Physiology for ED practice
Oxygen and carbon dioxide homeostasis
Variations at altitude and depth
Blood pressure homeostasis – a more complex mechanism
Haemostasis – an example of positive feedback with a cut-off mechanism
Shock – where homeostasis fails and uncontrolled positive feedback ensues
Homeostasis
A single-celled organism, such as an amoeba, requires warmth, oxygen, nutrients and fluids in order to survive and must be able to rid itself of waste products. It interacts directly with the outside world in order to achieve this (Fig. 23.1). The human body is a highly complex collection of millions of cells, very few of which are in direct contact with the outside world, and yet each individual cell has the same survival requirements as the amoeba – a constant supply of fluids, nutrients, oxygen and warmth in order to live and the ability to remove waste products. The external environment (the ‘outside world’) of the cells in the body is the interstitial fluid that surrounds them (see Fig. 23.2 for body fluid compartments) and this fluid must be kept supplied with all the components that the cells might need. Individual cells need to maintain a constant environment within relatively narrow limits in order to function optimally and this constant state must be maintained whatever is happening to the body as a whole. The term ‘homeostasis’, first used by an American physiologist, Walter Cannon, in 1932, refers to the physiological mechanisms that maintain the body in a relatively constant state despite changes in the environment. The word comes from the Greek and means ‘standing the same’, something of a misnomer since physiological function is never static but constantly fluctuating. Homeostasis is essential if the metabolic activities that occur constantly in all cells are to continue.
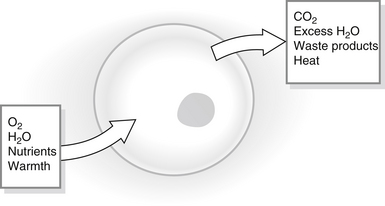
Figure 23.1 Cell homeostasis.
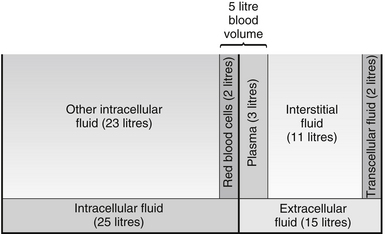
Figure 23.2 Body fluid compartments.
Throughout the body there are many self-regulating homeostatic mechanisms that aim to maintain an internal ‘steady state’. Most homeostatic mechanisms within the body work by ‘negative feedback’, where a deviation from normal will cause a response to restore the steady state – thus too little of something will cause more to be produced, too much of something will trigger mechanisms to reduce the amount. Once steady state is reached, the homeostatic mechanisms are switched off. An example of a see-saw is commonly used to illustrate this concept (Fig. 23.3). In order to function, homeostatic mechanisms require specialized receptors to detect deviations from the ‘steady state’; they also require a control centre to receive and process the information and the ability to stimulate appropriate body organs and structures to redress the imbalance.
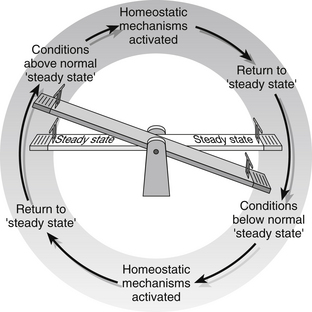
Figure 23.3 Homeostasis: maintaining a steady state.
Temperature control
Maintenance of a constant core body temperature, within the internal organs, is essential for optimal functioning of cellular enzymes. Humans are homeothermic and normally maintain a constant core temperature of 37 C regardless of the external temperature. The skin temperature may be several degrees different from the core temperature and varies between areas of the body, as those who always seem to have cold feet and hands will know. Body temperature is usually lower, by about 0.5°C at night and is 0.5–1°C higher in women during the second half of the menstrual cycle as a result of normal circadian rhythms. Children have higher core temperatures than neonates and the elderly, and core temperature can rise by up to 2°C during strenuous exercise. Despite all these normal variations, the body must maintain a careful balance between heat gained and heat lost. A summary of factors influencing heat gain and loss is given in Box 23.1.
Temperature homeostasis
• peripheral vasoconstriction mediated via the sympathetic nervous system closes down the surface blood vessels, ensuring that blood is kept closer to the warm core and heat loss through the skin is minimized
• shivering is initiated by the posterior hypothalamus and results in uncoordinated muscle activity that generates heat
• the thyroid gland is stimulated to produce the hormone thyroxin, which raises the basal metabolic rate of cells, thus increasing heat production
• information is relayed to the cerebral cortex and we become conscious of the cold and will take steps to warm ourselves such as putting on extra clothes, turning on the fire, exercising or having a warm drink.
A rise in core temperature above 37°C will stimulate responses aimed at losing heat:
• peripheral blood vessels are dilated under the influence of the sympathetic nervous system and heat is lost through the skin by radiation, conduction and convection
• sweat glands are stimulated, again via the sympathetic nervous system, to increase secretion, and heat is lost by evaporation. Evaporation of sweat is reduced when humidity is high and this is consequently a less effective means of reducing temperature in hot climates
• again the cerebral cortex receives information and we take steps to cool down – removing clothes, taking a cold shower, drinking iced drinks.
Once temperature returns to normal levels, the physiological mechanisms are switched off. A diagrammatic representation of thermoregulatory mechanisms is given in Fig. 23.4.
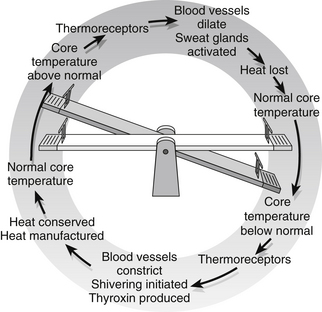
Figure 23.4 Thermoregulation.
Fluid and electrolyte balance
Fluid is either inside the cells (intracellular) or outside the cells (extracellular). Extracellular fluid includes blood plasma, interstitial or tissue fluid that bathes the cells (see above), and small amounts of transcellular fluid, found in body cavities such as intraocular, peritoneal and pleural fluid, cerebrospinal fluid and digestive juices. Figure 23.2 shows how these fluid compartments compare.
Intracellular fluid contains more positively charged potassium and magnesium and negatively charged protein and phosphate than extracellular fluid (which contains more positively charged sodium ions and negatively charged chloride ions) (Fig. 23.5). The ions are prevented from diffusing into other compartments by the selective permeability of the cell membranes and by the presence of a pumping mechanism within cell walls which actively pumps out sodium and exchanges it for potassium. This difference between intra- and extracellular fluids is essential in nerve and muscle cells (excitable tissues), since nerves would be unable to relay messages and muscles unable to contract without it.
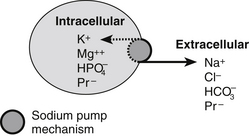
Figure 23.5 Electrolytes in fluid compartments.
Fluid and sodium balance are closely linked and hormonal responses are triggered by both changes in extracellular fluid volumes and changes in plasma osmolality. A diagrammatic representation is given in Fig. 23.6.
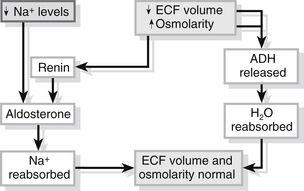
Figure 23.6 Water and sodium balance.
Calcium levels in the body are regulated by the secretion of parathyroid hormone from the four parathyroid glands. The hormone is released directly in response to low extracellular fluid concentrations of calcium and stimulates the release of calcium from bone and its reabsorption from the kidney tubules. In addition, vitamin D is activated and increases the amount of calcium absorbed in the gut from food. When calcium is reabsorbed, phosphate is lost. High calcium levels stimulate the release of calcitonin from the thyroid gland. Calcitonin inhibits the release of calcium from bone and increases its excretion through the kidney until levels return to normal and the mechanism is switched off (Fig. 23.7).
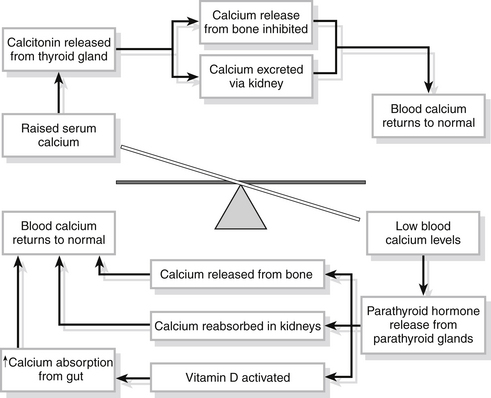
Figure 23.7 Calcium homeostasis.
Oxygen and carbon dioxide homeostasis
All cells require oxygen in order to function, and produce carbon dioxide as a result of metabolic activity. These gases are carried to and from the cells in the blood and their values can be measured as partial pressures (PO2 and PCO2). Variations in arterial PO2 and PCO2 are sensed by chemoreceptors. Peripheral chemoreceptors in the aortic arch and at the bifurcation of the common carotid artery are particularly sensitive to falls in arterial oxygen levels (PaO2), and rises in arterial carbon dioxide (PaCO2). Once altered levels are sensed, the respiratory centre in the medulla of the brain is stimulated, via the vagal and glossopharyngeal nerves, and stimulates the phrenic and intercostal nerves to the diaphragm and intercostal muscles. The result is that the rate and depth of respiration are increased and more oxygen is inhaled and delivered to the blood. Once arterial blood oxygen levels are restored to normal, the mechanism is switched off.
These homeostatic mechanisms are diagrammatically represented in Fig. 23.8.
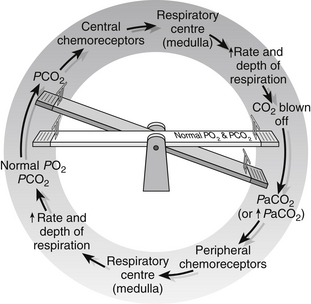
Figure 23.8 Respiratory homeostasis.
Oxygen transport
or
The PN2 and PCO2 can be similarly calculated.
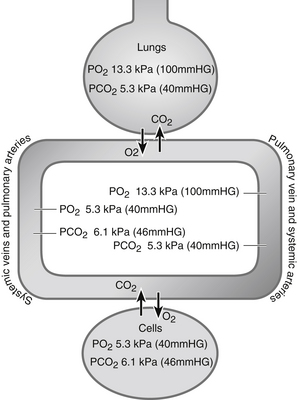
In the alveoli, gaseous exchange is possible because of the very thin pulmonary membrane between the alveoli and capillaries and the vast network of capillaries surrounding them. The existence of a pressure gradient, i.e., different pressures on either side of the membrane, results in movement of oxygen into the blood and carbon dioxide out of the blood and into the alveoli ready to be expired. Blood leaving the lungs for the heart contains oxygen and carbon dioxide at virtually the same partial pressures as those contained within the alveoli, so that the normal pulmonary vein and systemic arterial partial pressure of oxygen (PaO2) is 13.3 kPa (100 mmHg) and PaCO2 is 5.3 kPa (40 mmHg) (Figs 23.9 and 23.10); these quantities of gas are carried within the blood to the tissues.
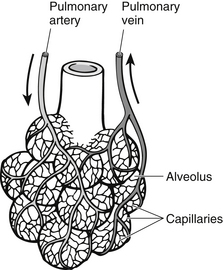
Figure 23.9 Capillary network surrounding the alveolus.
At the tissues, gases diffuse in the opposite direction across pressure gradients and thin membranes. Oxygen is given up to the tissues and replaced with carbon dioxide produced by the tissues. Partial pressures of oxygen and carbon dioxide within the cells are the same as those in blood arriving at the lungs (Fig. 23.10), since the gases cannot cross the thicker membranes of blood vessels in the rest of the circulation.
Haemoglobin is a conjugated protein found in red blood cells and consists of four haem groups containing iron and four polypeptide chains. Each of these haem groups can combine with one molecule of oxygen to form oxyhaemoglobin, which is bright red and gives arterial blood its distinctive colour. This process is known as oxygenation. Normal Hb is approximately 15 g/dL and each gram of Hb can carry 1.34 mL O2, so that the total oxygen capacity of the blood, i.e., the total amount that could be carried, is 15 × 1.34 = 20.1 mL/dL. This equation is simpler if SI units are used – normal Hb is 2.2 mmol/L blood and each molecule of Hb can combine with four molecules of O2, so the oxygen capacity is 8.8 mmol/L (1 mmol O2 = 23.4 mL). Amounts of oxygen carried bound to Hb can thus be far in excess of the normal requirements of the body. A simple sum will allow us to see that the body, which needs 250 mL of O2 each minute at rest, actually has theoretically available 8.8 × 23.4 (= 197.12 mL per litre) × 5 L pumped out of the heart each minute, i.e., 986 mL per minute.
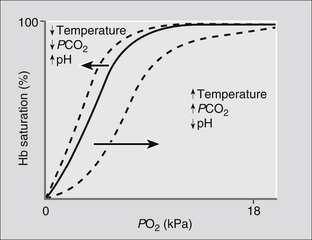
Oxygen does not bind to each haem molecule with the same ease, and a graph plotting Hb saturation against PO2 is not linear. The rate at which they bind is dependent on the PO2. The first haem group combines with O2 with relative difficulty, the second and third groups combine more readily and the fourth combines with the greatest difficulty of all. It will be seen from Fig. 23.10 that at a PO2 of 5.3 kPa, (40 mmHg) as in blood arriving at the lungs, almost 70 % of the Hb sites are bound with oxygen and exposure to a PO2 of 13.3 kPa (100 mmHg) at the alveoli will allow up to 98 % of the Hb to become saturated with O2. At the tissues, O2 is unloaded from the haem molecules in response to the fall in PO2, so that a tissue PO2 of 5.3 kPa (40 mmHg) will mean that oxygen from the 70–97% range can be removed for use.
The ‘s’ shape of the oxygen–haemoglobin dissociation curve is important physiologically for a number of reasons. Normal physiological function occurs over only a small part of this curve (Fig. 23.11) and a large reserve is available in the event of a fall in arterial PO2, such as in lung disease, during exercise or at altitude. Even at a PO2 of only 8 kPa (60 mmHg), 90 % saturation of Hb with oxygen will be achieved in blood leaving the lungs (point I in Fig. 23.11). During strenuous exercise, it is possible to achieve a PO2 at the tissues of as little as 2 kPa (15 mmHg) and this will allow 80 % of the bound oxygen to be released (point II in Fig. 23.11), thus supplying the increased amount of oxygen required by the tissues.
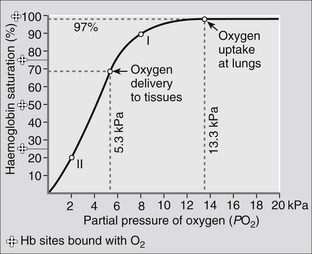
Figure 23.11 The oxygen dissociation curve.
Several factors affect the ease with which O2 binds with Hb and will influence the position of the oxygen–haemoglobin dissociation curve. The factors influencing ‘shifts’ in the curve are summarized in Fig. 23.12. The result of a shift to the left is that loading of Hb with O2 occurs more readily, i.e., at a lower PO2, while a shift to the right facilitates release of the O2 at the tissues.
Carbon dioxide transport
Carbon dioxide is transported around the body in three ways:
CO2 carried in this way does not exert a pressure within the blood and the equation reverses readily when blood arrives at the lungs so that CO2 is readily released to be blown off. Fig. 23.8 shows this process diagrammatically.
Oxygen therapy
The aim of oxygen therapy is to raise the PO2 in the lungs, thus increasing the pressure gradient across the alveolar capillary membrane and allowing more oxygen to enter the blood for transport to the tissues. There are, however, potential hazards that should be considered when oxygen therapy is indicated.
Blood glucose homeostasis
Cells need a constant supply of nutrients from which to extract energy for cell work and glucose plays an important role in this as it is a major substrate for the manufacture of adenosine triphosphate (ATP) within the cells. This is particularly true in the brain, where 90 % of the cellular energy required for metabolism is derived from glucose. Glucose is obtained from food and food substrates and the body has efficient glucose storage mechanisms for use in times of plentiful supply (for example, following a meal) and equally efficient means of releasing the stores during fasting states. Two hormones – insulin and glucagon – are responsible for maintaining blood glucose within relatively narrow limits so that cells throughout the body receive a constant and adequate supply (Fig. 23.13).
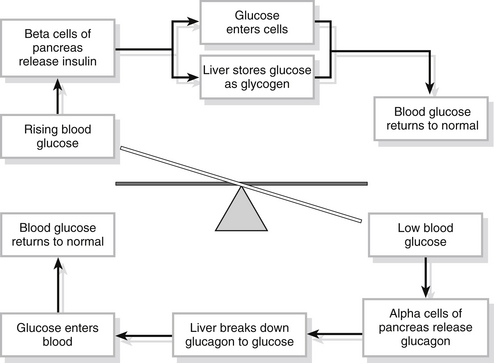
Figure 23.13 Glucose homeostasis.
• encourages the entry of glucose into cells throughout the body, especially the cells of skeletal muscle, to be used to manufacture ATP
• turns glucose into the storage form glycogen (this process is known as glycogenesis) for storage in cells and in the liver
• slows down the processes which turn fats and proteins into glucose.
If blood sugar is low, the alpha cells of the islets of Langerhans are stimulated and secrete the hormone glucagon. This leads to a number of physiological alterations aimed at raising the blood glucose levels:
• acts on liver cells to convert stored glycogen back to glucose (glycogenolysis) and release it back into the blood
• encourages the liver to manufacture glucose from lactic acid and from some amino acids (gluconeogenesis) and release it into the blood
• when blood levels have returned to normal, the mechanism is switched off.
Haemostasis – an example of positive feedback with a cut-off mechanism
1. Myogenic reflex – damaged vessels will normally dilate immediately after injury under the influence of histamine released by mast cells in response to the trauma. Within seconds the vessels constrict and the cut ends retract as platelets within the vessels begin to clump together and release powerful vasoconstrictors, serotonin (also called 5-hydroxytryptamine or 5HT) and thromboxane A. This so-called ‘myogenic reflex’ occurs even in large vessels and lasts for approximately 20 minutes, enough time for stages two and three to commence.
2. Platelet plug formation – when blood vessels are cut, filaments of collagen and elastin are exposed and attract passing platelets which adhere to them. This adherence causes the release of adenosine diphosphate (ADP) from the platelets, red blood cells and vessel walls. ADP triggers a change in the shape of the platelets which encourages them to clump together. Other substances, including serotonin, also encourage platelet clumping until a plug of platelets is formed which is large enough to close the wounded vessel. A platelet plug is formed within a few seconds of injury and is sufficiently strong to stop bleeding in smaller vessels. The plug must then be stabilized by fibrin fibres or it will break down after about 20 minutes and bleeding will start again.
3. Fibrin clot – fibrin is an insoluble protein that is laid down as a mesh of fine threads which adhere to one another and to blood cells and platelets. They become entangled in the platelet plug, attract more cells to plug the damaged area and gradually make the clot firmer and more stable. Fibrin is formed by a complex process initiated when tissues are damaged. The complexity of the process is important since clotting within undamaged vessels would be highly undesirable. The early stages of fibrin formation also trigger the complicated clotting cascade involving 13 different factors, mostly blood constituents, which ultimately results in a blood clot. Blood is prevented from clotting, or the process is prolonged, if any of the factors are absent (as in haemophilia) or by the use of anticoagulants (such as heparin or aspirin), which prevent their production. The positive feedback of this clotting mechanism stops once the cascade is complete.
4. Fibrinolysis – during this stage fibrin is broken down and removed by phagocytes. The enzyme plasmin, which is responsible for this process, may be activated by streptokinase and other fibrinolytic agents.
Shock – where homeostasis fails and uncontrolled positive feedback ensues
Classification of shock
There are three distinct mechanisms that may lead to hypoperfusion of the tissues:
• hypovolaemia – there is insufficient blood to carry the oxygen and nutrients needed
• pump failure (cardiogenic) – the heart is unable to pump the blood around the body effectively
• distribution problems – blood volume and cardiac output are essentially adequate but widespread vasoconstriction leads to pooling of blood and reduces venous return to the heart. Neurogenic, septic and anaphylactic shock all fall into this category.
Hypovolaemic shock
The causes of hypovolaemia are:
• loss of blood through haemorrhage
• loss of plasma as in severe burns and peritonitis
• loss of body fluids through diarrhoea, vomiting or sweating
Fifteen per cent or more of total blood volume may be lost before signs of hypovolaemic shock are noted in an adult.
Neurogenic shock
Intense pain and severe fright may also result in neurogenic shock.
Septic shock
Damage is caused by overwhelming bacterial infection, usually by Gram-negative bacilli such as Escherichia coli, Pseudomonas and Klebsiella. The bacteria cause damage by releasing endotoxins that cause vasodilatation and increased capillary permeability. Fluid leaks out of the capillaries, causing hypotension and ultimately hypovolaemia. Septic shock often has an insidious rather than a sudden onset and is sometimes referred to as ‘hot shock’ because sufferers are pyrexial as a result of the precipitating infection. ‘Toxic shock’ is a type of septic shock and is generally associated with menstruation and prolonged use of tampons. The causative organism in this instance is Staphylococcus aureus.
Anaphylactic shock
• by injection, e.g., animal or insect bites and stings, drug therapy and mismatched blood transfusion
• by ingestion – food (shellfish, cheese, egg, nuts are some common causes of allergic reaction) or orally administered drugs
It will be noted that, whatever the initial cause of shock, venous return will be increasingly reduced and the cardiac output will continue to fall – a clear example of positive feedback at work.
Physiology of shock
Whatever the initial cause of shock, the pathophysiological response is the same (Fig. 23.14). Cells throughout the body are deprived of oxygen, resulting in cell membrane damage. Histamines and kinins are released in response to the damage and cause vasodilation and increased capillary permeability. White blood cells leak out of the capillaries and proteins pass into the extracellular fluid. Oedema occurs within the cells and the interstitial fluid volume increases as the fluid compartments break down. The result is a decrease in the circulating blood volume and a consequent reduction in venous return, in the amount of blood available for oxygenation and in cardiac output. Metabolism continues within the cells despite the lack of oxygen, and lactic acid, produced as a result of cellular metabolism, builds up causing metabolic acidosis.
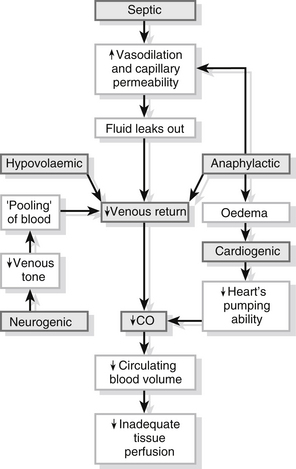
Figure 23.14 The causative mechanisms of shock.
Progressive shock – when compensatory mechanisms are not enough
• metabolic acidosis causes hyperventilation and this causes respiratory acidosis in addition as too much CO2 is blown off
• PCO2 falls, causing a reduction in blood flow to the brain and a reduced level of consciousness
• adrenaline (epinephrine) and noradrenaline (norepinephrine) are produced in response to sympathetic nervous stimulation and cause vasoconstriction, which causes further hypoxia by decreasing blood flow through the lungs. Surfactant production in the lungs starts to fail and the lungs begin to collapse, making breathing more difficult. Fluid leaks from the pulmonary capillaries and pulmonary oedema results
• reduced blood volume and flow result in poor renal perfusion with resultant oliguria
• in the liver, cells can eventually no longer conjugate bilirubin; it is returned to the circulation and jaundice becomes apparent
• poor blood flow through the gut leads to breakdown of the gut lumen. Gut contents cross into the circulation and blood passes into the gut – haematemesis and melaena are indications that this is happening
• disseminated intravascular coagulation occurs when the clotting system is activated by enzymes released from the breakdown of cells and this further reduces blood flow
• the heart’s pumping ability is so reduced that it is unable to supply even the needs of the cardiac muscle and it becomes weaker and weaker.
Irreversible shock – the final stage
The vicious circle described above, with its many positive-feedback mechanisms, is illustrated in Fig. 23.15. Early intervention may mean that homeostasis can be restored but once cell breakdown and acidosis reach a critical level, the damage is irreversible and death will ensue despite all intervention.
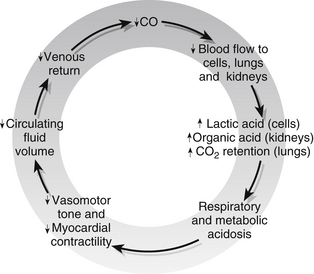
Figure 23.15 The vicious circle of irreversible shock.
Conclusion
A basic understanding of physiological mechanisms employed to maintain homeostasis is vital if the ED nurse is competently to manage the complex assaults on normal body systems that are regularly witnessed in patients attending ED.
Godfrey, H. Understanding the Human Body: Biological Perspectives for Healthcare. Edinburgh: Churchill Livingstone; 2004.
Montague, S.E., Watson, R., Herbert, R.A. Physiology for Nursing Practice, third ed. London: Baillière Tindall; 2005.
Silverthorn, D.U. Human Physiology: An Integrated Approach, fourth ed. New Jersey: Prentice Hall; 2009.
Sole, M.L., Goldenberg-Klein, D., Moseley, M.J. Introduction to Critical Care Nursing, fifth ed. Philadelphia: WB Saunders; 2009.
Thibodeau, G.A., Paton, K.T. The Human Body in Health and Disease, fifth ed. Missouri: Elsevier Mosby; 2009.
Urden, L.D., Stacey, K.M., Lough, M.E. Critical Care Nursing: Diagnosis and Management, sixth ed. Missouri: Elsevier Mosby; 2009.
Wingerd, B. The Human Body: Concepts of Anatomy and Physiology, third ed. Philadelphia: Lippincott Wilkins & Williams; 2013.

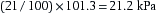
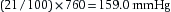
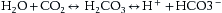
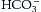 is then removed to the plasma where it is transported combined with sodium found in the plasma as sodium bicarbonate NaHCO3.
is then removed to the plasma where it is transported combined with sodium found in the plasma as sodium bicarbonate NaHCO3.



