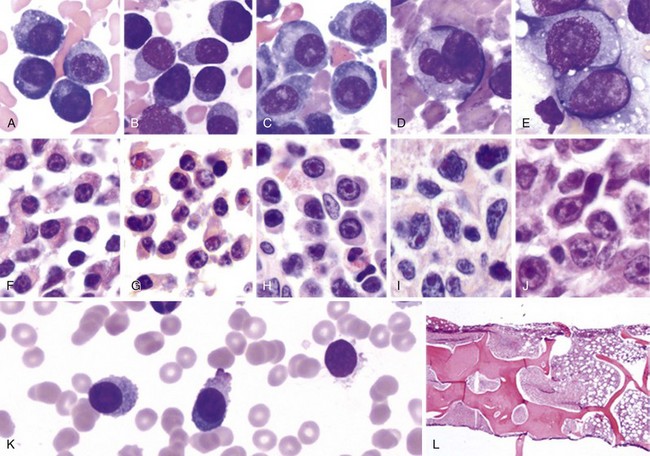
Chapter 38 Plasma Cell Neoplasms
Treatment of Relapsed Multiple Myeloma
Ensure that the patient has a relapse requiring intervention. The conversion from a negative immune-fixation analysis to a positive study result is not an indication to initiate salvage therapy. A clear clinical relapse with new or increased evidence of end-organ damage or a substantial or aggressive biochemical relapse as defined by criteria for progressive disease are indications for the initiation of treatment.
Selection of treatment depends on prior therapy received.
Table 38-1 Thalidomide Regimens in Relapsed or Refractory Multiple Myeloma
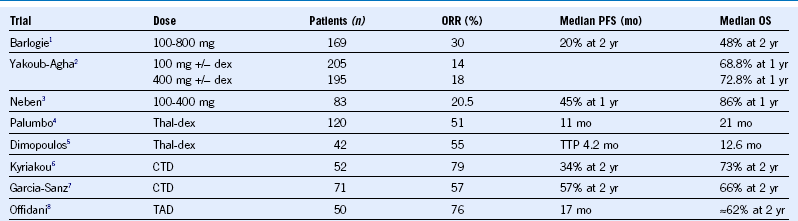
CTD, Cyclophosphamide, thalidomide, and dexamethasone dex, dexamethasone; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TAD, thalidomide, adriamycin, and dexamethasone; Thal, thalidomide; TTP, time to progression.
Table 38-2 Lenalidomide Regimens in Relapsed or Refractory Multiple Myeloma
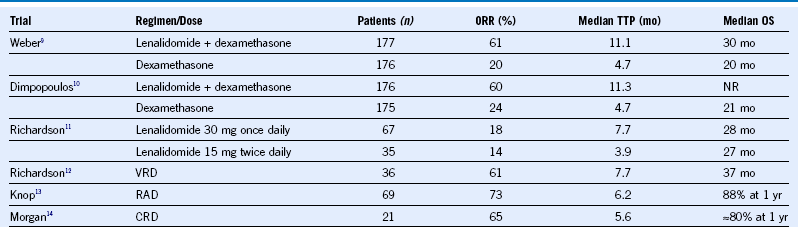
CRD, Carfilzomib, lenalidomide, dexamethasone; NR, not reported; ORR, overall response rate; OS, overall survival; RAD, lenalidomide, adriamycin, dexamethasone; TTP, time to progression; VRD, bortezomib, lenalidomide, dexamethasone.
Table 38-3 Bortezomib Regimens in Relapsed or Refractory Multiple Myeloma
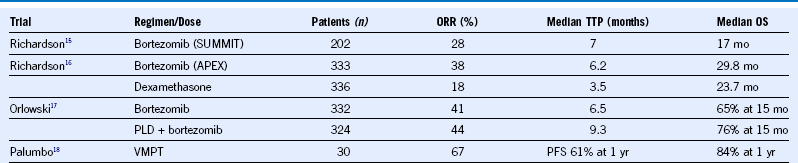
ORR, Overall response rate; OS, overall survival; PFS, progression-free survival; PLD, Liposomal doxorubicin; TTP, time to progression; VMPT, bortezomib, melphalan, prednisone, thalidomide.
Treatment of Newly Diagnosed Multiple Myeloma
Confirm that the patient has symptomatic myeloma. Patients with MGUS or SMM should not be treated outside of a clinical trial.
Perform risk stratification using ISS staging, FISH, or cytogenetics, and assessment of renal function and measurement of LDH level to develop long-term plan based on estimate of prognosis. Although initial treatment is not affected by risk category, later consideration of more aggressive consolidation or use of allogeneic SCT in eligible patients can be considered in high-risk younger patients.
Induction: Both transplant-eligible and ineligible patients can be treated with a triple-drug regimen (bortezomib and dexamethasone with lenalidomide, with thalidomide or cyclophosphamide, or cyclophosphamide, thalidomide, dexamethasone) for 3 to 6 cycles before transplant or 9 to 12 months for transplant-ineligible patients. For transplant-ineligible patients, additional options include a two-drug regimen (dexamethasone with lenalidomide, with bortezomib, or with thalidomide) or melphalan containing regimen such as melphalan with prednisone in combination with any of the newer agents, MPT, MPV, or MPR.
Drug Selection should be influenced depending on patient characteristics.
Transplant-eligible patients can undergo a single autologous transplant with melphalan with a second autologous transplant being optional depending on the overall response or on the protocol in which the patient is participating.
Maintenance: After transplant or optimal induction therapy (to best response), patients can receive maintenance with thalidomide, lenalidomide, or bortezomib. For high-risk patients, both bortezomib and lenalidomide or thalidomide combination can be used. Selection can be influenced based on their induction regimen.
Improving complete response rates is a key goal of current trials, but patients can live a long time with residual paraprotein.
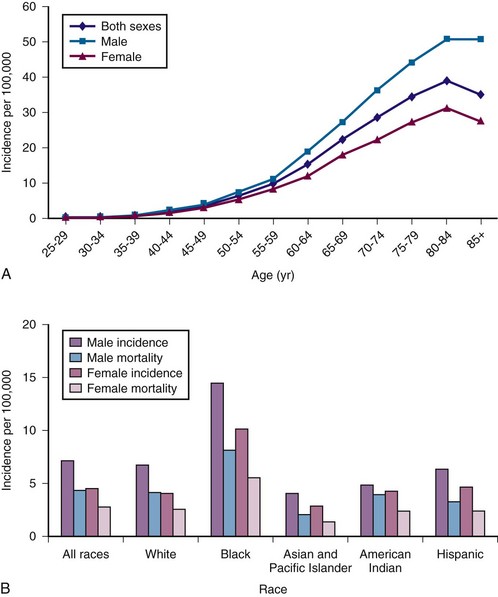
Figure 38-1 A, Multiple myeloma (MM) average annual age- and gender-specific incidence per 100,000 population in the United States in 2009. B, MM average annual race-specific incidence per 100,000 population in the United States in 2009. An increase in incidence is noted with advancing age, men are affected more than women, and a higher incidence is observed in blacks than whites.
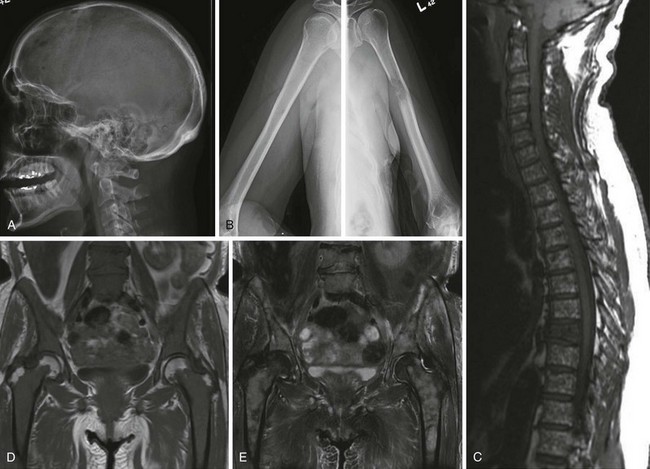
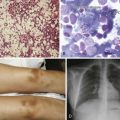
Figure 38-3 A and B, Typical skeletal changes on radiography. Example of “punched-out” lytic lesions in the skull and humerus. C to E, Magnetic resonance imaging pattern in multiple myeloma in the spine and pelvis showing diffuse involvement with focal lesions.
(Courtesy Dr. Nikhil Ramaiya, MD.)
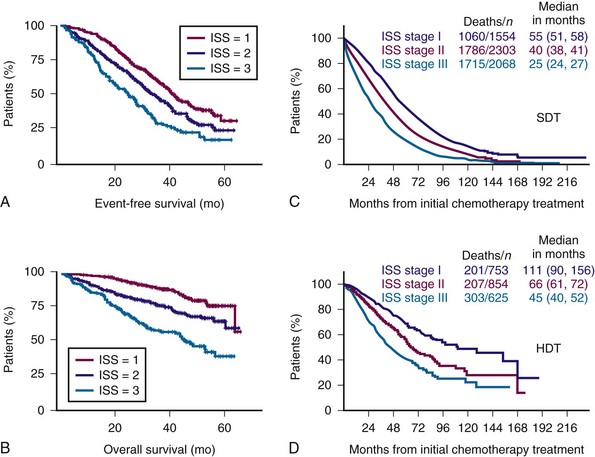
Figure 38-4 International Staging System (ISS) predicts both event-free survival (A) and overall survival (B) and overall survival with both standard-dose therapy (SDT) (C) and high-dose therapy (HDT) (D).
(A and B from Avet-Loiseau H, Attal M, Moreau P, et al: Genetic abnormalities and survival in multiple myeloma: The experience of the Intergroupe Francophone du Myélome. Blood 109:3489, 2007; C and D from Greipp PR, San Miguel J, Durie BG, et al: International staging system for multiple myeloma. J Clin Oncol 23:3412, 2005.)
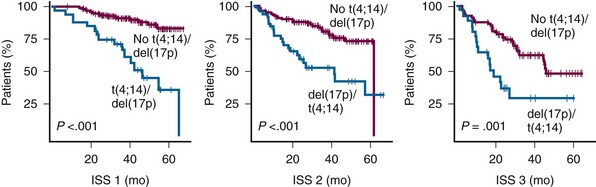
Figure 38-5 KAPLAN-MEIER ESTIMATES OF SURVIVAL ACCORDING TO THE INTERNATIONAL STAGING SYSTEM (ISS) STAGES AND t(4;14) OR del(17p).
(From Avet-Loiseau H, Attal M, Moreau P, et al: Genetic abnormalities and survival in multiple myeloma: The experience of the Intergroupe Francophone du Myélome. Blood 109:3489, 2007.)
| Adhesion Molecule | Normal Plasma Cells | Multiple Myeloma Cells |
|---|---|---|
| CD138 | + | + |
| CD19 | + | − |
| CD28 | − | − |
| CD38 | + | + |
| CD40 | + | + |
| CD45 | + | −* |
| CD27 | − | + |
| CD11a | + | − |
| CD11b | − | − |
| CD44 | + | + |
| CD54 | + | + |
| CD56 | − | + |
| CD58 | − | + |
| LFA-1 | − | −/+ |
| RHAMM | − | + |
| VLA-4 | + | + |
| VLA-5 | + | + |
RHAMM, Hyaluronan-mediated motility receptor; VLA, very late antigen.
*CD45 on immature myeloma cells.
Table 38-5 Clinical Features of Multiple Myeloma
| Bone Destruction |
|---|
Table 38-6 Diagnostic Criteria for Multiple Myeloma, Myeloma Variants, and Monoclonal Gammopathy of Unknown Significance
CT, Computed tomography; MM, multiple myeloma; MRI, magnetic resonance imaging.
*If flow cytometry is performed, most plasma cells (>90%) will show a neoplastic phenotype.
†A small M component may sometimes be present.
Table 38-7 Imaging Modalities for Disease Assessment in Myeloma
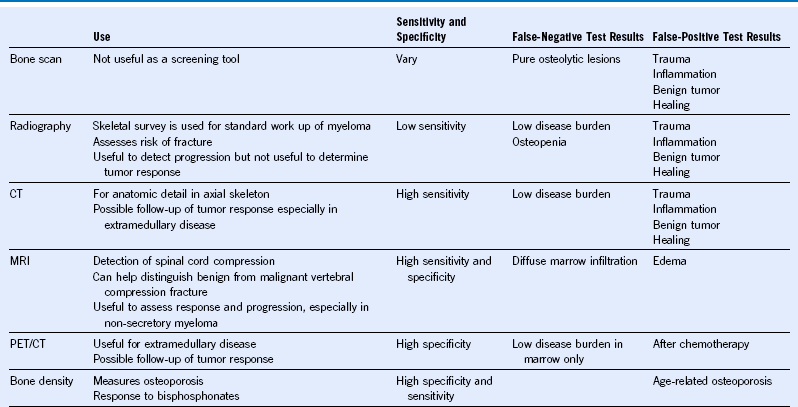
CT, Computed tomography; MM, multiple myeloma; MRI, magnetic resonance imaging; PET, positron emission tomography.
Table 38-8 Risk Stratification in Multiple Myeloma
| Investigation Recommended for Risk Stratification |
|---|
| Additional Investigation for Risk Stratification |
BM, Bone marrow; CGH, comparative genomic hydridization; FISH, fluorescent in situ hybridization; ISS, International Staging System; MRI, magnetic resonance imaging; PC, plasma cells; PET, spositron emission tomography; SNP, single nucleotide polymorphism.
Table 38-9 International Staging System of Multiple Myeloma Patients
| Stage | Criteria | Median Survival (mo) |
|---|---|---|
| I | Serum β2 microglobulin <3.5 mg/L | 62 |
| Serum albumin ≥3.5 g/dL | ||
| II* | Not stage I or III | 44 |
| III | Serum β2 microglobulin ≥5.5 mg/L | 29 |
*There are two categories for stage II: serum β2 microglobulin <3.5 mg/L but serum albumin <3.5 g/dL; or serum β2-microglobulin 3.5 to <5.5 mg/L irrespective of the serum albumin level.
Data from Greipp PR, San Miguel J, Durie BG, et al: International staging system for multiple myeloma. J Clin Oncol 23:3412, 2005.
Table 38-10 Evaluation of Multiple Myeloma Patients
| Evaluation for Diagnosis Evaluation for Monoclonal Protein |
|---|
| Evaluation for Clonal Plasma Cells |
| Other Investigations for Selected Patients |
BM, Bone marrow; CRP, C-reactive protein; FISH, fluorescent in situ hybridization; Ig, immunoglobulin; ISS, International Staging System; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging.
Table 38-11 Lenalidomide Regimens in Newly Diagnosed Multiple Myeloma
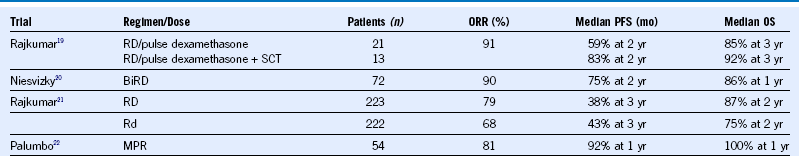
BiRD, Biaxin, lenalidomide, dexamethasone; ORR, overall response rate; PFS, progression-free survival; Rd, lenalidomide, low-dose dexamethasone; RD, lenalidomide, high-dose dexamethasone; SCT, stem cell transplantation.
Table 38-12 Bortezomib Regimens in Newly Diagnosed Multiple Myeloma
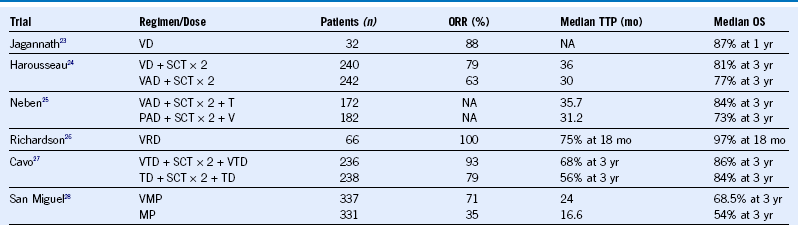
MP, Melphalan and prednisone; NA, not applicable; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; SCT, stem cell transplantation; TTP, time to progression; VAD, vincristine, dexamethasone, and Adriamycin.
Table 38-13 Single Versus Double ASCT for Newly Diagnosed Multiple Myeloma
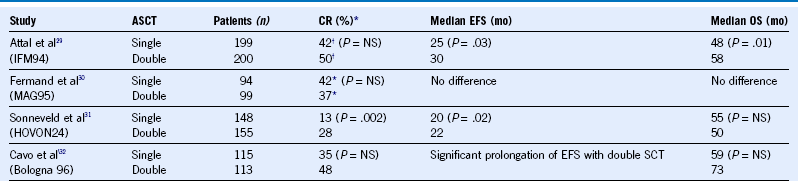
ASCT, Autologous stem cell transplant; CR, complete remission; EFS, event-free survival; NS, not significant; OS, overall survival; SCT, stem cell transplantation.
Table 38-14 Studies of Myeloablative and Reduced Intensity Allogeneic Stem Cell Transplantation for Newly Diagnosed Myeloma
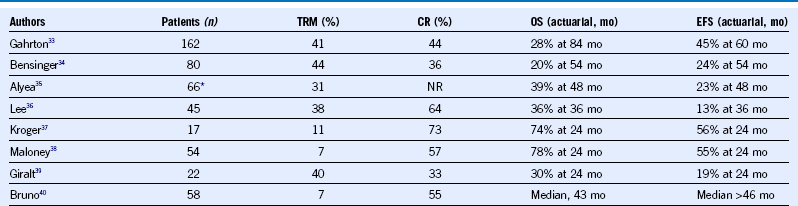
CR, Complete remission; DFS, disease-free survival; EFS, event-free survival; NR, not reported; OS, overall survival; PFS, progression-free survival; TRM, treatment-related mortality.
1 Barlogie B, Desikan R, Eddlemon P, et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: Identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98:492.
2 Yakoub-Agha I, Mary J, Hulin C, et al. Low-dose vs. high-dose thalidomide for advanced multiple myeloma: A prospective trial from the Intergroupe Francophone du Myélome. Eur J Haemato. 2012;l88:249.
3 Neben K, Moehler T, Benner A, et al. Dose-dependent effect of thalidomide on overall survival in relapsed multiple myeloma. Clin Cancer Res. 2002;8:3377.
4 Palumbo A, Bertola A, Falco P, et al. Efficacy of low-dose thalidomide and dexamethasone as first salvage regimen in multiple myeloma. Hematol J. 2004;5:318.
5 Dimopoulos MA, Zervas K, Kouvatseas G, et al. Thalidomide and dexamethasone combination for refractory multiple myeloma. Ann Oncol. 2001;12:991.
6 Kyriakou C, Thomson K, D’Sa S, et al. Low-dose thalidomide incombination with oral weekly cyclophosphamide and pulsed dexamethasoneis a well tolerated and effective regimen in patients with relapsed and refractory multiple myeloma. Br J Haematol. 2005;129:763.
7 García-Sanz R, González-Porras JR, Hernández JM, et al. The oral combination of thalidomide, cyclophosphamide and dexamethasone (ThaCyDex) is effective in relapsed/refractory multiple myeloma. Leukemia. 2004;18:856.
8 Offidani M, Corvatta L, Marconi M, et al. Low-dose thalidomide with pegylated liposomal doxorubicin and high-dose dexamethasone for relapsed/refractory multiple myeloma: A prospective, multicenter, phase II study. Haematologica. 2006;91:133.
9 Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133.
10 Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123.
11 Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458.
12 Richardson PG, Weller E, Jagannath S, et al. Multicenter, Phase I, Dose-Escalation Trial of Lenalidomide Plus Bortezomib for Relapsed and Relapsed/Refractory Multiple Myeloma. J Clin Oncol. 2009;27:5713.
13 Knop S, Gerecke C, Liebisch P, et al. Lenalidomide, adriamycin, and dexamethasone (RAD) in patients with relapsed and refractory multiple myeloma: a report from the German Myeloma Study Group DSMM (Deutsche Studiengruppe Multiples Myelom). Blood. 2009;113:4137.
14 Morgan GJ, Schey SA, Wu P, et al. Lenalidomide (Revlimid), in combination with cyclophosphamide and dexamethasone (RCD), is an effective and tolerated regimen for myeloma patients. Br J Haematol. 2007;137:268.
15 Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomibin relapsed, refractory myeloma. N Engl J Med. 2003;348:2609.
16 Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: Final time-to-event results of the APEX trial. Blood. 2007;110:3557.
17 Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized Phase III Study of Pegylated Liposomal Doxorubicin Plus Bortezomib Compared With Bortezomib Alone in Relapsed or Refractory Multiple Myeloma: Combination Therapy Improves Time to Progression. J Clin Oncol. 2007;25:3892.
18 Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-Melphalan-Prednisone-Thalidomide Followed by Maintenance With Bortezomib-Thalidomide Compared With Bortezomib-Melphalan-Prednisone for Initial Treatment of Multiple Myeloma: A Randomized Controlled Trial. J Clin Oncol. 2010;28:5101.
19 Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050.
20 Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naïve Q symptomatic multiple myeloma. Blood. 2007;111:1101.
21 Rajkumar SV, Jacobus S, Call NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010;11:29.
22 Palumbo A, Falco P, Corradini P, et al. Melphalan, Prednisone, and Lenalidomide Treatment for Newly Diagnosed Myeloma: A Report From the GIMEMA Italian Multiple Myeloma Network. J Clin Oncol. 2007;25:4459.
23 Jagannath S, Durie BGM, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776.
24 Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib Plus Dexamethasone Is Superior to Vincristine Plus Doxorubicin Plus Dexamethasone As Induction Treatment Prior to Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: Results of the IFM 2005-01 Phase III Trial. J Clin Oncol. 2010;28:4621.
25 Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:1.
26 Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679.
27 Cavo M, Pantani L, Petrucci MT, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120:9.
28 San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906.
29 Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495.
30 Fermand J, et al. Hematol J. 2003;4:S59. P10.2.2
31 Sonneveld P, van der Holt B, Segeren CM, et al. Intermediate-dose melphalan compared with myeloablative treatment in multiple myeloma: Long-term follow-up of the Dutch cooperative group HOVON 24 trial. Haematologica. 2007;92:928.
32 Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434.
33 Gahrton G, Tura S, Ljungman P, et al. Prognostic factors in allogeneic bone marrow transplantation for multiple myeloma. J Clin Oncol. 1995;13:1312.
34 Bensinger WI, Buckner CD, Anasetti C, et al. Allogeneic marrow transplantation for multiple myeloma: An analysis of risk factors on outcome. Blood. 1996;88:2787.
35 Alyea E, Weller E, Schlossman R, et al. Outcome after autologous and allogeneic stem cell transplantation for patients with multiple myeloma: Impact of graft-versus-myeloma effect. Bone Marrow Transplant. 2003;32:1.
36 Lee CK, Badros A, Barlogie B, et al. Prognostic factors in allogeneic transplantation for patients with high-risk multiple myeloma after reduced intensity conditioning. Experimental Hematology. 2003;31:73.
37 Kröger N, Schwerdtfeger R, Kiehl M, et al. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood. 2002;100:755.
38 Maloney DG, Molina AJ, Sahebi F, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447.
39 Giralt S, Aleman A, Anagnostopoulos A, et al. Fludarabine/melphalan conditioning for allogeneic transplantation in patients with multiple myeloma. Bone Marrow Transplantation. 2002;30:1.
40 Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110.