Michalke G., et al. Morphology and pathogenesis of pneumoconiosis in dental technicians. Pathol Res Pract. 1985;179:528-536.
82 Rom W.N., Lockey J.E., Lee J.S., et al. Pneumoconiosis and exposures of dental laboratory technicians. Am J Public Health. 1984;74:1252-1257.
83 Selden A., Sahle W., Johansson L., et al. Three cases of dental technician’s pneumoconiosis related to cobalt-chromium-molybdenum dust exposure. Chest. 1996;109:837-842.
84 Cullen M.R., Balmes J.R., Robins J.M., et al. Lipoid pneumonia caused by oil mist exposure from a steel rolling tandem mill. Am J Ind Med. 1981;2:51-58.
85 Skorodin M.S., Chandrasekhar A.J. An occupational cause of exogenous lipoid pneumonia. Arch Pathol Lab Med. 1983;107:610-611.
86 Jarvholm B. Cutting oil mist and bronchitis. Eur J Respir Dis Suppl. 1982;118:79-83.
87 Skyberg K., Ronneberg A., Kamoy J.I., et al. Pulmonary fibrosis in cable plant workers exposed to mist and vapor of petroleum distillates. Environ Res. 1986;40:261-273.
88 Beckett W., Kallay M., Sood A., et al. Hypersensitivity pneumonitis associated with environmental mycobacteria. Environ Health Perspect. 2005;113:767-770.
89 Kreiss K., Cox-Ganser J. Metalworking fluid-associated hypersensitivity pneumonitis: a workshop summary. Am J Ind Med. 1997;32:423-432.
90 Bernstein D.I., Lummus Z.L., Santilli G., et al. Machine operator’s lung. A hypersensitivity pneumonitis disorder associated with exposure to metalworking fluid aerosols. Chest. 1995;108:636-641.
91 Gupta A., Rosenman K.D. Hypersensitivity pneumonitis due to metal working fluids: sporadic or under reported? Am J Ind Med. 2006;49:423-433.
92 Eschenbacher W.L., Kreiss K., Lougheed M.D., et al. Nylon flock–associated interstitial lung disease. Am J Respir Crit Care Med. 1999;159:2003-2008.
93 Kern D.G., Kuhn C.3rd, Ely E.W., et al. Flock worker’s lung: broadening the spectrum of clinicopathology, narrowing the spectrum of suspected etiologies. Chest. 2000;117:251-259.
94 Kern D.G., Crausman R.S., Durand K.T., et al. Flock worker’s lung: chronic interstitial lung disease in the nylon flocking industry. Ann Intern Med. 1998;129:261-272.
95 Boag A.H., Colby T.V., Fraire A.E., et al. The pathology of interstitial lung disease in nylon flock workers. Am J Surg Pathol. 1999;23:1539-1545.
96 Kanwal R., Kullman G., Piacitelli C., et al. Evaluation of flavorings-related lung disease risk at six microwave popcorn plants. J Occup Environ Med. 2006;48:149-157.
97 van Rooy F.G., Rooyackers J.M., Prokop M., et al. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am J Respir Crit Care Med. 2007;176:498-504.
98 Kreiss K., Gomaa A., Kullman G., et al. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330-338.
99 Akpinar-Elci M., Travis W.D., Lynch D.A., et al. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur Respir J. 2004;24:298-302.
100 Hendrick D.J. “Popcorn worker’s lung” in Britain in a man making potato crisp flavouring. Thorax. 2008;63:267-268.
101 Hubbs A.F., Battelli L.A., Goldsmith W.T., et al. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol Appl Pharmacol. 2002;185:128-135.
102 Balakrishnan K., Sankar S., Parikh J., et al. Daily average exposures to respirable particulate matter from combustion of biomass fuels in rural households of southern India. Environ Health Perspect. 2002;110:1069-1075.
103 Balakrishnan K., Sambandam S., Ramaswamy P., et al. Exposure assessment for respirable particulates associated with household fuel use in rural districts of Andhra Pradesh, India. J Expo Anal Environ Epidemiol. 2004;14(suppl 1):S14-S25.
104 Grobbelaar J.P., Bateman E.D. Hut lung: a domestically acquired pneumoconiosis of mixed aetiology in rural women. Thorax. 1991;46:334-440.
105 Gold J.A., Jagirdar J., Hay J.G., et al. Hut lung. A domestically acquired particulate lung disease. Medicine (Baltimore). 2000;79:310-317.
106 Dennis R.J., Maldonado D., Norman S., et al. Woodsmoke exposure and risk for obstructive airways disease among women. Chest. 1996;109:115-119.
107 Bruce N., Perez-Padilla R., Albalak R. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ. 2000;78:1078-1092.
108 Perez-Padilla R., Regalado J., Vedal S., et al. Exposure to biomass smoke and chronic airway disease in Mexican women. A case-control study. Am J Respir Crit Care Med. 1996;154:701-706.
109 Ramirez-Venegas A., Sansores R.H., Perez-Padilla R., et al. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med. 2006;173:393-397.
110 Diaz J.V., Koff J., Gotway M.B., et al. Case report: a case of wood-smoke-related pulmonary disease. Environ Health Perspect. 2006;114:759-762.