Pleural ultrasound
Overview
Proper equipment setup is important to maximize diagnostic yield, increase image detail, and improve accuracy. Usually, two-dimensional imaging is adequate for a pleural sonographic examination, and color Doppler techniques are not generally required. A microconvex transducer (3.5 to 8 MHz) is ideal for pleural ultrasound examination because it allows the deeper structures to be visualized and has a small footprint such that it can easily fit between the intercostal spaces.1 In addition, high-frequency transducers can be used to allow more detailed imaging of the pleural surface.
In addition to transducer selection, proper orientation of the transducer is important when imaging the pleura and pulmonary structures. Imaging of the pleura should be performed in the longitudinal plane, and the marker of the transducer should always be directed to the head of the patient. By standard convention, imaging of the pleura will require the marker to be in the top left corner of the ultrasound screen.2 The operator is able to perform various adjustments to optimize the quality of acquired images as detailed in previous chapters.
Identification of anatomic structures
When performing a basic lung ultrasound examination, the thorax is usually divided in three zones:
• The anterior zone is defined medially by the sternum, superiorly by the clavicle, laterally by the anterior axillary line, and inferiorly by the diaphragm.
• The posterior zone extends from the posterior axillary line to the posterior midline.
• The lateral zone is located between the anterior and posterior zones.
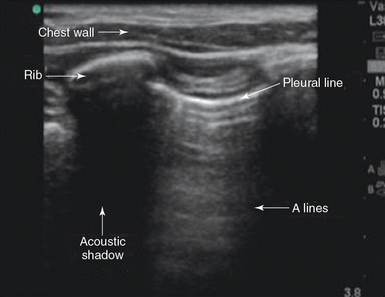
The ribs are easily identified because they cast an acoustic shadow. The examiner should move the transducer on a longitudinal plane to identify the pleura and lung tissue through the intercostal spaces while scrolling through the ribs (Figure 20-1).2
Lung sliding
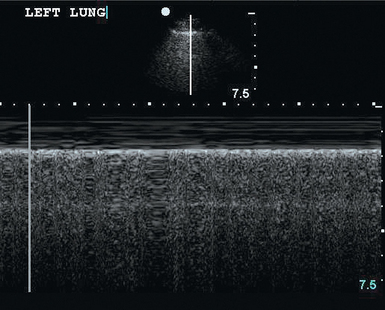
The pleura appear as a moving bright line on lung ultrasonography. When using a high-frequency transducer the visceral and parietal pleura can be visualized as separate linear structures that are closely apposed. Movement of the pleural line against a fixed chest wall is known as lung sliding. Visualization of lung sliding implies that the two pleural surfaces are mobile and in apposition with each other and that no air is opposed between them at the point of the transducer. It is very important to distinguish pleural sliding from chest wall movement.1,2 An alternative method of assessing lung sliding is M-mode. Pleural sliding gives a characteristic seashore sign (Figure 20-2). The presence of lung sliding implies with 100% certainty that no pneumothorax is present at the point of the probe.3
Lung pulse
Careful observation of the pleura reveals a subtle shimmering movement of the pleural line that coincides with cardiac pulsation; this is known as lung pulse. The finding of lung pulse is usually more prominent in the left hemithorax and in the lower aspects of the thorax.2 The presence of lung pulse indicates apposition of the parietal and visceral pleural surfaces at the point of the transducer; therefore the finding of lung pulse reliably excludes the presence of pneumothorax.
Pneumothorax
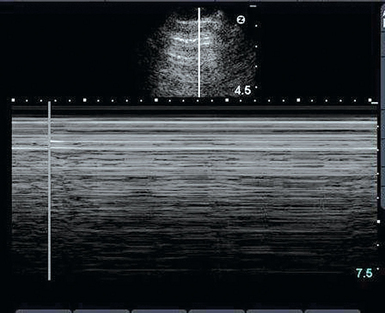
The ability to rule out pneumothorax quickly is a critical application of pleural ultrasonography, which is noninvasive procedure that has greater specificity than and equal sensitivity as chest radiography in diagnosing pneumothorax.4 It is also excellent for detecting residual pneumothorax after drainage.5 At the point on the chest where the pneumothorax is present there will be neither lung sliding and nor a seashore sign. Because the air rises to nondependent areas in the pleural cavity, ultrasound examination should begin in this area of the chest.2 The presence of lung sliding rules out pneumothorax. However, the opposite is not true, and the examiner should keep in mind that the absence of lung sliding is not specific for pneumothorax only. Lung sliding may be absent in other conditions such as atelectasis, pleural thickening, extensive pulmonary fibrosis, and pleural symphysis.1 Other findings that suggest the presence of pneumothorax are lung point, “barcode” sign (Figure 20-3), and B-lines (lung rockets), as detailed in Chapter 19.2,6,7 Hence the use of lung ultrasound after every procedure that carries high risk for pneumothorax, such as attempts at central venous catheterization, thoracentesis, or bronchoscopy with transbronchial biopsy, is a prudent strategy.
Pleural effusions
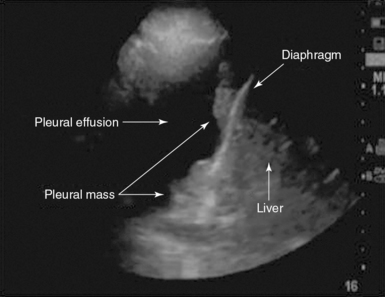
Identification of a pleural effusion is a straightforward application of pleural ultrasound. The latter easily detects fluid collections throughout the body as anechoic or hypoechoic structures.8 Ultrasound is superior to chest radiography in detecting pleural effusions. A free-flowing pleural effusion usually accumulates in the most dependent areas of the chest, and its position can change with changes in the patient’s posture or positioning in the bed. It is important to study the entire chest to detect pleural effusions that may be loculated and will not collect in dependent areas of the chest.2
There are three cardinal rules for diagnosing a pleural effusion:
1. Identification of the anatomic boundaries of a pleural effusion:
b. The diaphragm and subdiaphragmatic organs: liver (right side), spleen (left side), and the kidneys.
c. Lung should be identified and distinguished from the pleural effusion.
2. Identifying the relatively anechoic space that will constitute the pleural effusion in the context of the boundaries just listed (Figure 20-4).
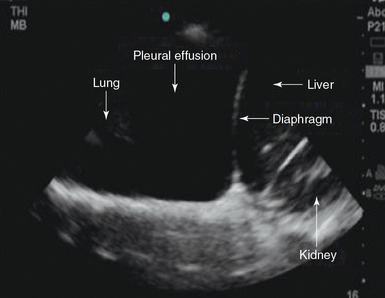
Figure 20-4 Pleural effusion. A typical anechoic fluid collection within normal anatomic boundaries is seen.
a. A flapping lung refers to the undulating movement of the compressed lung within the pleural effusion (jellyfish sign). The lung may not be compressed and a flapping lung may be absent with small effusions.
b. The curtain sign refers to aerated lung that moves into the scanning field and partially obscures the pleural effusion and adjacent compressed air during inspiration.
c. The sinusoid sign reflects the respiratory motion of the visceral pleura on M-mode as it moves toward and away from the chest wall with each breath. It represents movement of the pleural surface within the fluid-filled pleural space.2
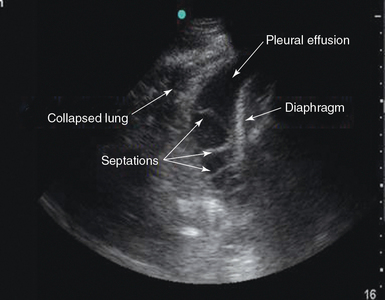
In addition, the size of a pleural effusion can be determined with pleural ultrasound. The easiest way to quantify the pleural effusion is to determine whether it is small, moderate, or large.8 Complex patterns within pleural effusions such as loculations, septations, and stranding patterns, which are commonly found in patients with complicated parapneumonic effusions and empyemas, can be featured by pleural ultrasound (Figure 20-5).
Types of pleural effusion
The echogenicity of the pleural effusion has clinical significance. The appearance of the fluid aids in determining the cause of the pleural effusion. Pleural fluid is usually anechoic but may have floating echogenic debris. An anechoic effusion can be either transudative or exudative. Homogeneously echogenic effusions consisting of diffuse internal echoes with a uniform gray appearance are usually exudative.9 Heterogeneously echogenic effusions (including debris, strands, fronds, or septations) may indicate a complicated parapneumonic effusion or empyema (see Figure 20-5). Heterogeneously echogenic effusions are identifiable by the presence of the following sonographic signs:
1. Hematocrit sign: usually seen with highly cellular effusions, it appears as a bilayer in which the most dependent part of the effusion is largely echogenic, where cells collect as a result of gravity.
2. Plankton sign: swirling debris that is agitated by the cardiac or respiratory cycle within a pleural effusion.
3. Undulating movements: strands or fronds that are agitated by cardiac or respiratory motion within the pleural effusion.2
Solid pleural abnormalities
Pleural ultrasound can detect solid pleural abnormalities. The latter can be malignant (e.g., a mesothelioma) or benign (e.g., lipomas) and appear as single or multiple lesions. When multiple pleural nodules are detected in the context of a malignant pleural effusion, they are highly indicative of pleural metastasis (Figure 20-6). Pleural ultrasound will guide experienced examiners in localizing and detecting these lesions and targeting pleural biopsy.
Pleuroparenchymal disorders
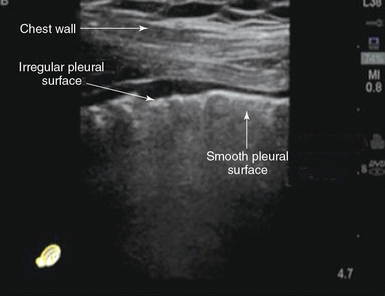
Ultrasound examination of the surface of the pleural line could aid in differentiating between acute cardiogenic pulmonary edema (APE) and acute respiratory distress syndrome (ARDS). Pleural line abnormalities are usually present in ARDS (Chapter 22) and consist of a reduction or even absence of pleural sliding, thickening, and an irregular appearance of the pleural line. In APE, the pleural line is usually regular and smooth with normal lung sliding (Figure 20-7).10
Diagnostic and interventional applications of pleural ultrasound
1. Thoracentesis. Ultrasound guidance improves the yield and reduces the complication rate of thoracentesis.11 Ultrasound allows superior localization and identification of the pleural space. Additionally, it enables safe identification of pleural effusions in mechanically ventilated patients, in whom localization of the effusion and body positioning can be challenging.12
2. Pleural biopsy. Biopsy of solid pleural findings that require further sampling to determine the diagnosis can be performed under direct visualization.
3. Chest tube placement. Ultrasound-guided placement of chest tubes and pigtail catheters for drainage of various pleural pathologic conditions has been proved to be safe and effective in draining both simple and complicated pleural effusions.13
4. Mechanical septal lysis. After placement of a chest tube, the latter can be effectively manipulated (its movement can be directed toward the transducer) to facilitate visualization of septations within a complex pleural space. Lysis of septations and better drainage of a complicated pleural space can therefore be achieved.
Pitfalls and limitations
Pleural imaging is challenging in morbidly obese and very muscular patients, as well as in those with extensive soft tissue edema, which may degrade image quality. The examiner may not be able to visualize the pleural surface adequately, and such situations may require expert-level operators.8
Pearls and highlights
• Imaging of the pleura should be performed in a longitudinal plane, and the marker of the transducer should be directed toward the head of the patient.
• Movement of the pleural line against a fixed chest wall is known as lung sliding. Its presence effectively rules out pneumothorax (at the point of the transducer).
• The presence of lung pulse indicates apposition of the parietal and visceral pleural (at the point of the transducer). Its presence also rules out pneumothorax.
• Pleural ultrasonography should be used after every procedure that carries a high risk for pneumothorax, such as attempts at central venous catheterization, thoracentesis, or bronchoscopy with transbronchial biopsy.
• A free-flowing pleural effusion usually accumulates in the most dependent areas of the chest.
• There are three cardinal rules for the sonographic diagnosis of a pleural effusion: identification of its anatomic boundaries, detection of the relatively anechoic space that will constitute the pleural effusion in the context of these boundaries, and visualization of dynamic signs (jellyfish, curtain, and sinusoid).
• Ultrasound guidance improves the yield and reduces the complication rate of thoracentesis.
References
1. Chandra, S, Narasimhan, M. Pleural ultrasonography. Open Crit Care Med J. 2009; 2:44–59.
2. Mayo, PH, Doelken, P. Pleural ultrasonography. Clin Chest Med. 2006; 27:215–227.
3. Lichtenstein, DA, Menu, Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. 1995. Chest. 1995; 108:1345–1348.
4. Ding, W, Shen, Y, Yang, J, et al. Diagnosis of pneumothorax by radiography and ultrasonography. A meta-analysis. Chest. 2011; 140:859–866.
5. Galbois, A, Ait-Oufella, H, Baudel, JL, et al. Pleural ultrasound compared with chest radiographic detection of pneumothorax resolution after drainage. Chest. 2010; 138:648–655.
6. Lichtenstein, DA, Mezière, G, Lascois, N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005; 33:1231–1238.
7. Lichtenstein, DA. General ultrasound in the critically ill. New York: Springer-Verlag; 2002.
8. Koenig, SJ, Narasimhan, M, Mayo, PH. Thoracic ultrasonography for the pulmonary specialist. Chest. 2011; 140:1332–1341.
9. Lichtenstein, DA. Ultrasound in the management of thoracic disease. Crit Care Med. 2007; 35:S250–S261.
10. Copetti, R, Soldati, G, Copetti, P, Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasoun. 2008; 6:16.
11. Feller-Kopman, D. Ultrasound guided thoracentesis. Chest. 2006; 129:1709–1714.
12. Mayo, PH, Goltz, HR, Tafreshi, M, et al. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004; 125:1059–1062.
13. Liu, Y, Lin, YC, Liang, SJ, et al. Ultrasound-guided pigtail catheters for drainage of various pleural diseases. Am J Emerg Med. 2010; 28:915–921.