Chapter 69 Pleural Effusion, Empyema, and Pneumothorax
Pleural Effusion
Epidemiology and Pathophysiology
Epidemiology
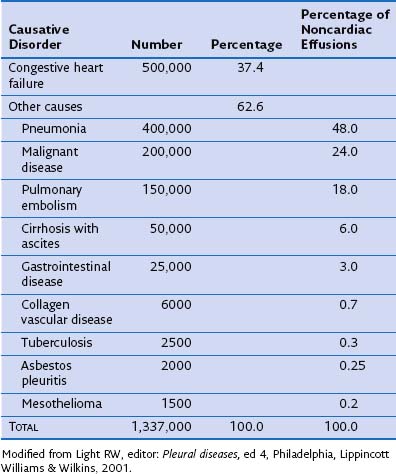
More than 60 causes of pleural effusions have been documented. The relative incidence of different types of effusion varies according to patient demographics and geographic areas. Heart failure is responsible for approximately one third of all pleural effusions (Table 69-1), with pleural infection and malignancy accounting for most exudative effusions.
Imaging
Thoracic Ultrasound Imaging
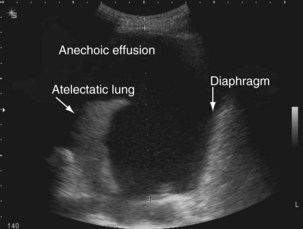
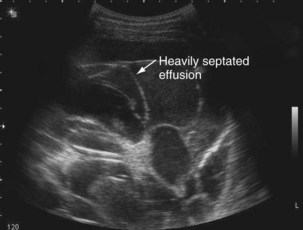
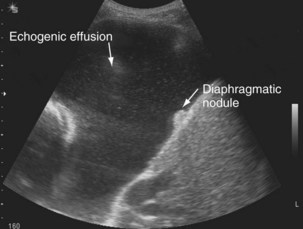
The availability of bedside thoracic ultrasound examination by clinicians has had a significant impact on pleural disease management in recent years. The 2010 British Thoracic Society Pleural Disease Guidelines strongly recommended the use of thoracic ultrasound imaging before procedures for pleural fluid. It is particularly useful for the detection (sensitivity approximately 100%), quantification (by depth), and characterization of pleural fluid (Figures 69-1 to 69-3; Table 69-2), as well as for guiding intervention. Ultrasonography is invaluable in the differentiation between pleural fluid and collapsed or consolidated lung, thereby avoiding unnecessary pleural procedures and associated complications.
| Sonographic Appearance | Significance |
|---|---|
| Anechoic (black fluid) (see Figure 69-1) | Transudative or exudative effusion |
| Septated (multiple lines within fluid) (see Figure 69-2) | Exudative effusion; may suggest possible difficulties inserting chest tube; effusion may drain poorly, although not necessarily |
| Echogenic (echoes, often swirling, within fluid) (see Figure 69-3) | Exudative effusion; heavily echogenic fluid suggestive of blood or pus |
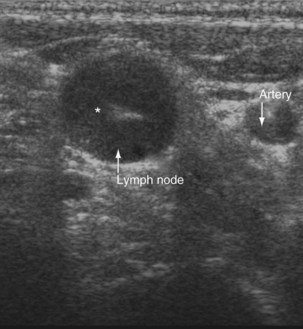
Ultrasound imaging has a high sensitivity (approximately 80%) for detecting pleural malignancy, which can manifest as thickening or nodularity on the visceral, parietal, and diaphragmatic pleural surfaces (see Figure 69-3). Detection of pleural nodularity mandates further investigation (e.g., with chest computed tomography [CT] and pleural biopsy), even if there are no further suspicious features. Ultrasonography also can identify abnormalities beyond the pleural cavity that may provide vital clues to the cause of the effusion, including peripheral lung tumors or abscesses, parenchymal consolidation and atelectasis, diaphragmatic paralysis or elevation, pericardial effusion, and rib and liver metastases and enables evaluation of supraclavicular and cervical lymphadenopathy (Figure 69-4).
Computed Tomography and Magnetic Resonance Imaging
CT with pleural phase contrast enhancement highlights pleural abnormalities and aids discrimination of benign from malignant disease (see Chapter 7). Specific “pleural” CT protocols should be adopted for optimal pleural enhancement and abnormality detection; recent data suggest that images should be acquired 60 seconds after injection of 150 mL of an intravenous contrast agent at 2.5 mL/second. The presence of contraction of the hemithorax, mediastinal pleural involvement, and circumferential pleural thickening (especially greater than 1 cm and with nodularity) all are suggestive of pleural malignancy (Figure 69-5) but cannot adequately differentiate mesothelioma from metastatic pleural cancers. Magnetic resonance imaging (MRI) can help delineate malignant chest wall involvement and is valuable in selected cases, particularly when (probably benign) pleural abnormalities are to be followed clinically by serial imaging in younger patients.
Positron Emission Tomography
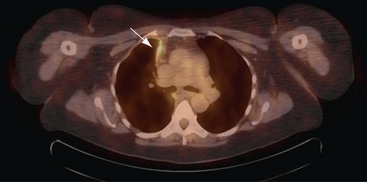
Positron emission tomography (PET)-CT scanning (see Chapter 8) is beginning to emerge as a useful tool in pleural disease management. PET-CT cannot adequately differentiate between benign and malignant effusions, because of the tracer 18F-fluorodeoxyglucose (FDG). FDG-enhanced PET imaging is confounded by avid pleural uptake of FDG in the presence of pleural inflammation (including that due to previous talc pleurodesis and pleural infection). However, FDG-PET may have a role in guiding pleural biopsy in patients with diffuse pleural abnormality to increase sensitivity (Figure 69-6). FDG-PET also may identify nonpleural sites that allow tissue sampling to confirm malignancy (e.g., lymphadenopathy or liver metastases). Recent data suggest a role for FDG-PET in monitoring disease response to therapy in malignant mesothelioma, as well as a potential prognostic role.
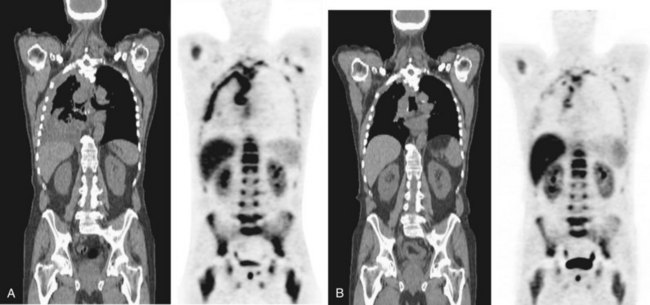
PET scanning using various novel molecular tracers is in early-phase trials for evaluation of pleural malignancies. For instance, PET scanning using labeled thymidine, essential for deoxyribonucleic acid (DNA) synthesis, can identify sites of cell proliferation activity and is not confounded by inflammation (Figure 69-7). New tracers targeting specific cell biology processes (e.g., annexin, a marker of apoptosis) are likely to provide valuable insight to disease pathobiology.
Diagnostic Approach
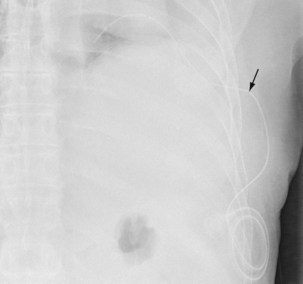
Investigation of a pleural effusion should be performed using a systematic approach (Figure 69-8), aiming to minimize the number of pleural procedures required to make a diagnosis and thereafter allow definitive treatment.
Thoracentesis, preferably imaging-guided, should be the initial investigation in pleural effusions of uncertain origin. If small (less than 1 cm in depth) effusions require sampling, this procedure should be undertaken using real-time radiologic guidance. Thoracentesis is generally safe and complications are uncommon but include vasovagal syncope (0.6%), pneumothorax, infection, and bleeding. Removal of large amounts of fluid may precipitate reexpansion pulmonary edema, often heralded by cough, chest discomfort (at which point the procedure must be terminated), or acute dyspnea. Pleural manometry has been advocated but is not widely available. If initial pleural fluid analysis is inconclusive, additional investigations are often required, including further imaging, repeat thoracentesis, and thoracoscopic or percutaneous pleural biopsy (see Chapters 13 and 74).
Pleural Fluid Analysis
Separation of Exudates and Transudates
Exudative pleural effusions most commonly are defined by Light’s criteria (Box 69-1), using the fluid-to-serum ratio of protein and lactate dehydrogenase, which has an accuracy of 96%. Numerous other markers and criteria have been tested (including measurement of pleural fluid cholesterol values), but none has proved superior. Distinguishing exudates from transudates may narrow the scope of the differential diagnosis and streamline further investigations, although such categorization has limitations: It does not provide the diagnosis and fails to identify concurrent transudative and exudative causes of fluid formation. Research in recent years has focused on disease-specific markers that may provide a definitive diagnosis.
Box 69-1
Light’s Criteria
A pleural fluid is an exudate if any of the following criteria are met:
Differential Leukocyte Count
The cellular portion of physiologic pleural fluid consists predominantly of macrophages and monocytes. In disease states, the differential cell count of the pleural fluid may be helpful in determining the cause (Box 69-2). Acute pleural inflammation or injury generates chemotaxins, such as interleukin 8, and attracts neutrophils to the pleural space. A neutrophil-predominant effusion is commonly seen with acute bacterial pneumonia or pulmonary infarction. A lymphocyte-rich fluid is more common in disease of insidious onset such as tuberculosis (TB) or malignancy. Tuberculous effusions occasionally (less than 10%) may be neutrophilic. An increased eosinophil count (more than 10% of total leukocytes) is often nonspecific. Most commonly, eosinophil effusions develop secondary to presence of intrapleural air or blood (including pneumothorax or previous interventions) but can also be associated with a range of other diseases, such as Churg-Strauss syndrome or drug-induced pleuritis.
pH and Glucose
Pleural fluid pH (or glucose) measurement can aid disease management. Low glucose levels are associated with a similar spectrum of diseases that give rise to low pH effusions (e.g., infection and connective tissue diseases) (Box 69-3) and are equally informative except in patients with hyperglycemia.
Pleural Biopsy
Histologic examination (with or without culture) of pleural tissue can aid in the diagnosis of specific pleural diseases. Tissue can be collected percutaneously (by “blind” or imaging-guided biopsy) or under direct vision (by thoracoscopy or thoracotomy), each with its relative merits (see Chapters 13 and 74).
Thoracoscopy
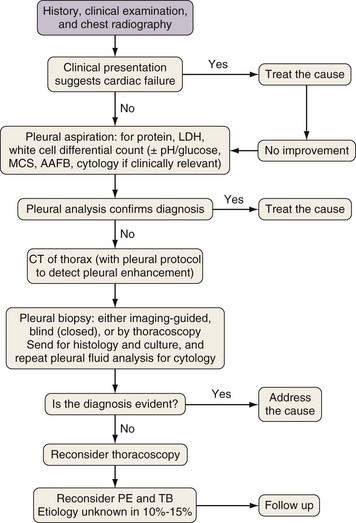
Thoracoscopy is indicated in patients with an undiagnosed exudative pleural effusion after a nondiagnostic thoracentesis. Thoracoscopy can be performed by physicians or surgeons either using local anesthesia and sedation (pleuroscopy) or with the patient under general anesthesia using single-lung ventilation (video-assisted thoracoscopic surgery, [VATS]). Pleuroscopy allows direct visualization of almost the entire pleural surface (Figure 69-9), drainage of fluid, parietal pleural biopsy, and pleurodesis by talc poudrage in the same procedure. Diagnostic sensitivity approaches 95% in malignant pleural diseases and almost 100% in tuberculous pleurisy. Complications are few, and mortality rates low (less than 0.5%). VATS has a similarly high diagnostic sensitivity but also allows other invasive interventions during surgery, including lung biopsy. For details of the technique, refer to Chapter 74.
Treatment
Symptom Control
Placement of Indwelling Pleural Catheters
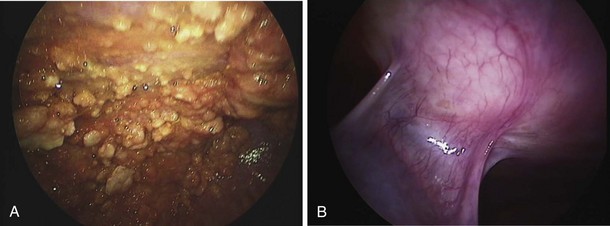
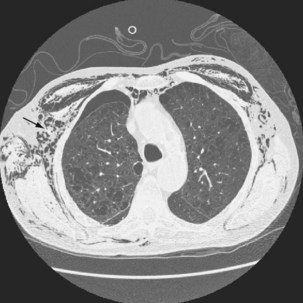
The insertion of a long-term tunneled indwelling pleural catheter (IPC) allows outpatient drainage of refractory malignant pleural effusions. The ambulatory catheters can be inserted as a “day case,” avoiding the need for hospital admission. Its presence induces a complete or partial pleurodesis in up to 70% of patients, and the patient (or the caregiver) can drain the effusion as guided by symptoms, averting hospitalization. Implantation of an IPC also is indicated in patients with a symptomatic effusion and underlying trapped lung, as described later. The catheters usually are well tolerated in clinical practice, but reported complications include development of pleural septations, pleural or soft tissue infection, local tumor invasion at the insertion site, and catheter displacement (Figure 69-10). A randomized trial is under way to evaluate the use of IPCs as first-line therapy in malignant effusions.
Preventing Fluid Reaccumulation
Pleurodesis
Pleurodesis is not indicated if close contact of the pleural layers cannot be achieved. The presence of a so-called trapped lung, when lung expansion is restricted either by visceral pleura tumor encasement or by endobronchial obstruction, prohibits effective pleurodesis (Figure 69-11). With “entrapment” of a significant (greater than 50%) proportion of the lung, insertion of an indwelling pleural catheter should be considered.
Specific Entities Associated with Pleural Effusion
Conditions Associated with Transudative Effusions
Conditions Associated with Exudative Effusions
Parapneumonic Effusion and Empyema
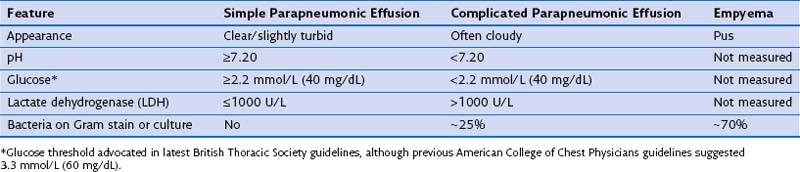
Pleural effusions occur in up to 57% of patients with pneumonia and range in size from small subcentimeter effusions to large effusions causing ventilatory embarrassment. A majority of these (approximately 60%) are sterile “simple” parapneumonic effusions reflecting vascular hyperpermeability from pleural inflammation secondary to the underlying pneumonia. “Complicated” parapneumonic effusions are characterized by neutrophilia, low pH and glucose, and often fibrinous septations (caused by fibrin deposition within the procoagulant milieu of the infected pleural space). The current belief is that complicated pleural effusion represents one end of the spectrum of pleural infection and should be treated as such. At the other end of the spectrum is empyema, characterized by frank pleural pus or presence of bacteria in the pleural fluid (Table 69-3).
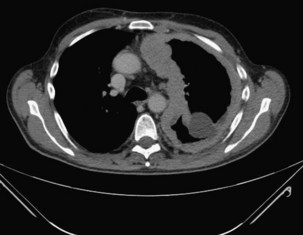
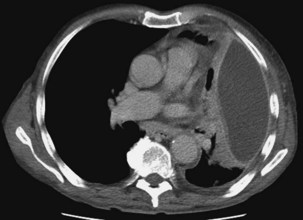
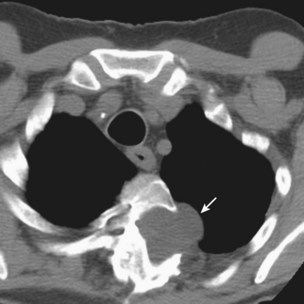
Chest radiography usually identifies the pleural collection and concomitant pneumonia. In a patient with sepsis, the finding of a new encapsulated effusion in a nonbasal position is strongly suggestive of pleural infection. Ultrasound imaging is of value in the evaluation of pleural infection, allowing fluid characterization and localization (which is particularly important for loculated fluid). In a patient with signs of infection, septations on ultrasound images suggest an infected pleural space with low pH, low glucose, and high lactate dehydrogenase (LDH); heavily echogenic fluid usually represents pus (or blood). Of note, however, the converse does not hold; absence of such sonographic features does not rule out pleural infection. Pleural-phase CT provides detailed information and discriminates between empyema and lung abscess. Empyemas usually are lenticular in shape, with compression of surrounding lung parenchyma. The “split pleura” sign, with enhanced parietal and visceral pleural tissue visible as the surfaces are separated by the pleural collection, is characteristic (Figure 69-12). Pleural thickening is seen in 86% to 100% of empyemas and in 56% of parapneumonic effusions. Pleural enhancement and increased attenuation of the extrapleural subcostal fat are suggestive of an infected pleural space.
Tuberculous Pleural Effusions
Spontaneous resolution frequently is seen in tuberculous effusions irrespective of antimycobacterial chemotherapy. Without appropriate treatment, however, approximately 60% of patients will have clinically overt TB develop elsewhere within 5 years. Mycobacterial resistance patterns in cases of tuberculous pleuritis usually are similar to the local resistance patterns of pulmonary TB cases. The treatment of TB is covered in Chapter 31. Thoracentesis may aid symptom relief, and steroids may help to reduce fluid volume, but neither alters the long-term outcome or the incidence of fibrothorax.
Effusions Secondary to Connective Tissue Disease
Drug-Induced Pleural Effusions
Drug-induced pleural effusions do occur, and a careful drug history may be required to make a diagnosis. Various prescribed medications, including amiodarone, nitrofurantoin, methotrexate, bromocriptine, clozapine, and phenytoin, may result in exudative pleural effusions and eventual pleural fibrosis (see www.pneumotox.com for further causes of drug-induced effusions). Withdrawal of the offending drug, with use of corticosteroids if needed, is sufficient in many cases. Decortication has been attempted but is of no established benefit.
Effusions Related to Abdominal Diseases
Chylothorax
Chylothorax develops when disruption of the thoracic duct results in passage of chyle (lymph rich in chylomicrons) into the pleural cavity. The pleural fluid characteristically is milky in appearance (Figure 69-13), except in starved patients. Chylothorax must be differentiated from pseudochylothorax and empyema fluid (see under “Pleural Fluid Analysis”).
Asbestos-Related Pleural Diseases
Asbestos fibers have a predisposition to localize to the pleura. Injury to the pleura can result in mesothelioma (see Chapter 70), as well as a range of benign pleural diseases—namely, pleural plaques, diffuse pleural thickening, benign asbestos–related pleural effusions, and round atelectasis.
Pneumothorax
Spontaneous pneumothoraces are divided into primary and secondary. Primary spontaneous pneumothoraces (PSPs) arise in apparently healthy people, whereas secondary spontaneous pneumothoraces are associated with underlying pulmonary disease, most commonly chronic obstructive pulmonary disease (COPD). Differentiation between these categories is important, because management and prognosis differ for the two groups (Box 69-4).
Primary Spontaneous Pneumothorax
Mortality rates for primary pneumothorax are extremely low.
Diagnosis
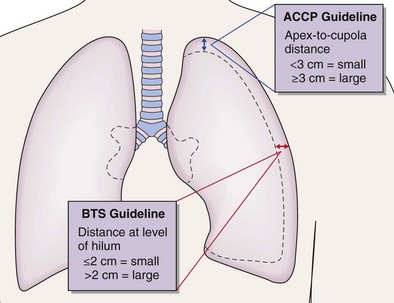
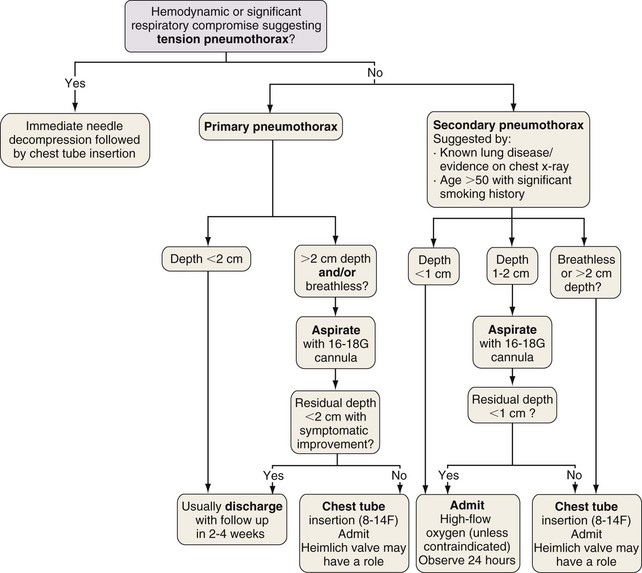
The “gold standard” for pneumothorax size assessment is CT scanning, but this modality is not used in routine practice. Pneumothorax size may be estimated using chest radiography and Light’s index: percent pneumothorax = 100 − [100 × (diameter of deflated lung)3/(diameter of hemithorax)3]. The latest British Thoracic Society guidelines advocate measurement of pneumothorax depth at the level of the hilum, but American College of Chest Physicians guidelines suggest apex-to-cupola depth measurements (Figure 69-14). A 2-cm depth is advocated as a sensible cutoff value for intervening in primary pneumothoraces, representing an approximately 50% pneumothorax. For patients with a secondary spontaneous pneumothorax, a 1-cm depth is advocated for intervention.
Management
Otherwise, treatment aims to remove air from the pleural space and decrease the risk of recurrence. The former may be achieved by simple aspiration or intercostal tube drainage with or without one-way (e.g., Heimlich) valve insertion. Prevention of recurrence involves removal of risk factors (e.g., smoking cessation) and induction of pleurodesis by means of chemical pleurodesis, thoracoscopy (usually VATS), or rarely, open thoracotomy. The latter two approaches allow identification and resection of abnormal areas (ELC treatment [e.g., bullectomy]) with or without chemical pleurodesis, pleural abrasion, or partial pleurectomy (Figure 69-15).
Secondary Spontaneous Pneumothorax
Secondary pneumothoraces occur in patients with underlying lung disease, often with impaired respiratory reserve. The reported incidence is similar to that of primary spontaneous pneumothorax, with highest rates (60 cases per 100,000 population per year) in men older than 75 years of age. COPD is a comorbid condition in 60% of patients. Pneumocystis jirovecii (i.e., “Pneumocystis pneumonia” [PCP]) infection in patients with acquired immunodeficiency syndrome (AIDS) is another risk factor. Other associated conditions are outlined in Box 69-4.
Strategies for Avoiding Recurrence
The effect of pleurodesing agents on future lung transplantation must be considered in selected patients. Although not an absolute contraindication, pleurodesis makes transplantation technically more difficult, thereby prolonging the period of donor organ ischemia. Excessive bleeding also is more common. Close liaison with the local transplantation unit is advised for all possible future transplant candidates in whom pleurodesis is contemplated (see Chapter 75).
Special Situations
Surgical/Subcutaneous Emphysema
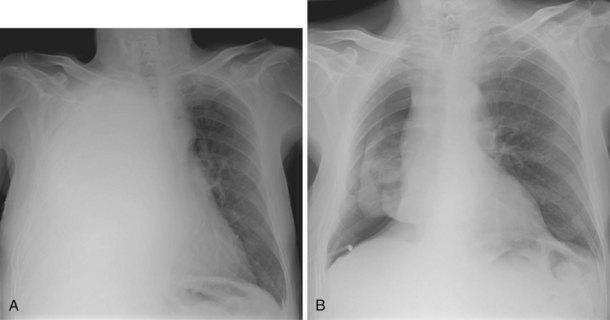
Surgical emphysema arises as air, under pressure from the pleural space, dissects through the subcutaneous tissue (Figure 69-16). This complication may result from misplacement of a drain with the air holes positioned outside the pleural cavity, from blockage of the drain, or in the presence of a large air leak.
Pneumothorax in Cystic Lung Diseases
Pulmonary Langerhans Cell Histiocytosis
Spontaneous pneumothorax may be the presenting feature in up to 25% of patients with Langerhans cell histiocytosis (LCH). Treatment options are those discussed previously for pneumothorax, together with management of the underlying LCH (see Chapter 54).
Lymphangioleiomyomatosis
In addition to chylothorax, patients with lymphangioleiomyomatosis (LAM) are at risk for development of pneumothorax. This complication is common in this population (affecting up to 80%) and may be the presenting feature of the underlying LAM. Recurrent pneumothoraces occur in two thirds of patients, and early definitive intervention is recommended to reduce recurrence rates. Pleurodesis may increase the rate of perioperative bleeding (occurring as a result of resultant adhesions) but does not preclude successful future lung transplantation (see Chapter 54).
Havelock T, Teoh R, Laws D, Gleeson, F, BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii61–ii76.
Light RW, Lee YCG. Textbook of pleural diseases, ed 2, London: Hodder Arnold, 2008.
Maskell NA, Davies CW, Nunn AJ, et al. U.K. controlled trial of intrapleural streptokinase for pleural infection (MIST1). N Engl J Med. 2005;352:865–874.
Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003;361:1326–1330.
Noppen M, Dekeukeleire T, Hanon S, et al. Fluorescein-enhanced autofluorescence thoracoscopy in patients with primary spontaneous pneumothorax and normal subjects. Am J Respir Crit Care Med. 2006;174:26–30.
Rahman NM, Maskell NA, West A, et al. Randomized trial of intrapleural tPA and DNase in pleural infection (MIST2). N Engl J Med. 2011;365:518–526.