Chapter 4 Physiology of Male Gametogenesis
INTRODUCTION
The contribution of the male to the biology of reproduction is to produce a genetically intact spermatozoa that will fertilize an oocyte. The end product of male gametogenesis, the mature spermatozoa, is designed for one purpose: to deliver the male contribution of the genetic makeup to the embryo. The biology of gamete production is different in males compared to females. Gamete production in females is intimately part of the endocrine responsibility of the ovary. If there are no gametes, then hormone production is drastically curtailed. Depletion of oocytes implies depletion of the major hormones of the ovary. In the male this is not the case. Androgen production will proceed normally without a single spermatozoa in the testes.
ORGANIZATION OF THE TESTES
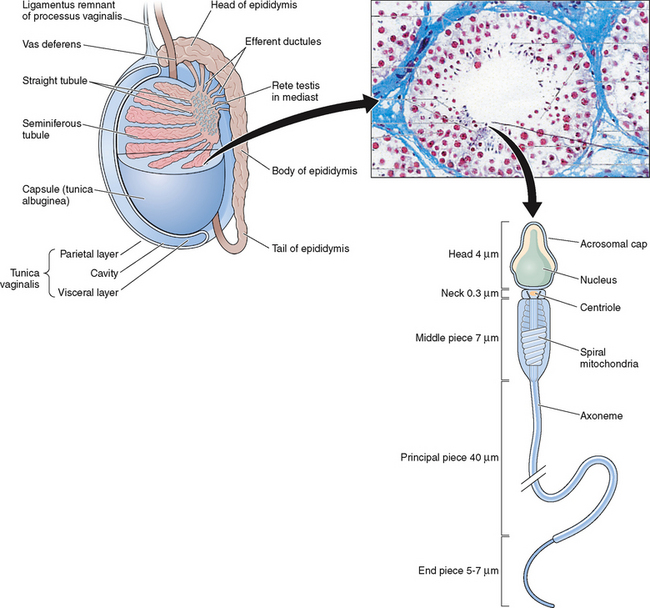
The testes are ellipsoid in shape, measuring 2.5 × 4 cm in diameter and engulfed by a capsule (tunica albuginea) of strong connective tissue.1 Along its posterior border, the testis is loosely connected to the epididymis, which gives rise to the vas deferens at its lower pole.2 The testis has two main functions: it produces hormones, in particular testosterone, and it produces the male gamete—the spermatozoa. The testis is incompletely divided into a series of lobules. Most of the volume of the testis is made up of seminiferous tubules, which are looped or blind-ended and packed in connective tissue within the confines of the fibrous septa (Fig. 4-1). The fibrous septae divide the parenchyma into about 370 conical lobules consisting of the seminiferous tubules and the intertubular tissue. The seminiferous tubules are separated by groups of Leydig cells, blood vessels, lymphatics, and nerves. The seminiferous tubules are the site of sperm production (see Fig. 4-1). The wall of each tubule is made up of myoid cells of limited contractility and also of fibrous tissue. Each seminiferous tubule is about 180 μm in diameter; the height of the germinal epithelium measures 80 μm, and the thickness of the peritubular tissue is about 8 μm.3 The germinal epithelium consists of cells at different stages of development located within the invaginations of Sertoli cells, namely spermatogonia, primary and secondary spermatocytes, and spermatids. Both ends of the seminiferous tubules open into the spaces of the rete testis4 (see Fig. 4-1). The fluid secreted by the seminiferous tubules is collected in the rete testis and delivered in the ductal system of the epididymis.
SUPPORTING CELLS
Leydig Cells
The Leydig cells are irregularly shaped cells with granular cytoplasm present individually or more often in groups within the connective tissue.5,6 Leydig cells are the prime source of the male sex hormone testosterone.7–9 The pituitary hormone luteinizing hormone (LH) acts on Leydig cells to stimulate the production of testosterone. This acts as a negative feedback on the pituitary to suppress or modulate further LH secretion.8 Compared with the testosterone levels in the blood, the intratesticular concentration of testosterone is many times higher, especially near the basement membrane of the seminiferous tubule.
The Sertoli Cell
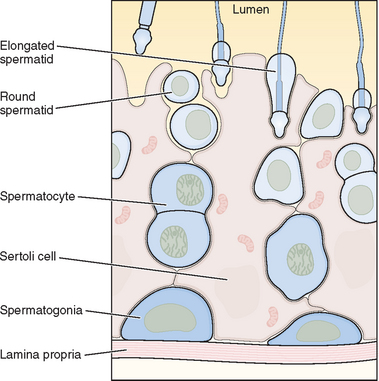
The seminiferous tubules are lined with highly specialized Sertoli cells that rest on the tubular basement membrane and extend into the lumen with a complex ramification of cytoplasm (Fig. 4-2). Spermatozoa are produced at puberty but are not recognized by the immune system that develops during the first year of life. The seminiferous tubule space is divided into basal (basement membrane) and luminal (lumen) compartments by strong intercellular junctional complexes called tight junctions. These anatomic arrangements, complemented by closely aligned myoid cells that surround the seminiferous tubule, form the basis for the blood–testis barrier. The blood–testis barrier provides a microenvironment for spermatogenesis to occur in an immunologically privileged site. Sertoli cells serve as “nurse” cells for spermatogenesis, nourishing germ cells as they develop. These also participate in germ cell phagocytosis. Multiple sites of communication exist between Sertoli cells and developing germ cells for the maintenance of spermatogenesis within an appropriate hormonal milieu. Follicle-stimulating hormone (FSH) binds to the high-affinity FSH receptors found on Sertoli cells, signaling the secretion of androgen-binding protein. High levels of androgens are also present within the seminiferous tubule.
The two most important hormones secreted by the Sertoli cells are antimüllerian hormone and inhibin. Antimüllerian hormone is a critical component of embryonic development and is involved in the regression of the müllerian ducts. Inhibin is a key macromolecule in pituitary FSH regulation. Some of the functions of the Sertoli cell are (1) maintenance of integrity of seminiferous epithelium, (2) compartmentalization of seminiferous epithelium, (3) secretion of fluid to form tubular lumen to transport sperm within the duct, (4) participation in spermiation, (5) phagocytosis and elimination of cytoplasm, (6) delivery of nutrients to germ cells, (7) steroidogenesis and steroid metabolism, (8) movement of cells within the epithelium, (9) secretion of inhibin and androgen-binding protein, (10) regulation of spermatogenic cycle, and (11) provision of a target for hormones LH, FSH, and testosterone receptors present on Sertoli cells.
SPERMATOGENESIS
The process of differentiation of a spermatogonium into a spermatid is known as spermatogenesis.4 It is a complex, temporal event whereby primitive, totipotent stem cells divide to either renew themselves or produce daughter cells that become specialized testicular spermatozoa over a span of weeks. Spermatogenesis involves both mitotic and meiotic proliferation as well as extensive cell remodeling. Spermatogenesis can be divided into three major phases: (1) proliferation and differentiation of spermatogonia, (2) meiosis, and (3) spermiogenesis, a complex metamorphosis that transforms round spermatids arising from the final division of meiosis into a complex structure called the spermatozoon. In humans, the process of spermatogenesis starts at puberty and continues throughout the entire lifespan of the individual. It takes place in the lumen of the seminiferous tubules. In fact, 90% of the testis volume is determined by the seminiferous tubules and their constituent germ cells at various stages of development. Once the gonocytes have differentiated into fetal spermatogonia, an active process of mitotic replication is initiated very early in embryonic development. This appears to be under FSH control and develops the baseline number of precursor cells of the testicle.
Proliferation and Differentiation of Spermatogonia
Within the seminiferous tubule, germ cells are arranged in a highly ordered sequence from the basement membrane to the lumen (see Fig. 4-2). Spermatogonia lie directly on the basement membrane, followed by primary spermatocytes, secondary spermatocytes, and spermatids as they progress toward the tubule lumen. The tight junction barrier supports spermatogonia and early spermatocytes within the basal compartment and all subsequent germ cells within the luminal compartment.
Types of Spermatogonia
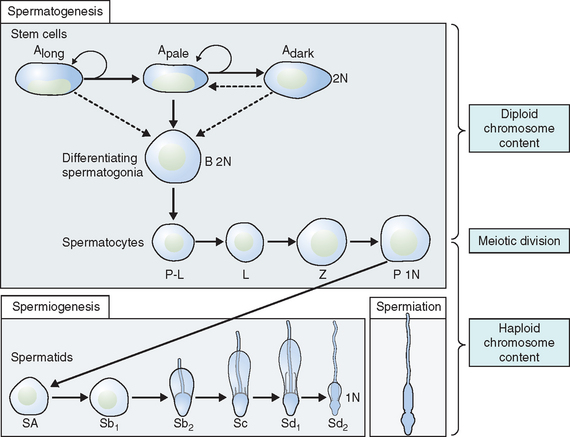
Spermatogonia represent a population of cells that divide by mitosis, providing both a renewing stem cell population as well as spermatogonia that are committed to enter the meiotic process. Germ cells are staged by their morphologic appearance; there are dark type A (Adark) and pale type A (Apale) and type B spermatogonia, primary spermatocytes (preloptotene, leptotene, zygotene, and pachytene), secondary spermatocytes, and spermatids (Sa, Sb, Sc, Sd1, and Sd2) (Fig. 4-3). Other proliferative spermatogonia include Apaired (Apr), resulting from dividing Aisolated (Ais), and subsequently dividing to form Aaligned (Aal). Differentiated spermatogonia include type A1, A2, A3, A4, intermediate, and type B, each a result of the cellular division of the previous type. In humans, four spermatogonial cell types have been identified; these are Along, Adark, Apale, and B.10–12 In the rat, type Ais is believed to be the stem cell13,14; however, it is not clear which human type A spermatogonia is the stem cell. Some investigators have proposed that the type Adark spermatogonia represent the reserve or nonproliferative spermatogonial population that gives rise to Apale.11,15,16 Spermatogonia do not separate completely after meiosis but remain joined by intercellular bridges, which persist throughout all stages of spermatogenesis and are thought to facilitate biochemical interactions, allowing synchrony of germ cell maturation.17
Type B spermatogonia possess considerably more chromatin within the inner nuclear envelope than intermediate or type A spermatogonia. Type B spermatogonia represent the cells that differentiate and enter into the process of meiosis during which they are called primary spermatocytes.11 They are the differential precursors to preleptotene spermatocytes. This last mitotic division helps maintain a pool of stem cells so the process can continue indefinitely.
Mitosis
Mitosis involves proliferation and maintenance of spermatogonia. It is a precise, well-orchestrated sequence of events involving duplication of the genetic material (chromosomes), breakdown of the nuclear envelope, and equal division of the chromosomes and cytoplasm into two daughter cells.18,19 DNA is also spatially organized into loop domains on which specific regulatory proteins interact during cellular replication.19–24 The mitotic phase involves spermatogonia (types A and B) and primary spermatocytes (spermatocytes I). Developing germ cells interconnected by intracellular bridges produce the primary spermatocyte through a series of mitotic divisions. Once the baseline number of spermatogonia is established after puberty, the mitotic component will proceed in order to continue to provide precursor cells and to start the process of differentiation and maturation.
Meiosis
Meiosis is a complex process with specific regulatory mechanisms of its own.25 The process commences when type B spermatogonia lose their contact with the basement membrane to form preleptotene primary spermatocytes. Thus, each primary spermatocyte can theoretically yield four spermatids, although fewer actually result, because some germ cells are lost due to the complexity of meiosis. The primary spermatocytes are the largest germ cells of the germinal epithelium. Meiosis is characterized by prophase, metaphase, anaphase, and telophase. In this, two successive cell divisions yield four haploid spermatids from one diploid primary spermatocyte. As a consequence, the daughter cells contain only half of the chromosome content of the parent cell. After the first meiotic division (reduction division), each daughter cell contains one partner of the homologous chromosome pair, and they are called secondary spermatocytes. These cells rapidly enter the second meiotic division (equational division), in which the chromatids then separate at the centromere to yield haploid early round spermatids. Meiosis assures genetic diversity and involves primary and secondary spermatocytes, which give rise to spermatids.
Spermiogenesis
Spermiogenesis is a process during which the morphologic changes occur during the differentiation of the spermatid into the spermatozoon. It begins once the process of meiosis is completed. Six different stages have been described in the process of spermatid maturation in humans: Sa-1 and Sa-2, Sb-1 and Sb-2, and Sc-1 and Sc-2 (see Fig. 4-3). Each of these stages can be identified by morphologic characteristics. During the Sa-1 stage, both the Golgi complex and mitochondria are well developed and differentiated, the acrosomal vesicle appears, the chromatid body develops in one pole of the cell opposite from the acrosomal vesicle, and the proximal centriole and the axial filament appears. During Sb-1 and Sb-2 stages, acrosome formation is completed, the intermediate piece is formed, and the tail develops. This process is completed during the Sc stages. During the postmeiotic phase, progressive condensation of the nucleus occurs with inactivation of the genome, the histones are converted into transitional proteins, and finally protamines are converted into well-developed disulfide bonds.
Spermiation
The process whereby a mature spermatid frees itself from the Sertoli cell and enters the lumen of the tubule as a spermatozoon is known as spermiation. The spermatids originating from the same spermatogonia remain connected by bridges to facilitate the transport of cytoplasmic products. Spermiation involves the active participation of the Sertoli cell. This may also involve actual cell movement as the spermatids advance toward the lumen of the seminiferous tubules.26 The mature spermatids close their intracellular bridges, disconnect their contact to the germinal epithelium, and become free cells called spermatozoa. Portions of the cytoplasm of the Sertoli cell known as the cytoplasmic droplet may remain as part of the spermatozoon during the process of spermiation. This is a morphologic feature present on immature sperm in semen.27
The Cycle or Wave of Seminiferous Epithelium
A cycle of spermatogenesis involves the division of primitive spermatogonial stem cells into subsequent germ cell types through the process of meiosis. Type A spermatogonial divisions occur at a shorter time interval than the entire process of spermatogenesis. Therefore, at any given time, several cycles of spermatogenesis coexist within the germinal epithelium. In humans, spermatocyte maturation takes 25.3 days, spermiogenesis 21.6 days, and the total estimated time for spermatogenesis is 74 days. Spermatogenesis is not random throughout the seminiferous epithelium. Germ cells are localized in spatial units referred to as stages and represent consistent associations of germ cell steps.28–31 In rodent spermatogenesis, one stage can be found in a cross-section of seminiferous tubule.
The cycle of spermatogenesis can be identified for each species, but the duration of the cycle varies.11 The stages of spermatogenesis are sequentially arranged along the length of the tubule. This arrangement of the stages of spermatogenesis is such that it results in a “wave of spermatogenesis” along the tubule. This wave is in space but the cycle is in time.31 Along the length of the seminiferous tubule there are only certain cross-sections where spermatozoa are released. In the rat, all stages are involved in spermatogenesis, but spermatozoa are released only in stage VIII.
Although it appears that the spatial organization is lacking or poor in the human seminiferous tubule, mathematic modeling indicates that these stages are tightly organized in an intricate spiral pattern.32 In addition to the steps being organized spatially within the seminiferous tubule, the stages are organized in time.31 Thus a position in the tubule that is occupied by cells comprising stage I will become stage II, followed by stage III, until the cycle repeats. In humans, the duration of the cycle is 16 days and the progression from spermatogonia to spermatozoa takes 70 days, or four and a half cycles of the seminiferous cycle. During spermatogenesis, cytoplasmic bridges link cohorts of germ cells that are at the same point in development, and these cells pass through the process together. Groups of such cells at different stages can be observed histologically on cross-section and many germ cell cohorts are seen only in association with certain other germ cells. This has led to the description of six stages of the seminiferous tubule epithelium in men. To add another level of complexity, the steps of the spermatogenic cycle within the space of the seminiferous tubules demonstrate a specific organization, termed spermatogenic waves. In humans, this wave appears to describe a spiral cellular arrangement as one progresses down the tubule. This spatial arrangement probably exists to ensure that sperm production is a continuous rather than a pulsatile process.
Efficiency of Spermatogenesis
Spermatogenetic efficiency varies between different species but appears to be relatively constant in man. The time for the differentiation of a spermatogonium into a mature spermatid is estimated to be 70 ± 4 days.33 In comparison to animals the spermatogenetic efficiency in man is poor. The daily rate of spermatozoa production is calculated at 3 to 4 million per gram of testicular tissue.34 A higher number of spermatozoa should be expected in the ejaculate than the 20 million/mL described by the World Health Organization.35 A majority of the cells developed (>75%) are lost as a result of apoptosis or degeneration; of the remaining, more than half are abnormal. Therefore, only about 12% of the spermatogenetic potential is available for reproduction.36 An age-related reduction in daily sperm production in men, which is associated with a loss of Sertoli cells, is also seen. This may result from an increase in germ cell degeneration during prophase of meiosis or from loss of primary spermatocytes. There is also a reduction in the number of Leydig cells, non-Leydig interstitial cells, myoid cells, and Sertoli cells.
Golgi Phase
The Golgi complex forms a caplike structure called the acrosome. The centrioles migrate from the cytoplasm to the base of the nucleus. The proximal centriole becomes the implantation apparatus to anchor the flagellum to the nucleus. The distal centriole becomes the axoneme.
Acrosomal Phase
The formation of the acrosome starts with the coalescence of a series of granules from the Golgi complex, which migrates to come into contact with the nuclear membrane, where it covers like a caplike structure over 30% to 50% of the nuclear surface.37 The acrosome covers the nucleus and contains the hydrolytic enzymes necessary for fertilization. The manchette is formed, and the spermatids are embedded in Sertoli cells.
SPERMATOZOA
Spermatozoa are highly specialized and condensed cells that do not grow or divide. A spermatozoon consists of the head, containing the paternal material (DNA), and the tail, which provides motility (see Fig. 4-1). The spermatozoon is endowed with a large nucleus but lacks the large cytoplasm characteristic of most body cells. Men are unique in the morphologic heterogeneity of the ejaculate.38–40
Head
The heads of stained spermatozoa are slightly smaller than heads of living spermatozoa in the original semen.41 The normal head is oval, measuring about 4.0 to 5.5 μm in length and 2.5 to 3.5 μm in width. The normal length-to-width ratio is about 1.50 to 1.70.41 Under bright field illumination, the most commonly observed aberrations include head shape/size defects, including large, small, tapering, piriform, amorphous, vacuolated (>20% of the head surface occupied by unstained vacuolar areas), and double heads, or any combination of these defects.42
Acrosome
The acrosome is represented by the Golgi complex and covers about two thirds of the anterior head area.39,40,42 The apical thickening seen in many other species is missing; however, the acrosome shows a uniform thickness/thinning toward the equatorial segment and covers about 40% to 70% of the sperm head. When observed under the scanning electron microscope, a furrow that completely encircles the head (i.e., acrosomal and postacrosomal regions) divides the sperm head unequally. An equatorial segment that is followed by the postacrosomal region is not very clearly visible on scanning electron microscope. The maximal thickness and width of the spermatozoon is seen in the postacrosomal region.
Under the electron microscope the sperm head is a flattened ovoid structure consisting primarily of the nucleus. The acrosome is a caplike structure covering the anterior two thirds of the sperm head, which arises from the Golgi complex of the spermatid as it differentiates into a spermatozoon. The acrosome contains several hydrolytic enzymes, including hyaluronidase and proacrosin, which are necessary for fertilization.38
During fertilization of the egg, the fusion of the outer acrosomal membrane with the plasma membrane at multiple sites releases the acrosomal enzymes at the time of the acrosome reaction. The anterior half of the head is devoid of plasma and an outer acrosomal membrane and is covered only by the inner acrosomal membrane.43 The posterior region of the sperm head is covered by a single membrane called the postnuclear cap. The overlap of the acrosome and the postnuclear cap results in an equatorial segment, which does not participate in the acrosome reaction. The nucleus, comprising 65% of the head, is composed of DNA conjugated with protein. The chromatin is tightly packed, and no distinct chromosomes are visible. The genetic information, including the sex-determining X or Y chromosome, is coded and stored in the DNA.38
Tail
The sperm tail arises at the spermatid stage. The centriole during spermatogenesis is differentiated into three parts: midpiece, the main or principal piece, and endpiece. The mitochondria reorganize around the midpiece. An axial core comprising of two central fibrils is surrounded by a concentric ring of nine double fibrils, which continue to the end of the tail. The additional outer ring is comprised of nine coarse fibrils. The principal piece is comprised of nine coarse outer fibrils diminishing in thickness and finally only the inner 11 fibrils of the axial core surrounded by a fibrous sheath. The mitochondrial sheath of the midpiece is relatively short but slightly longer than the combined length of the head and neck.38
Endpiece
The endpiece is not distinctly visible by light microscopy. Both tail sheath and coarse filaments are absent. The tail, containing all the motility apparatus, is 40 to50 μm long and arises from the spermatid centriole. It propels by waves that are generated in the neck region and pass distally along like a whiplash. Under bright field illumination, common neck and midpiece aberrations include their absence, bent tails, distended or irregular/ bent midpiece, abnormally thin midpiece (no mitochondrial sheath), or any of these combinations.42 Tail aberrations include short, multiple, hairpin, and broken tails; tails of irregular width; coiling tails with terminal droplets; or any of these combinations.42 Cytoplasmic droplets greater than one third the area of a normal sperm head are considered abnormal. They are usually located in the neck/midpiece region of the tail, although some immature spermatozoa may have a cytoplasmic droplet at other locations along the tail.40,42
Under scanning electron microscopy the tail can be subdivided intro three distinct parts (i.e., midpiece, principal piece, and endpiece. In the midpiece the mitochondrial spirals can be clearly visualized. This ends abruptly at the beginning of the midpiece. The midpiece narrows toward the posterior end. A longitudinal column along with transverse ribs is visible. The short endpiece has a small diameter due to the absence of the outer fibers.38 Under transmission electron microscopy, the midpiece possesses a cytoplasmic portion and a lipid-rich mitochondrial sheath that consists of several spiral mitochondria, surrounding the axial filament in a helical fashion. The midpiece provides the sperm with the energy necessary for motility. An additional outer ring of nine coarser fibrils surrounds the central core of 11 fibrils. Individual mitochondria are wrapped around these fibrils in a spiral manner to form the mitochondrial sheath, which contains the enzymes needed in the oxidative metabolism of the sperm. The mitochondrial sheath of the midpiece is relatively short, slightly longer than the combined length of the head and neck.38
The principal piece, the longest part of the tail, provides most of the propellant machinery. The coarse nine fibrils of the outer ring diminish in thickness and finally disappear, leaving only the inner fibrils in the axial core for most of the length of the principal piece.44 The fibrils of the principal piece are surrounded by a fibrous tail sheath, which consist of branching and anastomosing semicircular strands or ribs, held together by their attachment to two bands that run lengthwise along opposite sides of the tail.38 The tail terminates in the endpiece with a length of 4 to 10 μm and diameter of less than 1 μM. The small diameter is due to the absence of the outer fibrous sheath and a distal fading of the microtubules.
REGULATION OF SPERMATOGENESIS
The spermatogenic process is maintained by different intrinsic and extrinsic influences.
Intrinsic Regulation
Leydig cells secrete hormone (testosterone), neurotransmitters (neuroendocrine substances), and growth factors to neighboring Leydig cells, blood vessels, lamina propria of the seminiferous tubules, and Sertoli cells.5,36,45 They help maintain the nutrition of the Sertoli cells and the cells of the peritubular tissue and influence the contractility of myofibroblasts, thereby regulating the peristaltic movements of the seminiferous tubules and transportation of the spermatozoa. Leydig cells also help in the regulation of blood flow in the intertubular microvasculature.1 In addition, different growth factors are delivered from Sertoli cells and various germ cells participating in a complicated regulation of cell functions and developmental processes of germ cells. Altogether these factors represent an independent intratesticular regulation of spermatogenesis.
Extrinsic influences
The local regulation of spermatogenesis is controlled by the hypothalamus and hypophysis. Pulsatile secretion of gonadotropin-releasing hormone of the hypothalamus initiates the release of LH from the hypophysis; in response the Leydig cells produce testosterone. Testosterone not only influences spermatogenesis, but is also distributed throughout the body. It thus provides feedback to the hypophysis that regulates the secretory activity of Leydig cells. Stimulation of Sertoli cells by FSH is necessary for maturation of the germ cells. Complete qualitative spermatogenesis requires both FSH and LH. The interaction between the endocrine and paracrine mechanisms determines the functions within the testis.46–48 Inhibin secreted by Sertoli cells functions in the feedback mechanism directed to the hypophysis. These extratesticular influences are necessary for the regulation of intratesticular functions. Thus growth and differentiation of testicular germ cells involve a series of complex interactions between both somatic and germinal elements.47,49,50
IMMUNE STATUS OF THE TESTIS
The spermatozoa, late pachytene spermatocytes, and spermatids express unique antigens. These antigens are not formed until puberty; therefore, immune tolerance is not developed. The blood–testis barrier develops as these autoantigens develop. The testis is considered to be an immune-privileged site (i.e., transplanted foreign tissue can survive for a period of time without immunologic rejection). An immune surveillance is present in the testis and the epididymis, which shows an active immunoregulation to prevent autoimmune disease.51,52
DISTURBANCES OF SPERMATOGENESIS
Both proliferation and differentiation of the male germ cells and the intratesticular and extratesticular mechanisms regulating spermatogenesis can be disturbed. These disturbances may occur as a result of environmental influences or due to diseases that directly or indirectly affect spermatogenesis.53,54 Additionally, nutrition, therapeutic drugs, hormones and their metabolites, increased scrotal temperature, toxic substances, or x-radiation may reduce or destroy spermatogenesis. All these events can result in reduced spermatogenesis.
SPERM TRANSPORT IN THE EPIDIDYMIS
The epididymis lies along the dorsolateral border of each testis. It is made up of the efferent ductules, which emanate from the rete testis, and the epididymal ducts (see Fig. 4-1). The epididymis opens into the vas deferens, which then passes through the inguinal canal into the peritoneal cavity and opens into the urethra adjacent to the prostate. The primary function of the epididymis is post-testicular maturation and storage of spermatozoa during their passage from the testis to the vas deferens. The epididymal epithelium is androgen-dependent and has both absorptive and secretory functions.
The epididymis is divided into three functionally distinct regions: head, body, and tail or caput epididymis, corpus epididymis, and cauda epididymis. Their functions can be described simplistically as increasing the concentration, maturation, and storage of the spermatozoa. Much of the testicular fluid that transports spermatozoa from the seminiferous tubules is resorbed in the caput, increasing the concentration of the spermatozoa by 10-fold to 100-fold. The epididymal epithelium secretes the epididymal plasma in which the spermatozoa are suspended. As the newly developed spermatozoa pass through these regions of the epididymis, many changes occur, including alterations in net surface charge, membrane protein composition, immunoreactivity, phospholipid and fatty acid content, and adenylate cyclase activity. Many of these changes are thought to improve the structural integrity of the sperm membrane and also increase the fertilization ability of the spermatozoa. The capacities for protein secretion and storage within the epididymis are known to be extremely sensitive to temperature and reproductive hormone levels, including estrogens. It is a complex fluid whose composition changes along the length of the epididymis; and spermatozoa experience a series of sophisticated microenvironments that regulate their maturation.
Role of the Epididymis in Sperm Maturation
As spermatozoa traverse the epididymis, they are exposed to a continuously changing milieu of the luminal fluid derived from the rete testis and modified by the secretory and absorptive activity of the epididymal epithelium. In nonhuman mammals there is compelling evidence that the epididymal epithelium does provide essential factors for sperm maturation.55–58 In humans most of the information is obtained from treatment of pathologic cases rather than from normal fertile men. Sperm maturation occurs outside the testis. Spermatozoa within the testis have very poor or no motility and are incapable of fertilizing an egg. Both epididymal maturation and capacitation is necessary before fertilization. Capacitation, the final step required to acquire the ability to fertilize, may be an evolutionary consequence of the development of a storage system for inactive sperm in the caudal epididymis. Preservation of optimal sperm function during this period of storage requires adequate testosterone; levels in the circulation.
The epididymis is limited to a storage role because spermatozoa that have never passed through the epididymis and that have been obtained from the efferent ductules in men with congenital absence of vas deferens can fertilize the human oocyte in vitro and result in pregnancy with live birth (as well as with intracytoplasmic sperm injection with sperm obtained after testicular biopsy). A direct involvement of the epididymis comes from the results of epididymovasostomy bypass operations for epididymal obstruction. However, these results are not consistent, whereas significantly reduced fertility was reported in anastomosis of vas deferens to the proximal 10 mm of the duct compared to anastomosis of the more distal region of the tract59; others have reported pregnancies in anastomosis of the vas deferens to the efferent ductules60 or from spermatozoa retrieved from the efferent ductules and proximal caput regions of men with congenital absence of vas deferens and used for successful in vitro fertilization.60 More direct evidence of epididymal involvement comes from experiments in which immature epididymal sperm recovered from fertile men were incubated in the presence of human epithelial cell cultures.61 These spermatozoa showed improved motility and a significant increase in their capacity to attach to human zona in vitro.
SPERM ENTRY INTO CERVICAL MUCUS
At the moment of ejaculation, spermatozoa from the cauda epididymis are mixed with secretions of the various accessory glands in a specific sequence and deposited around the external cervical os and in the posterior fornix of the vagina. Spermatozoa in the first fraction of the ejaculate have significantly better motility and survival than those in the later fractions. The majority of the spermatozoa penetrate cervical mucus within 15 to 20 minutes of ejaculation.62,63 The ability to migrate across the semen–mucus interphase is highly dependent on the specific movement pattern of the spermatozoa.64 At the time of sperm penetration into the cervical mucus, further selection of the spermatozoa occurs based on the differential motility of normal versus abnormal spermatozoa. This is further modified once the vanguard spermatozoon is within the cervical mucus.65 The receptivity of the cervical mucus to penetration by the spermatozoa is cyclic, increasing over a period of about 4 days before ovulation and decreasing rapidly after ovulation. Maximum receptivity is seen the day before and on the day of the LH peak.66 Spermatozoa enter the uterine cavity from the internal cervical os by virtue of their own motility.67 From here the spermatozoa traverse to the site of fertilization in the ampulla of the fallopian tube or the oviduct.
CAPACITATION AND THE ACROSOME REACTION
Animal studies in rats and rabbits indicate that spermatozoa that are stored in the female tract are unable to penetrate the ova. They have to spend time in the female tract before they acquire this ability. Capacitation is a series of cellular or physiologic changes that spermatozoa must undergo to fertilize.68,69 It is characterized by the ability to undergo the acrosome reaction, to bind to the zona pellucida, and to acquire hypermotility. Capacitation may be an evolutionary consequence of the development of a storage system for inactive sperm in the caudal epididymis.
Capacitation per se does not involve any morphologic changes even at the ultrastructural level. It represents a change in the molecular organization of the intact sperm plasmalemma that then confers on spermatozoa the ability to undergo the acrosome reaction in response to induction of the stimulus. Capacitation involves the removal of seminal plasma factors that coat the surface of the sperm; modification of the surface charge; modification of the sperm membrane and of the sterols, lipids, and glycoproteins and the outer acrosomal membrane lying immediately under it. It also involves an increase in intracellular free calcium.70,71 Changes in sperm metabolism, increase in 3′, 5′-cyclic monophosphate, and activation of acrosomal enzymes are believed to be components of capacitation. Sperm capacitation may be initiated in vivo during migration through cervical mucus.72 This is finally followed by the acquisition of hypermotility, displayed by an increase in velocity and flagellar beat amplitude. This may be necessary for avoiding attachment to the tubal epithelium and penetrating the cumulus and zona pellucida.
The acrosome reaction confers the ability to penetrate the zona pellucida and also confers the fusogenic state in the plasmalemma overlying the nonreactive equatorial segment, which is needed for interaction with the oolemma. There are distinct fusion points between the outer acrosomal membrane and the plasma membrane. The fusion begins posteriorly around the anterior border of the equatorial segment, which is always excluded from the reaction. The changes termed as acrosome reaction prepare the sperm to fuse with the egg membrane. The removal of cholesterol from the surface membrane prepares the sperm membrane for the acrosome reaction.73,74 In addition D-mannose-binding lectins are also involved in the binding of human sperm to the zona pellucida.75,76
1 Middendorff R, Muller D, Mewe M, et al. The tunica albuginea of the human testis is characterized by complex contraction and relaxation activities regulated by cyclic GMP. J Clin Endocrinol Metab. 2002;87:3486-3499.
2 de Kretser DM, Temple-Smith PD, Kerr JB. Anatomical and functional aspects of the male reproductive organs. In: Bandhauer K, Fricks J, editors. Handbook of Urology. Berlin: Springer-Verlag; 1982:1-131.
3 Davidoff MS, Breucker H, Holstein AF, Seidel K. Cellular architecture of the lamina propria of human tubules. Cell Tissue Res. 1990;262:253-261.
4 Roosen-Runge EC, Holstein A. The human rete testis. Cell Tissue Res. 1978;189:409-433.
5 de Kretser DM, Kerr JB. The cytology of the testis. In: Knobill E, Neil JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994:1177-1290.
6 Christensen AK. Leydig cells. In: Hamilton DW, Greep RO, editors. Handbook of Physiology. Baltimore: Williams and Wilkins; 1975:57-94.
7 Payne AH, Downing JR, Wong K-L. Lutenizing hormone receptors and testosterone synthesis in two distinct populations of Leydig cells. Endocrinol. 1980;106:1424-1429.
8 Ewing LL, Keeney DS. Leydig cells: Structure and function. In: Desjardins C, Ewin LL, editors. Cell and Molecular Biology of the Testis. New York: Oxford University Press; 1993:137-165.
9 Glover TD, Barratt CLR, JJP Tyler (eds): Human Male Fertility. London and San Diego, Academic Press, p 247.
10 Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat. 1963;112:35-51.
11 Clermont Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198-236.
12 Schulze C. Morphological characteristics of the spermatogonial stem cells in man. Cell Tissue Res. 1974;198:191-199.
13 Clermont Y, Bustos-Obregon E. Re-examination of spermatogonial renewal in the rat by means of seminiferous tubules mounted “in toto.”. Am J Anat. 1968;122:237-247.
14 Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec. 1971;169:533-557.
15 Clermont Y. Two classes of spermatogonial stem cells in the monkey (Cercopithecus aethiops). Am J Anat. 1969;126:57-71.
16 van Alphen MM, van de Kant HJ, de Rooij DG. Depletion of the spermatogonia from the seminiferous epithelium of the rhesus monkey after X irradiation. Radiat Res. 1988;113:473-486.
17 Dym M, Fawcett DW. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4:195-215.
18 Berezney R, Coffey DS. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977;73:616-637.
19 Paulson JR, Laemmli UK. The structure of histone-depleted metaphase chromosomes. Cell. 1977;12:817-828.
20 Mirkovitch J, Mirault ME, Laemmli UK. Organization of the higher-order chromatin loop: Specific DNA attachment sites on nuclear scaffold. Cell. 1984;39:223-232.
21 Gasse S. Studies on scaffold attachment sites and their relation to genome function. Int Rev Cytol. 1989;119:57.
22 Izaurralde E, Kas E, Laemmli UK. Highly preferential nucleation of histone H1 assembly on scaffold-associated regions. J Mol Biol. 1989;210:573-585.
23 Adachi Y, Kas E, Laemmli UK. Preferential cooperative binding of DNA topoisomerase II to scaffold-associated regions. Embo J. 1989;13:3997.
24 Dickinson LA, Joh T, Kohwi Y, Kohwi-Shigematsu T. A tissue-specific MAR/SAR DNA-binding protein with unusual binding site recognition. Cell. 1992;70:631-645.
25 Giroux CN. Meiosis: Components and process in nuclear differentiation. Dev Genet. 1992;13:387-391.
26 Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater, Fla.: Cache Press, 1993.
27 Breucker H, Schafer E, Holstein AF. Morphogenesis and fate of the residual body in human spermiogenesis. Cell Tissue Res. 1985;240:303-309.
28 Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat. 1952;90:167-215.
29 Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952;55:548-573.
30 Clermont Y, Perey B. The stages of the cycle of the seminiferous epithelium of the rat: Practical definitions in PA-Schiff-hematoxylin and hematoxylin-eosin stained sections. Rev Can Biol. 1957;16:451-462.
31 Perey B, Clermont Y, LeBlonde CP. The wave of seminiferous epithelium in the rat. Am J Anat. 1961;108:47-77.
32 Schulze W, Rehder U. Organization and morphogenesis of the human seminiferous epithelium. Cell Tissue Res. 1984;237:395-407.
33 Heller CH, Clermont Y. Kinetics of the germinal epithelium in man. Recent Prog Horm Res. 1964;20:545-575.
34 Sculze W, Salzbrunn A. Spatial and quantitative aspects of spermatogenetic tissue in primates. In: Neischlag E, Habenicht U, editors. Spermatogenesis-Fertilization-Contraception. Berlin, Heidelberg, New York: Springer; 1992:267-283.
35 Rowe PJ, Comhaire F, Hargreave TB, Mellows HJ, editors. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple. Cambridge: Cambridge University Press, 1993.
36 Sharpe RM. Regulation of spermatogenesis. In: Knobill E, Neil JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994:1363-1434.
37 De Kretser DM. Ultrastructural features of human spermiogenesis. Z Zellforsch Mikrosk Anat. 1969;98:477-505.
38 Hafez ESE, editor. Human Semen and Fertility. St. Louis: Mosby, 1976.
39 Kruger TF, Menkveld R, Stander FS, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118-1123.
40 Menkveld R, Stander FS, Kotze TJ, et al. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586-592.
41 Katz DF, Overstreet JW, Samuels SJ, et al. Morphometric analysis of spermatozoa in the assessment of human male fertility. J Androl. 1986;7:203-210.
42 World Health Organization. Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction, 4th ed. New York: Cambridge University Press, 1999.
43 Barros C, Franklin B. Behaviour of the gamete membranes during sperm entry into the mammalian egg. J Cell Biol. 1968;37:13.
44 White IG. Mammalian sperm. In Hafez ESE, editor: Reproduction of Farm Animals, 3rd ed., Philadelphia: Lea & Febiger, 1974.
45 Jegou B. The Sertoli cell. Baillieres Clin Endocrinol Metab. 1992;6:273-311.
46 de Kretser DM, Sun Y, Drummond AE, et al, (eds): Serono Symposia. USA, pp 19–30.
47 Bellve AR, Zheng W. Growth factors as autocrine and paracrine modulators of male gonadal functions. J Reprod Fertil. 1989;85:771-793.
48 Skinner MK. Cell–cell interactions in the testis. Endocr Rev. 1991;12:45-77.
49 Sharpe T. Intratesticular control of steroidogenesis. Clin Endocrinol. 1990;33:787-807.
50 Sharpe RM. Monitoring of spermatogenesis in man—measurement of Sertoli cell- or germ cell-secreted proteins in semen or blood. Int J Androl. 1992;15:201-210.
51 Mahi-Brown CA, Yule TD, Tung KS. Evidence for active immunological regulation in prevention of testicular autoimmune disease independent of the blood–testis barrier. Am J Reprod Immunol Microbiol. 1988;16:165-170.
52 Barratt CL, Bolton AE, Cooke ID. Functional significance of white blood cells in the male and female reproductive tract. Hum Reprod. 1990;5:639-648.
53 Holstein AF, Schulze W, Breucker H. Histopathology of human testicular and epididymal tissue. In: Hargreave TB, editor. Male Infertility. London, Berlin, Heidelberg, New York: Springer; 1994:105-148.
54 Nieschlag E, Behre H, editors. Andrology: Male Reproductive Health and Dysfunction. Berlin, Heidelberg, New York: Springer, 2001.
55 Bedford JM. Effect of duct ligation on the fertilizing capacity of spermatozoa in the epididymis. J Exp Zool. 1967;166:271-281.
56 Orgebin-Crist M. Maturation of spermatozoa in the rabbit epididymis: Fertilizing ability and embryonic mortality in does inseminated with epididymal spermatozoa. Ann Biol Anim Biochem Biophy. 1967;7:373-379.
57 Moore HD, Hartman TD. In vitro development of the fertilizing ability of hamster epididymal spermatozoa after co-culture with epithelium from the proximal cauda epididymis. J Reprod Fertil. 1986;78:347-352.
58 Temple-Smith PD, Southwich GJ, Herrera Casteneda E, et al. Surgical manipulation of the epididymis: An experimental approach to sperm maturation. In: Serio M, editor. Perspectives in Andrology. New York: Raven Press; 1989:281.
59 Schoysman RJ, Bedford JM. The role of the human epididymis in sperm maturation and sperm storage as reflected in the consequences of epididymovasostomy. Fertil Steril. 1986;46:293-299.
60 Silber SJ, Balmaceda J, Borrero C, et al. Pregnancy with sperm aspiration from the proximal head of the epididymis: A new treatment for congenital absence of the vas deferens. Fertil Steril. 1988;50:525-528.
61 Moore HD, Curry MR, Penfold LM, Pryor JP. The culture of human epididymal epithelium and in vitro maturation of epididymal spermatozoa. Fertil Steril. 1992;58:776-783.
62 Tredway DR, Settlage DS, Nakamura RM, et al. Significance of timing for the postcoital evaluation of cervical mucus. Am J Obstet Gynecol. 1975;121:387-393.
63 Tredway DR, Buchanan GC, Drake TS. Comparison of the fractional postcoital test and semen analysis. Am J Obstet Gynecol. 1978;130:647-652.
64 Mortimer D. Objective analysis of sperm motility and kinematics. In: Keel BA, Webster BW, editors. Handbook of Laboratory Diagnosis and Treatment of Infertility. Boca Raton: CRC Press; 1990:97-133.
65 Katz DF, Drobnis E, Overstreet JW. Factors regulating mammalian sperm migration through the female reproductive tract and oocyte vestments. Gamet Res. 1989;22:443-469.
66 Mortimer D. Sperm transport in the human female reproductive tract. In: Finn CA, editor. Oxford Reviews of Reproductive Biology. Oxford: Oxford University Press; 1983:30-61.
67 Settlage DSF, Motoshima M, Tredway DR. Sperm transport from the external cervical os to the fallopian tubes in women: A time and quantitation study. In: Hafez ESE, Thibault CG, editors. Sperm Transport, Survival and Fertilizing Ability in Vertebrates. Paris: INSERM; 1974:201-217.
68 Eddy EM, O’Brien DA. The spermatozoon. In: Knobill EO, O’Neill JD, editors. The Physiology of Reproduction. 2nd ed. New York: Raven Press; 1988:135-185.
69 Yanagamachi R. Mammalian fertilization. In: Knobill E, O’Brien NJ, editors. The Physiology of Reproduction. New York: Raven Press, 1994.
70 Thomas P, Meizel S. Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochem J. 1989;264:539-546.
71 Mahanes MS, Ochs DL, Eng LA. Cell calcium of ejaculated rabbit spermatozoa before and following in vitro capacitation. Biochem Biophys Res Commun. 1986;134:664-670.
72 Overstreet JW, Katz DF, Yudin AI. Cervical mucus and sperm transport in reproduction. Semin Perinatol. 1991;15:149-155.
73 Parks JE, Ehrenwalt E. Cholesterol efflux from mammalian sperm and its potential role in capacitation. In: Bavister BD, Cummins J, Raldon E, editors. Fertilization in Mammals. Norwell, Mass.: Serono Symposia, 1990.
74 Ravnik SE, Zarutskie PW, Muller CH. Purification and characterization of a human follicular fluid lipid transfer protein that stimulates human sperm capacitation. Biol Reprod. 1992;47:1126-1133.
75 Benoff S, Cooper GW, Hurley I, et al. Antisperm antibody binding to human sperm inhibits capacitation induced changes in the levels of plasma membrane sterols. Am J Reprod Immunol. 1993;30:113-130.
76 Benoff S, Hurley I, Cooper GW, et al. Fertilization potential in vitro is correlated with head-specific mannose-ligand receptor expression, acrosome status and membrane cholesterol content. Hum Reprod. 1993;8:2155-2166.