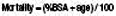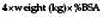18 Plastic and reconstructive surgery
Introduction
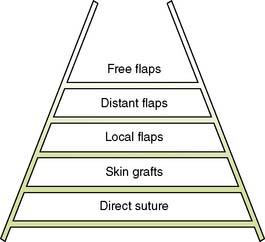
Plastic and reconstructive surgery is concerned with the restitution of form and function after trauma and ablative surgery. The techniques by which this is achieved are applicable to virtually every surgical subspecialty and are not limited to any single anatomical region or system. The ‘reconstructive ladder’ is broad, simple and widely applicable at its base, but narrow, technically demanding and complex at its top (Fig. 18.1). It is important to distinguish plastic and reconstructive surgery from cosmetic, or aesthetic, surgery. In the latter, the techniques of the former are applied to improve appearance but not physical function, although there may be considerable psychological benefit.
Structure and functions of skin
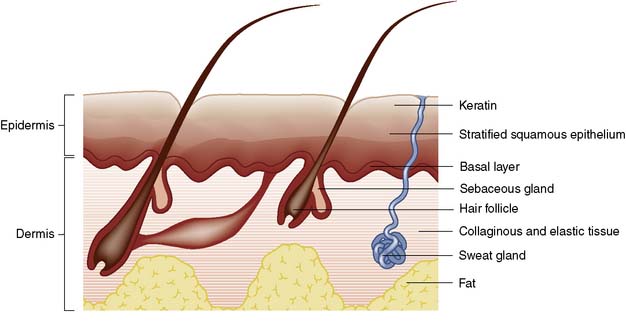
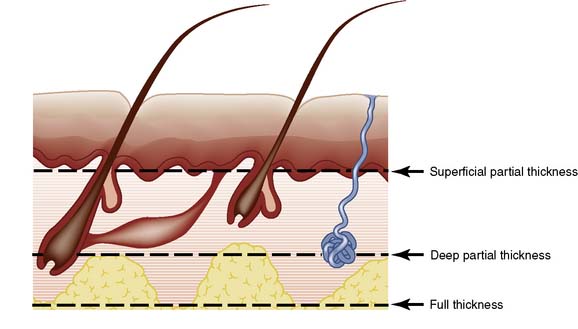
Skin consists of epidermis and dermis. The epidermis is a layer of keratinized, stratified squamous epithelium (Fig. 18.2) that sends three appendages (hair follicles, sweat glands and sebaceous glands) into the underlying dermis. Because of their deep location, the appendages escape destruction in partial-thickness burns and are a source of new cells for reconstitution of the epidermis. The basal germinal layer of the epidermis generates keratin-producing cells (keratinocytes), which become increasingly keratinized and flattened as they migrate to the surface, where they are shed. The basal layer also contains pigment cells (melanocytes) that produce melanin, which is passed to the keratinocytes and protects the basal layer from ultraviolet light.
Wounds
Types of wound
Wounds can be classified according to the mechanism of injury:
• Incised wounds. A sharp instrument causes these; if there is associated tearing of tissues, the wound is said to be lacerated
• Abrasions. These result from friction damage and are characterized by superficial bruising and loss of a varying thickness of skin and underlying tissue. Dirt and foreign bodies are frequently embedded in the tissues and can give rise to traumatic tattooing
• Crush injuries. These are due to severe pressure. Even though the skin may not be breached, there can be massive tissue destruction. Oedema can make wound closure impossible. Increasing pressure within fascial compartments can cause ischaemic necrosis of muscle and other structures (compartment syndrome)
• Degloving injuries. These result from shearing forces that cause parallel tissue planes to move against each other: for example, when a hand is caught between rollers or in moving machinery. Large areas of apparently intact skin may be deprived of their blood supply by rupture of feeding vessels
• Gunshot wounds. These may be low-velocity (e.g. shotguns) or high-velocity (e.g. military rifles). Bullets fired from high-velocity rifles cause massive tissue destruction after skin penetration
• Burns. These are caused not only by heat but also by electricity, irradiation and chemicals.
Principles of wound healing
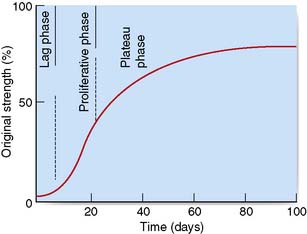
The essential features of healing are common to wounds of almost all soft tissues, and result in the formation of a scar. Soft tissue healing can be subdivided into three phases (Table 18.1) according to the development of tensile strength (Fig. 18.3).
| Lag phase (2–3 days) |
Primary and secondary intention
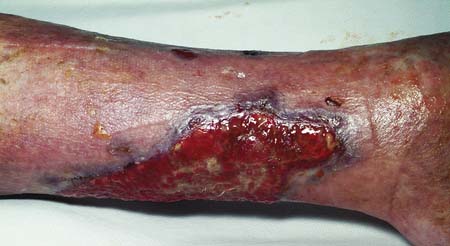
Wounds may heal by primary intention if the edges are closely approximated: for example, by accurate suturing. Epithelial cover is quickly achieved and healing produces a fine scar (Fig. 18.4). If the wound edges are not apposed, the defect fills with granulation tissue and the restoration of epidermal continuity takes much longer. The advance of epithelial cells across the denuded area may be hindered by infection. This is known as healing by secondary intention and usually results in delayed healing, excessive fibrosis and an ugly scar (Fig. 18.5). If a wound has begun to heal by secondary intention, it may still be possible to speed healing by excising the wound edges and bringing them into apposition, or by covering the defect with a skin graft.
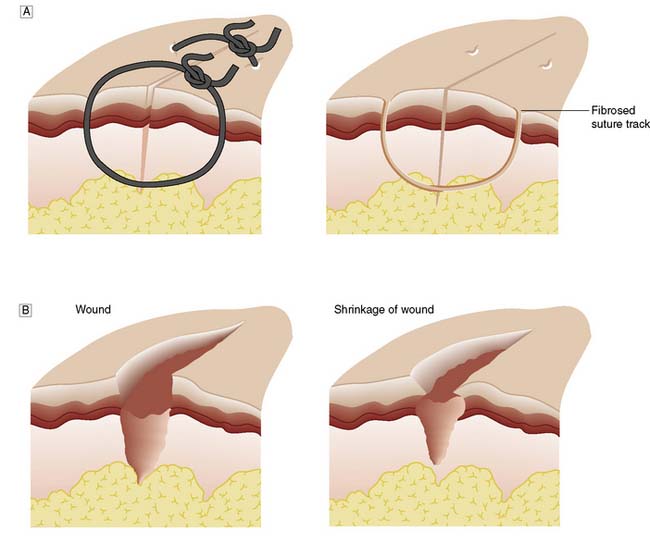
Fig. 18.4 Wound healing.
A Healing by primary intention. B Healing by secondary intention, showing shrinkage of the wound.
Summary Box 18.1 Classification of wound healing
• Healing by first intention is the most efficient method and results when a clean incised surgical wound is meticulously apposed and heals with minimal scarring
• Healing by second intention occurs when wound edges are not apposed and the defect fills with granulation tissue. In the time taken to restore epithelial cover, infection supervenes, fibrosis is excessive and the resulting scar is unsightly
• The term ‘healing by third intention’ describes the situation where a wound healing by second intention (e.g. a neglected traumatic wound or a burn) is treated by excising its margins and then apposing them or covering the area with a skin graft. The final cosmetic result may be better than if the wound had been left to heal by second intention.
Factors influencing wound healing
Wound infection
Classification
• Clean procedures are those in which wound contamination is not expected and should not occur. An incision for a clean elective procedure should not become infected. In clean operations, the wound infection rate should be less than 1%.
Summary Box 18.2 Factors affecting wound healing
Wounds with a good blood supply (e.g. head and neck wounds) heal well
Infection is a major adverse factor and the risk of infection is influenced by:
Intercurrent disease may impair healing. Important factors include:
Surgical technical factors that have a major influence on wound healing include:
• Clean-contaminated procedures are those in which no frank focus of infection is encountered but where a significant risk of infection is nevertheless present, perhaps because of the opening of a viscus, such as the colon. Infection rates in excess of 5% may suggest a breakdown in ward and operating theatre routine.
• Contaminated or ‘dirty’ wounds are those in which gross contamination is inevitable and the risk of wound infection is high; an example is emergency surgery for perforated diverticular disease, or drainage of a subphrenic abscess.
Antibiotic prophylaxis is appropriate for the latter two types of operation.
Involvement of other structures
Summary Box 18.3 Principles of management of contaminated traumatic wounds
• Contaminated wounds should be debrided under general anaesthesia
• The contaminated wound and its margins must be cleansed thoroughly, and grit, soil and foreign bodies/materials removed
• Devitalized tissue is formally excised until bleeding is encountered
• Primary closure is avoided if there has been gross contamination and when treatment has been delayed for more than 6 hours. Inappropriate attempts to achieve primary closure increase the risk of wound infection and expose the patient to the risks of anaerobic infection (tetanus and gas gangrene)
• Wounds left open may be suitable for delayed primary suture after 2–3 days, or for later excision and secondary suture (with or without skin grafting)
• Appropriate protection against tetanus must be afforded and the use of antibiotics should be considered.
involves a limb, the distal circulation must be checked. Where appropriate, X-rays will help to establish whether peritoneal, pericardial or pleural cavities have been entered, and whether there is underlying bony injury.
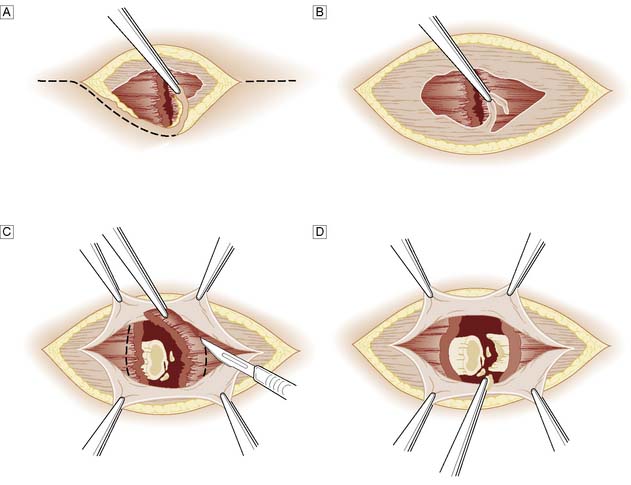
More extensive or severely contaminated wounds usually require inpatient treatment, with exploration and debridement under general anaesthesia. The wound and its margins are cleansed and all obvious foreign material picked out. Devitalized tissue is trimmed back until bleeding occurs. In areas of poor vascularity such as the leg, or if there is severe contamination, crushing or a fracture, the wound margins are formally excised (Fig. 18.6). Bleeding from the wound margin is not a certain indication of its ultimate survival, as impaired venous drainage can lead to progressive necrosis, particularly after a crushing or degloving injury. If there is any doubt, the wound should not be sutured and a ‘second-look’ dressing change should be undertaken under anaesthesia after 48 hours.
Devitalized skin flaps
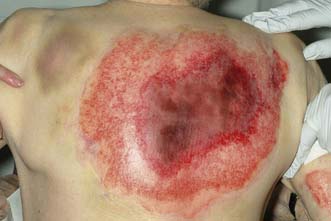
A common emergency problem is posed by the patient, usually an elderly woman, who falls and raises a triangular flap over the surface of the tibia (pretibial laceration). In some cases, the flap is blue-black in colour and obviously non-viable, but in most cases viability is uncertain. Similar injuries can occur elsewhere in the body. The wound must be cleansed and non-viable tissue excised. No attempt should be made to suture the flap back into place; because of the post-traumatic oedema this would only be possible under tension, and would lead to death of the flap. A small defect can be treated conservatively on an out-patient basis by wound dressing and an elastic supporting bandage providing the arterial circulation is normal; Ch. 21) and the patient is kept ambulant. The wound will normally take several weeks to heal. A larger defect may require a split-skin graft, either immediately or as a delayed primary procedure.
Wounds with skin loss
Flaps
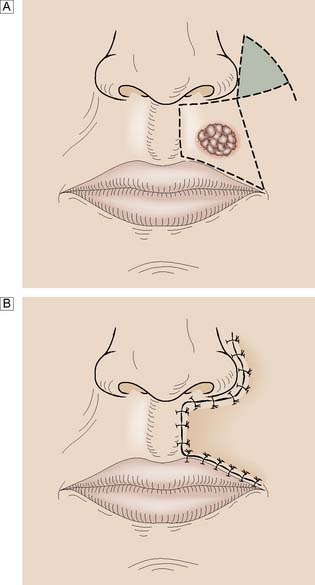
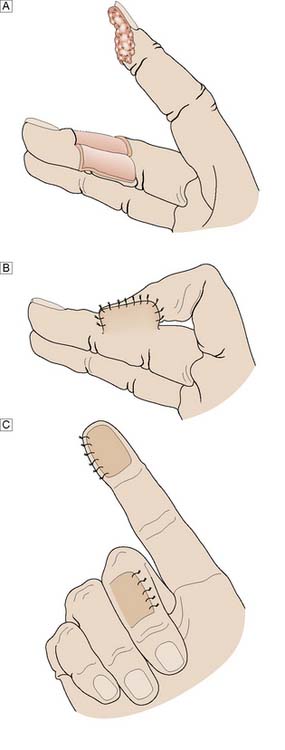
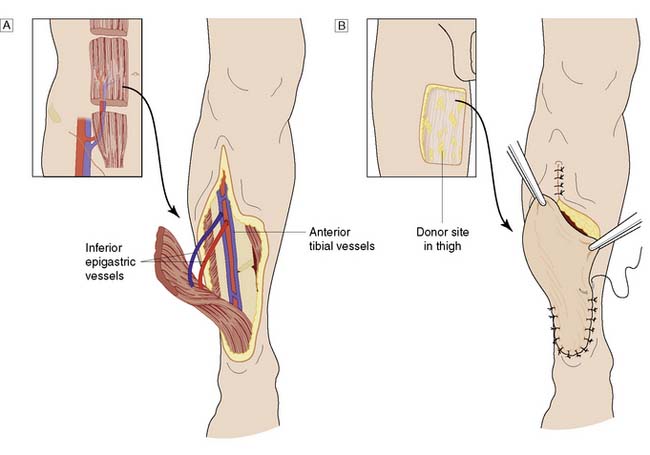
Whereas grafts require a vascular bed to survive, flaps bring their own blood supply to the new site. They can therefore be thicker and stronger than grafts and can be applied to avascular areas such as exposed bone, tendon or joints. They are used in acute trauma only if closure is not possible by direct suture or skin grafting, and are more usually reserved for the reconstruction of surgical defects and for secondary reconstruction after trauma. The simplest flaps use local skin and fat (local flaps), and are often a good alternative to grafting for small defects such as those left after the excision of facial tumours (Fig. 18.7). A flap may have to be brought from a distance (distant flap) and remain attached temporarily to its original blood supply until it has picked up a new one locally (Fig. 18.8). This usually takes 2–3 weeks, after which the pedicle can be divided. Advances in our knowledge of the blood supply to the skin and underlying muscles have led to the development of many large skin, muscle and composite flaps, which have revolutionized plastic and reconstructive surgery. One example is the use of the transverse rectus abdominis musculocutaneous (TRAM) flap for reconstruction of the breast. The ability to join small blood vessels under the operating microscope now allows the surgeon to close defects in a single stage, even when there is no local tissue available, by free tissue transfer (Fig. 18.9). Other tissues, such as bone, cartilage, nerve and tendon, can also be grafted to restore function and correct deformity after tissue damage or loss.
Burns
Local effects of burn injury
The local effects result from destruction of the more superficial tissues and the inflammatory response of the deeper tissues (Table 18.2). Fluid is lost from the surface or trapped in blisters, the magnitude of loss depending on the extent of injury. Loss is greatly increased by leakage of fluid from the circulation (see below) where, instead of the normal insensible loss of 15 ml/m2 body surface/hour, as much as 200 ml/m2 may be lost during the first few hours. With deeper injuries, the epidermis and dermis are converted into a coagulum of dead tissue known as eschar. In its least severe form, the dermal inflammatory response consists of capillary dilatation, as in the erythema of sunburn. With deeper burns, the damaged capillaries become permeable to protein, and an exudate forms with an electrolytic and protein content only slightly less than that of plasma. Lymphatic drainage fails to keep pace with the rate of exudation and interstitial oedema leads to a reduction in circulating fluid volume. An increase of 2 cm in the diameter of the leg represents the accumulation of over 2 litres of excess interstitial fluid. Exudation is maximal in the first 12 hours, capillary permeability returning to normal within 48 hours.
| Destruction of tissue |
General effects of burn injury
Summary Box 18.4 Consequences of burns
• hypovolaemia (loss of protein, fluid and electrolytes)
• metabolic derangements (hyponatraemia followed by risk of hypernatraemia, hyperkalaemia followed by hypokalaemia)
• sepsis, which may be both local and generalized
Classification
Burns are classified according to depth as either partial- or full-thickness (Fig. 18.10).
Prognosis
Age and general condition
Infants, the elderly, alcoholics and those with other co-morbidity fare less well than healthy young adults (see EBM 18.1).
Extent of the burn
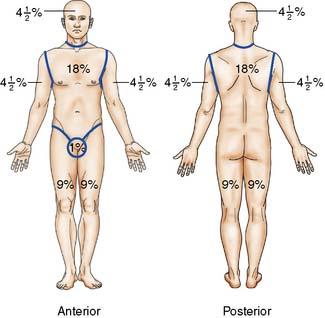
The approximate extent of the burn can be quickly calculated in adults by using the ‘rule of nines’ (Fig. 18.11). Tables are available for more accurate estimations of burn area. The patient’s hand and fingers together constitute about 1% of body surface area (BSA). Hypovolaemic shock is anticipated if more than 15% of the surface is burned in adults, or more than 10% in a child. The ‘rule of nines’ cannot be used in children because of the relatively large head size (about 20% of body surface at birth) and the relatively small limbs (legs are about 13%).
Management
First aid
Prompt effective action prevents further damage and may save life or prevent months of suffering. The key principles are to arrest the burning process, ensure an adequate airway and avoid wound contamination (Table 18.3).
Initial assessment and management
Once airway patency is assured, the time of injury, the type of burn, its previous treatment, and its extent and depth are established (Fig. 18.12). If the burn is over 15% in extent (10% in children), establishing an intravenous infusion takes priority over a detailed history and physical examination. Intravenous therapy may be needed for many days, but there may be few veins available and they must be treated with great respect. It is best to start with the most peripheral vein available in the upper limb, but in shocked patients with vasoconstriction, cannulation of the internal jugular or subclavian vein may be needed. Blood is withdrawn for cross-matching and for determination of haematocrit and urea and electrolyte concentrations. Arterial blood gas analyses are performed and carboxyhaemoglobin levels measured if there is concern about the airway and smoke inhalation. Once an infusion has been established, the pulse rate, blood pressure and core/peripheral temperature difference are monitored. In patients with burns of more than 20%, a catheter is inserted to measure hourly urine output. Severe pain is relieved by intravenous opiates. Tetanus can complicate burns, and tetanus toxoid is given if the patient has not received it recently. In general, patients with burns involving more than 5% of body surface should be admitted to hospital, as should all those with significant full-thickness injury or burns in sites likely to pose particular management problems.
Prevention and treatment of burn shock
The aim of management is to prevent hypovolaemic shock by prompt and adequate fluid replacement (Table 18.4). Opinions vary as to the relative amounts of colloid and crystalloid that should be used. Various formulae are available to help calculate replacement needs, but all are merely guides and the amounts of fluid given must be adjusted in the light of the patient’s response to resuscitation.
Organ failure and burn shock
Organ failure and shock are discussed in detail in Chapter 1.
Prevention of contamination
Skin and soft tissue lesions
Diagnosis of skin swellings
Summary Box 18.5 Key questions when examining skin swellings
• Is the swelling located in the skin or in the subcutaneous tissues, i.e. can the overlying skin be pinched up and moved independently of the swelling?
• Is the swelling epidermal or dermal? Epithelial swellings create irregularity of skin surface, whereas dermal swellings do not
• Is the swelling pigmented? Pigmentation most often (although not always) indicates melanocytic activity.
Cysts
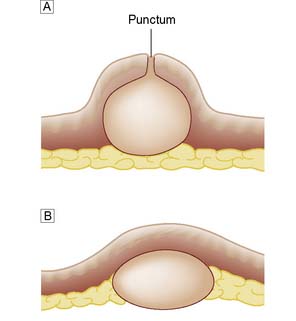
Sebaceous cysts
Sebaceous (or epidermoid) cysts are dermal swellings covered by epidermis (Fig. 18.13). They have a thin wall of flattened epidermal cells and contain cheesy white epithelial debris and sebum. They form soft smooth hemispherical swellings over which the skin cannot be moved. A small surface punctum is often visible. If infection supervenes, the cyst becomes hot, red and painful. Infected cysts are incised to allow the infected material to escape. Excision is deferred until the inflammation has settled. In some cases, the inflammation destroys the cyst lining so that excision is not necessary.
Tumours of the skin
Epidermal tumours are common and can arise from basal germinal cells or melanocytes, whereas dermal tumours arising from connective tissue elements are rare (Table 18.5).
| Epidermal neoplasms (common) | |
|---|---|
| From basal germinal cells: | From melanocytes: |
| Papilloma | Benign pigmented mole |
| Infective wart | Common mole |
| Senile wart | Giant hairy mole |
| Pedunculated papilloma | Blue naevus |
| Keratoacanthoma | Halo naevus |
| Premalignant keratosis | Malignant melanoma |
| Carcinoma in situ | Melanotic freckle (lentigo maligna) |
| Epidermoid cancer | Superficial spreading melanoma |
| Basal cell cancer (rodent ulcer) | Nodular melanoma |
| Squamous cell cancer | Other forms of melanoma |
| Dermal neoplasms (rare) | |
|---|---|
| Fibroma Lipoma Neurofibroma |
Epidermal neoplasms arising from basal germinal cells
Papillomas
Papillomas (or warts) are common benign skin neoplasms.
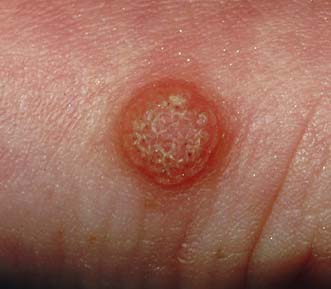
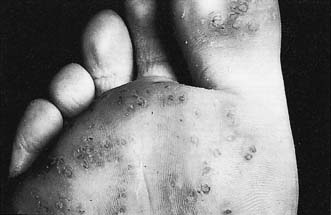
Infective warts
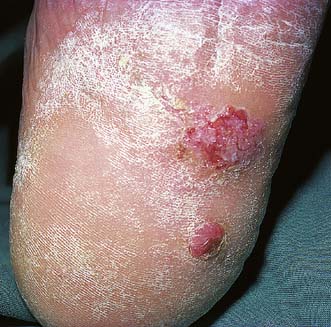
These are caused by viral infection. and are found most commonly on the hands and fingers of young children and adults. They spread by direct inoculation and are often multiple. They form greyish-brown, round or oval elevated lesions with a filiform surface and keratinized projections (Fig. 18.14), and may be studded with spots of blood. They often regress spontaneously but can be removed by caustics (acetic acid) or freezing (liquid nitrogen or CO2 snow). Plantar warts (verruca plantaris) are particularly troublesome infective warts acquired in swimming pools and showers. They are found under the heel and metatarsal heads. They are flush with the surface (Fig. 18.15) and may be intensely painful. If persistent, they are treated by curettage or freezing. Infective warts in the perineum and on the penis may be of venereal origin and are associated with gonorrhoea, syphilis, HIV infection and lymphogranuloma. Infective warts are also common in immunosuppressed patients.
Senile warts
These are basal cell papillomas and are common in the elderly (Fig. 18.16). They form a yellowish-brown or black greasy plaque (synonym: seborrhoeic keratosis) with a cracked surface that falls off in pieces. Senile warts are often multiple, commonly affect the upper back and trunk, and are best treated by curettage.
Keratoacanthoma (molluscum sebaceum)
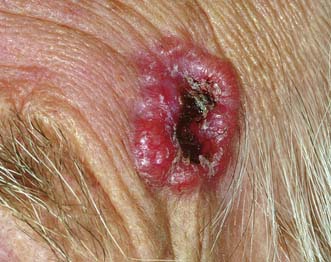
This lesion can be confused with squamous cancer because of its clinical appearance. It grows rapidly over 4–6 weeks and then involutes. Histologically, it has a well-defined ‘shoulder’, but even under the microscope it may resemble a squamous carcinoma. The distinction between the two is the history. Keratoacanthoma occurs most commonly on the face as a hemispherical nodule with a friable red centre crusted with keratin (Fig. 18.17). It is found mainly in those over 50 years of age. It heals after shedding its central core, but can also be eradicated by curettage.
Actinic (solar) keratosis
This is a premalignant keratosis and is characterized by small, single or multiple, firm warty spots on the face, back of the neck and hands (Fig. 18.18). Such keratoses are particularly common in older, fair-skinned people who have been exposed to excessive sunlight. The scaly lesions drop off periodically to leave a shallow premalignant ulcer. The keratoses should be biopsied to exclude frank malignancy, and then treated by freezing.
Cancer of the epidermis
Basal cell carcinoma (rodent ulcer)
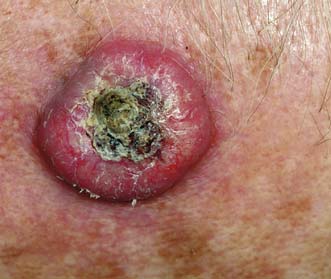
Rodent ulcers are slow-growing, locally invasive and never metastasize. They commonly arise in the skin of the middle third of the face, typically on the nose, inner canthus of the eye, forehead and eyelids (Fig. 18.19). The earliest lesion is a hard pearly nodule, dimpled in its centre and covered by thin telangiectatic skin. Cystic degeneration may make the lesion raised and translucent. Clinical types are described as cystic, nodular, sclerosing, morphoeic, centrally healing and ‘field fire’. Over a period of years, the rodent ulcer repeatedly scales over and breaks down. Growth is extremely slow. Occasionally, the tumour is highly invasive and can burrow deeply, despite little apparent surface activity. All suspicious lesions must be biopsied. Surgical excision or radiotherapy can be used for definitive treatment, but the latter is contraindicated if the lesion is close to the eye or overlies cartilage. Complex reconstructive surgery may be needed to restore structure and function in patients who present late.
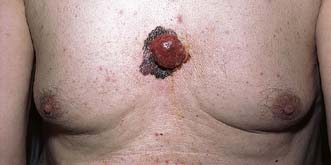
Squamous cell carcinoma
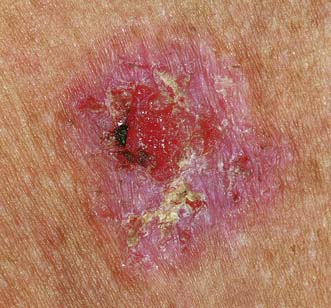
This tumour may affect any area (Fig. 18.20) but is particularly common on exposed parts such as the ear, cheeks, lower lips and backs of the hands. It commonly develops in an area of epithelial hyperplasia or keratosis. In mucosa, such as the lips, the analogous change is leucoplakia. The lesion starts as a hard erythematous nodule, which proliferates to form a cauliflower-like excrescence or ulcerates to form a malignant ulcer with a raised fixed hard edge. The cancer grows more quickly than a rodent ulcer but more slowly than a keratoacanthoma. The regional nodes can be involved early. The choice of treatment (surgery or radiotherapy) depends on the tumour’s size, site and aggressiveness. Palpable lymph nodes require regional lymphadenectomy by block dissection. Adjuvant radiotherapy may be required if histology shows extracapsular spread.
Epidermal neoplasms arising from melanocytes
Benign pigmented moles
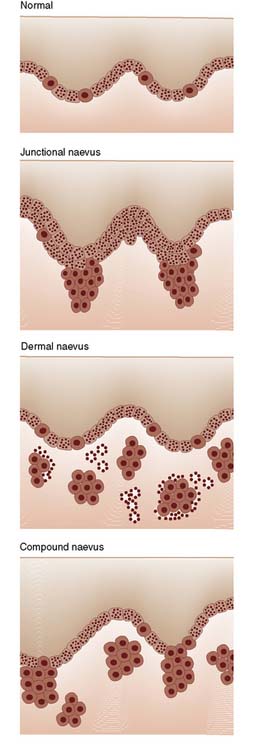
The number of melanocytes is relatively fixed (approximately 2000 million), regardless of the colour of the individual, but the amount of pigment produced varies greatly. As a developmental abnormality, conglomerates of melanocytes may migrate to the dermis or epidermis to form a melanocytic naevus or mole. The naevus cells can cause a variety of pigmented spots and swellings (naevi) according to their site and activity (Fig. 18.21). Moles showing melanocyte activity at the junction of epidermis and dermis (junctional change) are common in childhood; all moles on the soles and palms are of this type. Migration of sheets of naevus cells to the dermis produces a dermal naevus; migration to both dermis and epidermis produces a compound naevus.
Halo naevus
Summary Box 18.6 Epidermal neoplasms arising from melanocytes
• A mole is due to a conglomeration of melanocytes
• Melanocyte activity at the junction of epidermis and dermis (i.e. junctional activity) is common in childhood
• Migration of melanocytes into the dermis produces a dermal naevus, while migration to both dermis and epidermis produces a compound naevus
• Only 1 in 100 000 moles becomes malignant, so that the presence of a mole is not in itself an indication for removal. Active growth in childhood need not cause concern, but growth thereafter should
• Excision is indicated if a mole shows an increase in pigmentation, scaliness, itching or bleeding. A 3 mm excision margin is adequate in the first instance.
Malignant melanoma
Hutchison’s melanotic freckle (lentigo maligna)
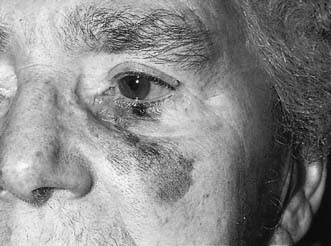
One in 10 malignant melanomas arises in a melanotic or senile freckle. They occur most commonly on the face of elderly women (Fig. 18.22), beginning as a brown-red patch that grows slowly, advancing and receding over the years. The edge of the lesion appears serrated but its margin with normal skin remains abrupt. Kaleidoscopic pigmentation of the surface is typical. This premalignant phase may last for 10–15 years. The first sign of malignancy is a brownish-red papule that develops eccentrically within the freckle and indicates vertical extension of melanocytes into the dermis in the form of a lentigo maligna melanoma.
Superficial spreading melanoma
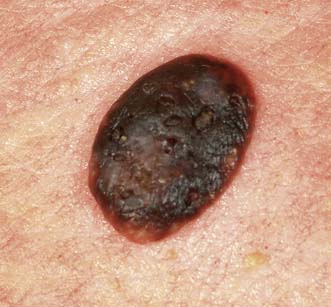
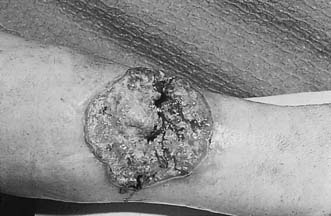
This is the most common type of malignant melanoma (Fig. 18.23). It occurs on the trunk and exposed parts, and is most common in middle age. During a pre-invasive phase, which lasts for at most 1 or 2 years, malignant cells spread outwards (horizontal growth phase) in the epidermis in all directions. The surface is slightly raised, the outline is indistinct, pigmentation is patchy and there may be a wide range of colours. Invasion of the dermis (vertical growth phase) occurs while the lesion is still relatively small and produces an indurated nodule, which soon ulcerates or bleeds.
Other types of malignant melanoma
Amelanotic melanomas are rare, pale pink lesions that can grow rapidly. Careful histological examination will demonstrate pigment in virtually every case. Acral lentiginous melanoma is seen on the soles and palms (Fig. 18.24). It resembles superficial spreading melanoma in its behaviour, although the thick skin of the affected regions may mask some of the features and cause late presentation, with nodularity and ulceration. Subungual melanomas typically affect the thumb or great toe of the middle-aged and elderly, causing chronic inflammation beneath the nail. Pigmentation is not usually visible in the early stages and the lesion is often misdiagnosed as a paronychia or ingrowing toenail.
Spread of malignant melanoma
Summary Box 18.7 Malignant melanoma
• Malignant melanoma is predominantly, but not exclusively, a disease of fair-skinned individuals
• Exposure to sunlight is the key aetiological factor
• The lesion is more common in females, reflecting the higher incidence of malignant melanomas of the lower leg
• 50% of all malignant melanomas arise in a pre-existing naevus
• The essential feature of malignancy is invasion of the dermis by proliferating melanocytes (which show large nuclei, prominent nucleoli and frequent mitoses)
• Malignant melanoma spreads rapidly by the lymphatic system and the bloodstream. ‘In transit’ metastases may develop in the lymphatics of the skin and subcutaneous tissues.
Clinical and pathological staging
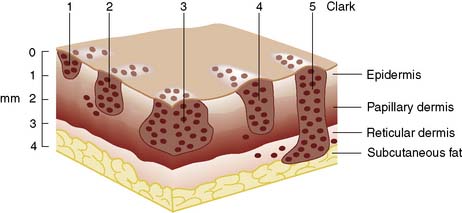
Three clinical stages are recognized and staging has major prognostic implications (Table 18.6). For lesions in clinical stage I, the most reliable prognostic indicator is the depth of the lesion (Fig. 18.25); the more superficial the lesion, the better the prognosis. Depth can be measured by reference to the normal layers of skin (Clark) or by a micrometer gauge (Breslow). As the skin layers may be distorted by the tumour, the Breslow system is usually preferred. Mitotic activity also influences prognosis, and tumours can be graded according to the number of mitotic figures in each field. Lymphocytic response and features of regression can also influence prognosis. Melanotic freckles and superficial spreading melanomas tend to remain superficial and so have a better prognosis than nodular melanomas.
Table 18.6 Prognosis in relation to the stage and depth of malignant melanoma
| Clinical stage | 5-year survival rate (%) |
|---|---|
| I Primary lesion only | |
| Breslow depth (mm) | 70 |
| < 1.5 | 93 |
| 1.5–3.5 | 60 |
| > 3.5 | 48 |
| II Primary lesion + regional lymph node or satellite deposit |
30 |
| III Metastatic disease | 0 |
Management of malignant melanoma
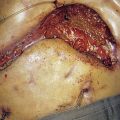
A biopsy is essential to confirm the diagnosis. Thereafter, the depth and stage of the disease are assessed to define the most appropriate form of treatment. Small pigmented lesions are excised with a margin of 3 mm of normal skin, usually under local anaesthesia. Surgical excision is used to treat stage I lesions. Wide excision with a margin of normal skin of at least 5 cm was once routine but has been shown to be unnecessary, particularly for the more superficial melanomas. Breslow depth is now used as the determinant of clearance margin, using a formula of 1 cm clearance for every millimetre of depth up to 3 cm. A smaller margin may be acceptable to avoid mutilation: for example, on the face. The tumour and surrounding skin are excised down to the deep fascia so that the entire depth of subcutaneous fat can be removed. Smaller defects can usually be closed primarily. Large defects have to be covered with a split-skin graft or flap. A block dissection of regional lymph nodes carries significant morbidity and is no longer carried out routinely. However, if the nodes are involved (clinical stage II), or if the primary tumour overlies the nodes, block dissection can be performed at the time of primary surgery. Isolated limb perfusion with cytotoxic drugs can be used in patients with recurrent disease in a single limb. The treatment of metastatic melanoma remains unsatisfactory. The key to the successful management of malignant melanoma is early diagnosis and appropriate surgical excision (EBM 18.2), with reconstruction as appropriate.
Vascular neoplasms (haemangiomas)
Summary Box 18.8 Management of malignant melanoma
• The depth of the lesion is a key prognostic factor and can be assessed by micrometer (Breslow) or by reference to normal layers of the skin (Clark). Superficial spreading melanomas and melanotic freckles have a better prognosis than nodular melanomas
• Excision biopsy is essential to confirm malignancy, assess depth and stage, and define the optimal method of treatment
• Once malignancy is confirmed, an excision margin of 1 cm, up to 3cms, for every 1 mm of Breslow depth is advised. The lesion is excised down to the deep fascia to remove all subcutaneous fat. Skin grafting may be required to close the defect
• Lymph node or satellite deposits reduce 5-year survival rates from 70% to 30%, but patients with distant metastases are not expected to survive for 5 years
• Block dissection of regional lymph nodes is no longer practised routinely but is indicated if the nodes are obviously involved or there is a positive sentinel lymph node biopsy.