CHAPTER 121 Piriformis Syndrome
BACKGROUND
In 1928, Yeoman1 ascribed 36% of cases of sciatica to sacroiliac arthritis transmitted via the piriformis muscle. Later articles by Freiberg and Vinkle in 19342 and by Freiberg3 also supported the notion of sciatica secondary to sacroiliac arthritis and compression by the piriformis muscle and fascia. In 1934, Mixter and Barr4 described the herniated disc as a cause of sciatica, which brought into question the original etiologies of sciatica proposed by Yeoman and Freiberg. Despite this compelling new evidence implicating a herniated disc as a major cause of sciatica, the literature continued to reflect the belief that the piriformis muscle can be involved in the etiology of sciatica. In 1936, Thiele5 described piriformis muscle pain secondary to spasm and hypertrophy irritating the sciatic nerve. In 1936, Shordania6 coined the term ‘piriformitis’ based on his observations of 37 women with sciatica. In 1937 and 1938, Beaton and Anson7 ascribed coccydynia to anatomic variations in the sciatic nerve and piriformis muscle. However, it was Robinson8 who, in 1947, first coined the term ‘piriformis syndrome’ and described six associated typical features. Robinson, like his predecessors, reported that the piriformis muscle and fascial tissues could cause sciatica. Despite this historical evolution of the diagnosis and modern advances in techniques of electrophysiologic testing and magnetic resonance imaging (MRI), piriformis syndrome remains a diagnosis of exclusion and is poorly understood and controversial. A recent survey of physiatrists revealed a lack of consensus on whether the diagnosis of piriformis syndrome exists, and if it does exist, how to make the diagnosis.9 Much of the controversy stems from the relative rarity of the diagnosis when compared to the more easily recognized and treatable causes of sciatica stemming from the lumbar spine.10
EPIDEMIOLOGY
Low back pain and sciatica, described as pain or numbness within the buttock and posterior thigh with occasional radiation into the foot, are common complaints, with a reported lifetime incidence as high as 60–90%.11 The total cost of low back pain and sciatica is significant, exceeding US$16 billion in both direct and indirect costs. Given the lack of agreement on exactly how to diagnose piriformis syndrome, estimates of frequency of sciatica caused by piriformis syndrome vary from rare to approximately 6% of sciatica cases seen in a general family practice.12,13 There is no clear age or gender preference, with reports varying from a 6:1 female-to-male predominance, while others suggest a 1.4:1 male-to-female ratio.13,14
ANATOMY
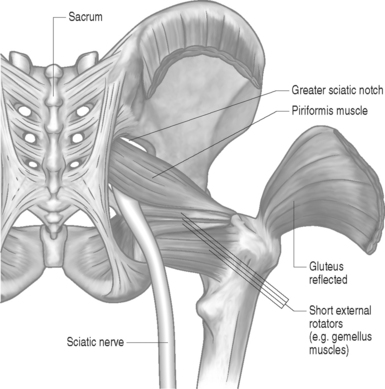
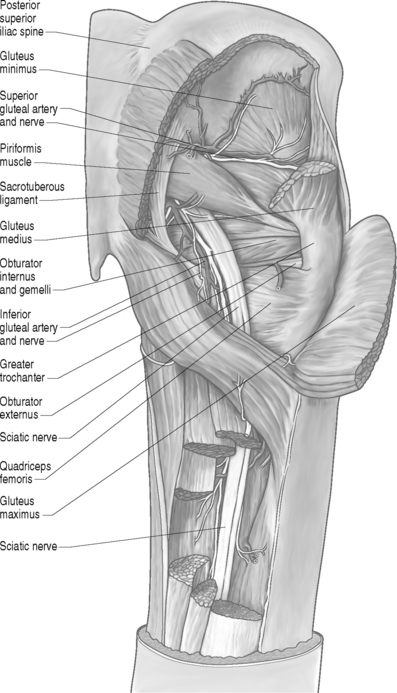
The piriformis muscle is flat, pyramid-shaped, and broadly originates from the ventrolateral surface of the sacrum from the S2–4 vertebrae. The piriformis muscle originates from the anterior surface of the sacrum between and lateral to the anterior sacral foramen, capsule of the sacroiliac articulation, the margin of the greater sciatic foramen, and the sacrotuberous ligament. From its point of origin, it passes through the greater notch and dorsal to the sciatic nerve before inserting on the superior-medial aspect of the greater trochanter of the femur (Fig. 121.1).
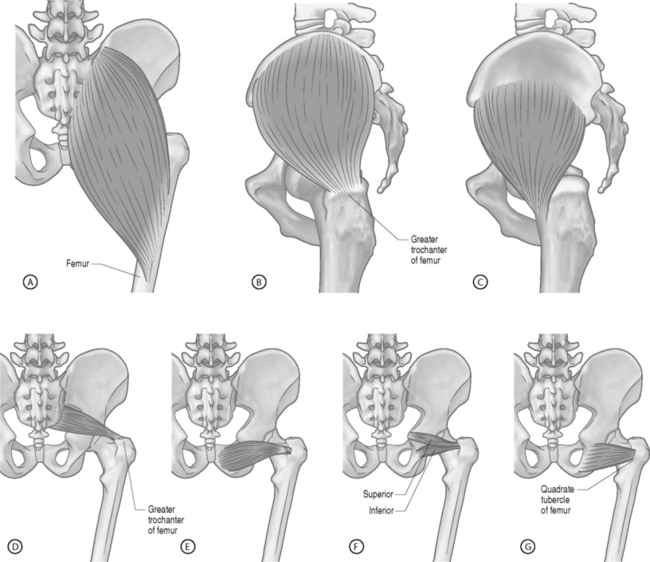
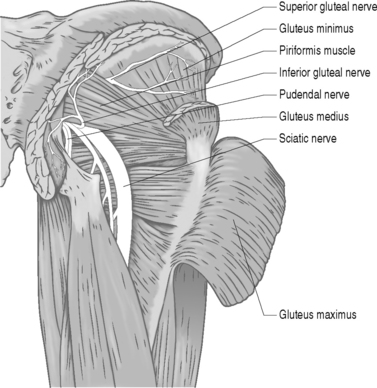
The piriformis muscle is the most proximal of the hip external rotators. With the hip extended, the piriformis muscle externally rotates the hip; however, with the hip flexed, the piriformis muscle becomes a hip abductor.15 Branches from L5, S1, and S2 nerve roots innervate the piriformis muscle. The superior gemellus, inferior gemellus, quadratus femoris, and obturator internus act as synergists with the piriformis muscle (Fig. 121.2, 121.3).
Many developmental variations of the relationship between the sciatic nerve in the pelvis and piriformis muscle have been observed. The sciatic nerve consists of branches from the L3 to S3 nerve roots and usually courses beneath the piriformis muscle and dorsal to the gemellus muscles after exiting the pelvis through the greater sciatic foramen (Fig. 121.4).16 In approximately 20% of the population, the piriformis muscle belly is split with one or more parts of the sciatic nerve dividing the muscle belly itself. In 10% of the population, the tibial and peroneal divisions of the sciatic nerve are not enclosed in a sheath. Usually, the peroneal portion splits the piriformis muscle belly, while it is rare for the tibial division to split the piriformis muscle belly.16
PATHOPHYSIOLOGY
Based on its etiology, piriformis syndrome may be divided into primary or secondary causes (Table 121.1). Primary causes occur from direct nerve compression such as trauma or due to factors that are intrinsic to the piriformis muscle and include anomalous variations in the muscle anatomy, muscle hypertrophy, chronic inflammation of the muscle, and secondary changes from trauma such as adhesions. Secondary causes may include symptoms due to pelvic mass lesions, infections, and anomalous vessels or fibrous bands crossing the nerve, bursitis of the piriformis tendon, sacroiliac inflammation, and possibly myofascial trigger points. Other causes of symptoms may include pseudoaneurysms of the inferior gluteal artery adjacent to the piriformis muscle, bilateral piriformis syndrome due to prolonged sitting during extended procedures, cerebral palsy due to hypertonicity and contractures, total hip arthroplasty as discussed below, and myositis ossificans.
| Primary | Secondary |
|---|---|
| Trauma | Hematoma |
| Pyomyositis | Bursitis |
| Myositis ossificans | Pseudoaneurysm |
| Dystonia musculorum deformans | Excessive pronation |
| Hypertrophy | Fibrous bands |
| Adhesions | Mass lesions |
| Fibrosis | Anomalous vessels |
| Anatomic variations |
Lumbar hyperlordosis and hip flexion contractures increase piriformis muscle strain and seem to predispose individuals with these to developing symptoms. Altered gait biomechanics has also been theorized to lead to piriformis muscle hypertrophy and chronic inflammation, which can cause piriformis syndrome. Patients with weak abductors or leg-length discrepancies are particularly prone. During the stance phase of gait, the piriformis is stretched as the weight-bearing hip is maintained in internal rotation. As the hip then enters the swing phase, the piriformis muscle contracts, aiding in external rotation. The piriformis muscle is under strain during the entire cycle of gait and may be more prone to hypertrophy than other muscles in that region.10,17–19 Any gait abnormality that maintains the affected hip in a position of increased internal rotation or adduction may increase muscle strain even more.
Blunt injury may cause hematoma formation and subsequent scarring between the sciatic nerve and short external rotators, which may lead to symptoms. In a study of 15 patients with post-traumatic piriformis syndrome as a result of a direct blow to the buttock, all patients had a release of the piriformis tendon and sciatic neurolysis with return to normal activity 2 months after surgery.20
A lower lumbar radiculopathy also may cause secondary irritation of the piriformis muscle, which may complicate the diagnosis and hinder physical treatment methods, as the treatment for a lower lumbar radiculopathy involves stretching of the lower back and hip musculature which may, in fact, increase the symptoms in a patient with piriformis syndrome. Even though there are case reports linking the superior gluteal nerve to piriformis syndrome, there is insufficient evidence to conclude that this nerve is involved in true cases of piriformis syndrome.21 Furthermore, the superior gluteal nerve usually leaves the sciatic nerve trunk and passes through the canal above the piriformis muscle.
CLINICAL PRESENTATION
However, piriformis syndrome is often a diagnosis made after excluding other causes of sciatica emanating from the lumbar spine or a pelvic mass causing extrinsic compression of the sciatic nerve. Attempts have been made to derive a set of features that could strongly suggest the diagnosis by history and examination. Robinson was the first to do so when he described six typical features: (1) a history of fall on the buttocks; (2) pain in the sacroiliac joint, greater sciatic notch, and the piriformis; (3) acute exacerbations brought on by stooping or lifting; (4) presence of a palpable mass over the piriformis; (5) positive Laseque’s sign; and (6) gluteal atrophy.8
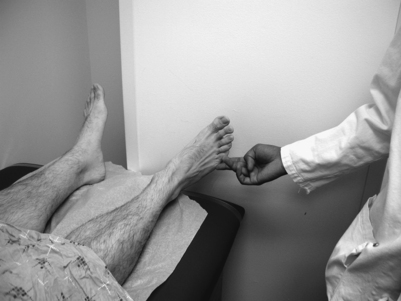
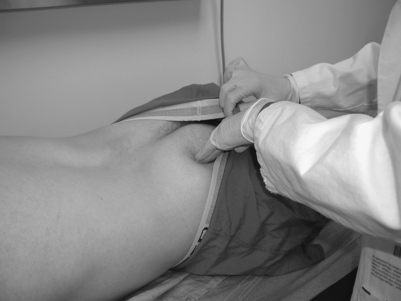
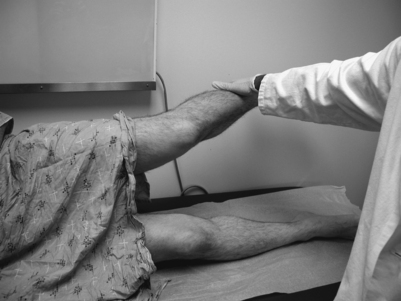
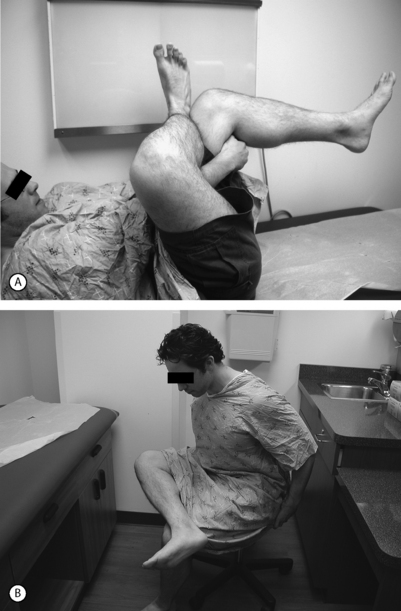
While there are no pathognomonic tests on physical examination to diagnose piriformis syndrome, there are a number of findings that may aid the diagnosis. In the supine position, the patient may have a tendency to keep the affected leg slightly elevated and externally rotated (positive piriformis sign) (Fig. 121.5).17 Piriformis muscle spasm can be detected by careful deep palpation over the location where the piriformis muscle crosses the sciatic nerve (Fig. 121.6). Locating the midpoint of a straight line drawn between the coccyx and the greater trochanter can approximate this position. Digital rectal examination may reveal tenderness over the lateral pelvic wall that reproduces the syndrome. Reproduction of sciatica-type pain with weakness is noted by resisted abduction/external rotation (Pace test) (Fig. 121.7). The Freiberg test is another diagnostic sign that elicits pain upon forced internal rotation of the extended thigh. Beatty described a technique that attempts to distinguish between lumbar radiculopathy, primary hip disease, and pain caused by piriformis syndrome.22 However, it was noted that the Beatty test can be positive in patients with herniated lumbar discs or hip osteoarthritis and therefore has a low specificity. The patient is made to lie on his or her side with the affected leg facing up and is then asked to hold the affected knee in the air for several minutes. Deep buttock pain was reported to be consistent with piriformis syndrome (Fig. 121.8). A painful point may be palpated at the lateral margin of the sacrum. On occasion, weak abductors or shortening of the involved extremity may be seen. Careful examination of the ipsilateral foot may reveal that the second metatarsal is longer than the first (Morton’s foot) causing a rocking motion during step-off and increased adduction and hip stress. While in the authors’ experience not one of these physical exam maneuvers is pathognomonic, the authors do feel that a combination of positive findings in a patient with the appropriate history and diagnostic work-up is consistent with piriformis syndrome.
It has been described that piriformis syndrome can be distinguished from a herniated intervertebral disc due to a lack of a neurologic deficit in piriformis syndrome.13 This concept was supported by Steiner’s study which stated that lack of a true neurologic deficit was the most important criterion in distinguishing piriformis syndrome from sciatica.23 However, a further examination of the literature reveals eleven of 28 reported cases (40%) where patients had evidence of neurologic deficits on physical examination.9,15,24–28
DIFFERENTIAL DIAGNOSIS
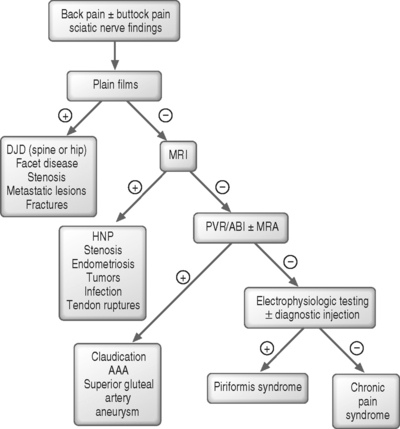
Since there is no described pathognomonic sign of piriformis syndrome, a broad differential diagnosis should be considered in patients presenting with sciatica before patients are given the diagnosis of piriformis syndrome (Fig. 121.9). Other possible diagnoses should include but not be limited to lumbar pathology such as herniated disc, degenerative disc disease, and facet arthropathy, sacroiliitis, myofascial pain, and trochanteric bursitis.
The diagnosis of piriformis syndrome remains one of exclusion.10 Generally, laboratory studies and imaging have a limited role in diagnosing piriformis syndrome but diagnostic imaging of the lumbar spine and pelvis should be done in all cases to rule out other causes of sciatica. Although there are reports in the literature of nuclear diagnostic imaging and MRI of the pelvis assisting in the primary diagnosis of piriformis syndrome, neither test is considered reliable in making the diagnosis.16 However, future improvements in resolution and image technology may allow visualization of subtle changes in the piriformis muscle signal intensity on MRI or computed tomograms to signify edema or inflammation.29
Electrophysiological testing is a promising area of development to aid in the diagnosis of piriformis syndrome. A 1.86-msec prolongation of the H-reflex with the flexion, adduction, and internal rotation test (FAIR) on the ipsilateral lower extremity has been shown to be a sensitive electrophysiologic criterion for diagnosing pyriformis syndrome.30,31 Some authors have suggested that the H-reflex is an electrically stimulated version of the Achilles reflex. The H-reflex crosses the piriformis muscle twice – in afferent and efferent orthodromic conductions. A growing body of work by Fishman et al.30–32 suggests evidence that piriformis syndrome is a mechanical and functional impingement. However, this methodology is theoretical and far from proven. Further clinical studies and evidence-based methodologies are required to delineate the ramifications of Fishman’s work. Changes in amplitude and latencies of recorded potentials from an epidural electrode inserted into the lumbar spine at L3–4 with stress to the affected leg have also been seen in patients with piriformis syndrome.33
Despite efforts to develop an objective diagnostic test, the diagnosis of piriformis syndrome is still one of exclusion based primarily on a constellation of signs and symptoms stemming from the history, physical examination, and diagnostic tests (Table 121.2).
| History | Physical examination | Diagnostic tests |
|---|---|---|
| Unilateral | Pain | Scintography |
| Pain | Localized | MRI of piriformis muscle |
| Buttock | Bending over | |
| Greater trochanter | Lifting | CT scan of piriformis muscle |
| Posterior thigh | Squatting | |
| Prolonged sitting | Limp on affected side | H-reflex |
| At night | Digital rectal examination | F wave |
| Dyspareunia | ||
| Failed back surgery | Pace test | |
| Trauma | Freiberg test |
TREATMENT
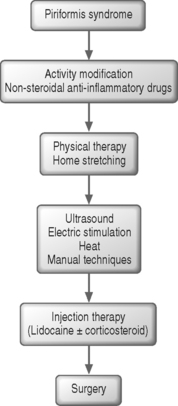
Without a reproducible and accurate method to diagnose piriformis syndrome, current therapeutic treatment regimens are controversial without substantive outcomes data that have been subjected to randomized, blind controlled trials. Despite this fact, numerous treatment strategies exist for patients with piriformis syndrome (Fig. 121.10). The first approach and the mainstay of treatment consists of rehabilitation measures beginning with activity modification and physical therapy. The emphasis of the latter component involves piriformis and hip abductor/adductor stretching and strengthening. Physical therapy aims at stretching the muscle and reducing the vicious cycle of pain and spasm. Stretching several times throughout the day can be complemented with manual techniques, heat, ultrasound, or electrical stimulation. When these additional techniques are performed prior to the initiation of piriformis stretching, they may allow the hip joint capsule to be mobilized anteriorly and posteriorly and lengthen the muscle belly, thus gaining greater excursion of the piriformis tendon and more effective stretching.
A home stretching program must be provided. Frequent and repeated stretching throughout the day is an essential component of the treatment program. During the acute phase of treatment stretching, at a minimum every 6 hours (while awake), is strongly recommended. Prolonged stretching of the piriformis muscle is accomplished in either a supine or orthostatic position with the involved extremity flexed and passively adducted/internally rotated (see Fig. 121.11).
Injection therapy can be incorporated if the situation is refractory to the aforementioned treatment program. Injections are usually applied at the origin of the sacroiliac joint with fluoroscopy, along the course of the muscle belly, or at the insertion of the piriformis.34 For effective injection, the piriformis muscle must be localized, but this can be difficult. Considerations should be given to use image-guided technology such as ultrasound, MRI, computed tomography (CT) scans if available, by a knowledgeable user, or manually by palpation over the point of maximal tenderness or by digital rectal examination. Once localized, the piriformis muscle is injected using a 3.5-inch spinal needle or a longer needle in obese patients. Care is taken to avoid direct injection of the sciatic nerve by asking the patient to report any changes in symptoms during the procedure. Traditional injections have included steroids and/or local anesthetics. Dosages may vary but the authors suggest 2–10 mL of 1% lidocaine and 80 mg of triamcinolone as a mixture or individually. Some researchers believe that there is little to no inflammatory component and, as such, have proposed using only 10 cc of 1% lidocaine followed immediately by piriformis stretching These injections without steroid can be performed on a weekly basis over a period of 4–5 weeks to assess benefit and the need for surgery. It is important to avoid longer-acting anesthetics in case the sciatic nerve is inadvertently injected. Preliminary studies using 12 500 units of botulinum neurotoxin B or a similar dose of botulinum toxin A and physical therapy to treat piriformis syndrome have been conducted. In a few cases patients describe relief for up to 3 months.30,32 Given that nearly 50% of patients suffered side effects including dry mouth and dysphagia, and the lack of compelling scientific evidence proving its efficacy, such treatment remains investigational.
Surgical management is the treatment of last resort, but can produce dramatic results. While conservative treatment has been noted to be effective, a review of the 28 reported cases in the literature revealed that 13 (46%) went on to require surgical intervention.9,19,23–27,35,36 Surgery for this condition involves resection of the muscle itself or the muscle tendon near its insertion at the superior-medial aspect of the greater trochanter of the femur.37 The authors’ technique involves a combination of both by first sectioning the tendon from its insertion and then through the muscle belly as it emerges from the greater sciatic notch to remove the muscle and completely decompress the sciatic nerve. The authors believe this offers the best chance to ensure complete decompression and to decrease the likelihood of recurrent compression from scarring of the muscle on the nerve.
SURGICAL TECHNIQUE
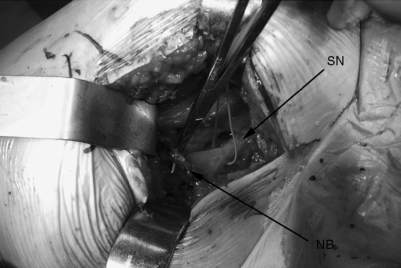
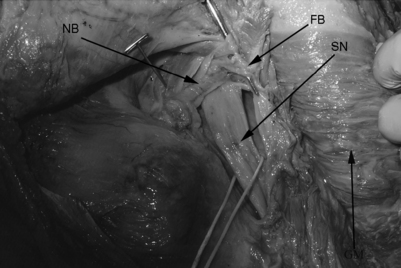
The patient is placed on a regular operating room table in the lateral decubitus position with the affected hip facing up. The authors limit their incision to the proximal third of the standard posterolateral incision used for total hip replacements. They believe in a minimally invasive approach and so use a microscope to completely resect the piriformis, in contrast to the endoscopic technique that has been described for releasing the piriformis muscle.38 The approach begins with a 4 cm skin incision that is followed by blunt splitting of the gluteus maximus muscle fibers under the microscope. This should be done slowly and meticulously to avoid injury to the sciatic nerve which can appear unprotected in more distal dissections. A self-retractor system is used to retract the gluteus maximus fibers and the deep adipose tissues are meticulously dissected to locate the piriformis muscle and its tendinous insertion on the greater trochanter. Internal rotation of the hip can facilitate identification of the piriformis tendon as it is placed under tension. The sciatic nerve should be identified and a Penrose drain placed around the nerve as a constant reminder of its presence (Fig. 121.12). The hip is internally rotated and the piriformis tendon is sectioned and used to retract the muscle off the sciatic nerve. The muscle belly is then sectioned to reveal the sciatic nerve more clearly as far back as the greater sciatic notch. The muscle is sectioned at its most proximal location as it emerges from the greater sciatic notch. The sciatic nerve is explored and decompressed to ensure that there are no residual fibrous bands, neurovascular bundles, or other constrictions compressing the nerve (Fig. 121.13). Patients are fully weight-bearing with crutches postoperatively, with physical therapy for abductor/adductor strengthening and gait training. Patients remain overnight in the hospital and may experience dramatic relief the first postoperative day. The authors’ experience with this method is promising as they await longer-term follow-up (Fig. 121.14).
1 Yeoman W. The relation of arthritis of the sacroiliac joint to sciatica. Lancet. 1928;2:1119-1122.
2 Freiberg AH, Vinkle TH. Sciatica and the sacro-iliac joint. J Bone Joint Surg [Am]. 1934;16:126-136.
3 Freiberg AH. Sciatica pain and its relief by operation on muscle and fascia. Arch Surg. 1937;34:337-350.
4 Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-214.
5 Thiele GH. Coccygodynia and pain in the superior gluteal region. JAMA. 1937;109:1271-1275.
6 Shordania JF. Die chronische Entzundung des Musculus pirifmoris – die Piriformitis – als eine der Ursachen von Kreuzschmerzen bei Frauen. Med Welt. 1936;X:999.
7 Beaton LE, Anson BJ. The sciatic nerve and the piriformis muscle: their interrelation a possible cause of coccygodynia. J Bone Joint Surg [Am]. 1938;20:686-688.
8 Robinson DR. Piriformis syndrome in relation to sciatic pain. Am J Surg. 1947;73:435-439.
9 Silver JK, Leadbetter WB. Piriformis syndrome: assessment of current practice and literature review. Orthopedics. 1998;21(10):1133-1135.
10 Rodrigue T, Hardy RW. Diagnosis and treatment of piriformis syndrome. Neurosurg Clin N Am. 2001;12(2):311-319.
11 Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318(5):291-300.
12 Bernard TNJr, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop. 1987;217:266-280.
13 Pace JB, Nagle D. Piriform syndrome. West J Med. 1976;124(6):435-439.
14 Durrani Z, Winnie AP. Piriformis muscle syndrome: an underdiagnosed cause of sciatica. J Pain Symptom Manage. 1991;6(6):374-379.
15 Brown JA, Braun MA, Namey TC. Piriformis syndrome in a 10-year-old boy as a complication of operation with the patient in the sitting position. Neurosurgery. 1988;23(1):117-119.
16 Jankiewicz JJ, Hennrikus WL, Houkom JA. The appearance of the piriformis muscle syndrome in computed tomography and magnetic resonance imaging. A case report and review of the literature. Clin Orthop. 1991;262:205-209.
17 Retzlaff EW, Berry AH, Haight AS, et al. The piriformis muscle syndrome. J Am Osteopath Assoc. 1974;73(10):799-807.
18 Parziale JR, Hudgins TH, Fishman LM. The piriformis syndrome. Am J Orthop. 1996;25(12):819-823.
19 Barton PM. Piriformis syndrome: a rational approach to management. Pain. 1991;47(3):345-352.
20 Benson ER, Schutzer SF. Posttraumatic piriformis syndrome: diagnosis and results of operative treatment. J Bone Joint Surg [Am]. 1999;81(7):941-949.
21 Rask MR. Superior gluteal nerve entrapment syndrome. Muscle Nerve. 1980;3(4):304-307.
22 Beatty RA. The piriformis muscle syndrome: a simple diagnostic maneuver. Neurosurgery. 1994;34(3):512-514. discussion 514
23 Steiner C, Staubs C, Ganon M, et al. Piriformis syndrome: pathogenesis, diagnosis, and treatment. J Am Osteopath Assoc. 1987;87(4):318-323.
24 Chen WS. Sciatica due to piriformis pyomyositis. Report of a case. J Bone Joint Surg [Am]. 1992;74(10):1546-1548.
25 Chen WS, Wan YL. Sciatica caused by piriformis muscle syndrome: report of two cases. J Formos Med Assoc. 1992;91(6):647-650.
26 Vandertop WP, Bosma NJ. The piriformis syndrome. A case report. J Bone Joint Surg [Am]. 1991;73(7):1095-1097.
27 Papadopoulos SM, McGillicuddy JE, Albers JW. Unusual cause of ‘piriformis muscle syndrome’. Arch Neurol. 1990;47(10):1144-1146.
28 Tesio L, Bassi L, Galardi G. Transient palsy of hip abductors after a fall on the buttocks. Arch Orthop Trauma Surg. 1990;109(3):164-165.
29 Rossi P, Cardinali P, Serrao M, et al. Magnetic resonance imaging findings in piriformis syndrome: a case report. Arch Phys Med Rehabil. 2001;82(4):519-521.
30 Fishman LM, Konnoth C, Rozner B. Botulinum neurotoxin type B and physical therapy in the treatment of piriformis syndrome: a dose-finding study. Am J Phys Med Rehabil. 2004;83(1):42-50. quiz 51–53
31 Fishman LM, Zybert PA. Electrophysiologic evidence of piriformis syndrome. Arch Phys Med Rehabil. 1992;73(4):359-364.
32 Fishman LM, Anderson C, Rosner B. BOTOX and physical therapy in the treatment of piriformis syndrome. Am J Phys Med Rehabil. 2002;81(12):936-942.
33 Nakamura H, Seki M, Konishi S, et al. Piriformis syndrome diagnosed by cauda equina action potentials: report of two cases. Spine. 2003;28(2):E37-E40.
34 Foster MR. Piriformis syndrome. Orthopedics. 2002;25(8):821-825.
35 Lam AW, Thompson JF, McCarthy WH. Unilateral piriformis syndrome in a patient with previous melanoma. Aust NZ J Surg. 1993;63(2):152-153.
36 Sayson SC, Ducey JP, Maybrey JB, et al. Sciatic entrapment neuropathy associated with an anomalous piriformis muscle. Pain. 1994;59(1):149-152.
37 Mizuguchi T. Division of the piriformis muscle for the treatment of sciatica. Postlaminectomy syndrome and osteoarthritis of the spine. Arch Surg. 1976;111(6):719-722.
38 Dezawa A, Kusano S, Miki H. Arthroscopic release of the piriformis muscle under local anesthesia for piriformis syndrome. Arthroscopy. 2003;19(5):554-557.