Chapter 90 Photic Retinal Injuries
Mechanisms, Hazards, and Prevention
Photic injuries occur when light is absorbed by tissue chromophores. Melanin and hemoglobin are the most effective retinal light absorbers.1–3 Light is also absorbed by lipofuscin, macular pigment, and photopigments for visual and nonvisual photoreception.4,5 Absorption spectra describe how light absorption varies by wavelength. Melanin and lipofuscin absorption increase steadily with decreasing wavelength. Other absorption spectra peak at specific wavelengths.1,2,4,5
Optical radiation includes 400–700 nm visible light and shorter wavelength ultraviolet (UV) radiation (UV-C, 100–280 nm; UV-B, 280–320 nm; UV-A, 320–400 nm). The cornea shields the retina from UV radiation below 300 nm.6 The crystalline lens protects it from most UV-B and UV-A radiation, but crystalline lenses under 30 years of age may transmit a small amount of potentially harmful UV-B radiation.6–8 Other ocular defense mechanisms against UV radiation and intense visible light include eyebrow shadowing, corneal reflection of light not incident perpendicular to its surface (Fresnel’s law), and the pupillary, aversion, squint, and blink responses.9–11
Retinal light exposure is specified using parameters including optical energy (joule), exposure (joule/cm2), power (watt) and irradiance (watt/cm2).3 Optical power is high when energy is delivered quickly in brief exposures. Exposure and irradiance are high when optical energy and power are confined to small areas, respectively.
Light can produce beneficial or harmful photomechanical, photothermal, and/or photochemical retinal effects.12–16
Photomechanical effects
The most common signs of acute photomechanical retinal trauma are retinal hemorrhages and/or holes.
Photomechanical mechanisms
Surgical photomechanical effects include photodisruption, photofragmentation, and photovaporization.16 Photodisruption occurs in Nd:YAG laser capsulotomy and lamellar keratectomy when infrared laser energy ionizes target tissue molecules, producing plasma and a rapidly expanding shock wave that dissects target tissue.17 Photofragmentation occurs in excimer laser photorefractive keratectomy when ultraviolet laser energy breaks bonds in corneal surface molecules and residual energy volatilizes molecular fragments. Photovaporization occurs in holmium laser sclerostomy and erbium laser phacolysis when rapid water vapor expansion excavates target tissues.
Most accidental photomechanical retinal injuries are caused by very brief laser exposures ranging in duration from a hundred femtoseconds (10−15 sec) to microseconds (10−6 sec).18–22 Their very high retinal irradiances (power densities) produce tissue heating and expansion that cause immediate thermomechanical chorioretinal distortion and bleeding. Photovaporization may occur, but retinal irradiances are generally far too low for other photomechanical effects. Most victims experience a brilliant light flash followed by immediate monocular vision loss. An audible sound (pop) and/or momentary pain occur infrequently at the time of the laser accident.
Photomechanical retinal injuries
A small number of photomechanical laser accidents occur each year.19–22 Most are caused by laboratory research lasers and military rangefinders or target designators.20–22 Injuries can be prevented by effective laser safety training and proper protective eyewear. Surgical Q-switched and femtosecond laser systems have numerous safeguards that limit high optical irradiances to small, restricted spatial volumes.
Initial vision loss depends on a laser injury’s retinal location and associated chorioretinal disruption and bleeding.21 Blood can spread laterally in subhyaloid, subretinal, or sub-RPE (retinal pigment epithelium) spaces. Chorioretinal scars form and evolve. Vision may improve over days to months. Prognosis is excellent for less severe injuries that do not involve the fovea.
Optical coherence tomography (OCT) and fluorescein angiography are valuable for evaluating, managing, and documenting real and alleged injuries. Anti-inflammatory and neuroprotective drugs for reducing laser damage have been studied experimentally,23,24 but clinical trials of their efficacy are impractical because injuries are uncommon. Accident victims should be followed for macular holes and choroidal neovascularization that can develop in the months following an injury.25–29 Macular holes may close and choroidal neovascularization (CNV) may resolve spontaneously but conventional macular hole surgery and CNV therapy are potentially useful when needed.25–29
1. the light source is usually known,
2. typical chorioretinal damage occurs,
3. there is an unambiguous temporal relationship between the laser incident and serious visual symptoms,
4. the severity of visual symptoms is commensurate with the extent of retinal damage demonstrable with retinal imaging and examination, and
5. typical chorioretinal remodeling occurs after the injury.30
Most laser injuries and noninjurious laser exposures are painless.30 Eye rubbing after a laser incident can cause painful self-inflicted corneal abrasions, sometimes falsely attributed to the laser exposure.30–32 Real retinal laser injuries do not cause chronic headache or other somatic complaints, including head, neck or jaw pain.30
The ease of laser injury diagnosis is directly proportional to the severity of the laser injury.30 In ambiguous cases, subtle retinal findings have an excellent visual prognosis. When a retinal laser injury is alleged and objective findings are absent or within normal limits, diagnosis of laser injury should be deferred pending a rigorous review of the patient’s retinal findings, ophthalmic and systemic tests, clinical course, and past medical history. Such an analysis may take weeks or even months to perform if there is a complex past medical history. A guideline has been published for this type of analysis.30
Pressure from patients or attorneys to reach quick conclusions in alleged but inapparent laser injuries should be resisted.30,33 There are numerous potential psychiatric, financial or other explanations for complaints of nonorganic origin.30,33 Differentiating between those origins is challenging, but organic retinal laser injuries do not cause chronic pain, and if a significant visual abnormality is present, it should be reproducible and consistent with a significant chorioretinal abnormality.30
Photothermal effects
Photothermal mechanisms
Photocoagulation occurs when intense light is absorbed mostly by melanin in the RPE and choroid. Light energy is converted into heat, increasing the temperature of directly exposed pigmented tissues.2,3,34 Heat conduction spreads temperature elevation to adjacent sites. Overlying neural retina damaged by heat conduction loses its transparency and becomes visible as a focal white lesion (“burn”) because it scatters white fundus illumination light back at an observer. Retinal burns increase in size over time due to postexposure scarring and collateral chorioretinal damage that is not apparent immediately after the exposure.2
Standard clinical photocoagulation produces immediately visible burns, with retinal temperature increases exceeding 20 °C.1 Photomechanical effects occur at roughly three times the laser exposure needed for a visible lesion. Invisible lesions that are apparent only with fluorescein or autofluorescence imaging occur at half to one-quarter of the exposure needed to produce ophthalmoscopically visible lesions. In clinical parlance, “subthreshold” means ophthalmoscopically invisible or “subvisible.”3 Subthreshold photocoagulation can produce beneficial therapeutic effects with low temperature rises that produce minimal or no apparent retinal damage.35
Most accidental photothermal retinal injuries are caused by pulsed laser exposures ranging in duration from a microsecond to a few seconds. Retinal irradiance is high enough for photocoagulation but too low for photomechanical effects. The magnitude and duration of chorioretinal temperature elevation determine the severity of a retinal burn, along with lesion size, fundus pigmentation, and chorioretinal sequelae.3,21 Photothermal and photomechanical retinal injuries are managed similarly. Injuries should be followed for retinal holes and CNV that can develop within a few months of the injury.27,36
Photothermal retinal injuries
Operating room or medical office injuries
Most medical laser accidents go unreported for legal reasons.30 Indirect ophthalmoscope photocoagulator beams and their reflections are potentially hazardous for many meters. Bystanders should put on protective eyewear before these systems are switched from standby to treatment mode. Protective operating microscope filters should be in place and the laser delivery probe inside a patient’s eye before an endoscopic photocoagulator is switched to treatment mode.37
Slit-lamp photocoagulators
Slit-lamp photocoagulator operators are protected from backscattered laser light by optical filters.38 Laser beam reflections are theoretically hazardous for bystanders up to 2 meters from a flat-surfaced contact lens,39 so persons within that range should wear laser safety glasses or goggles effective for the laser treatment wavelength. No injury of this type has ever been reported.
Laser pointers and other consumer laser devices
Laser pointers marketed in the United States are regulated by the Food and Drug Administration (FDA).31,32,40 They are supposed to produce less than 5 mW (milliwatt) of power (Class 3A) and have warning labels cautioning users not to stare into the laser beam. The low cost of laser pointers has made them available to children, adults who do not observe warning labels, and rioters.
Staring deliberately into a laser pointer beam for more than 10 seconds is hazardous and has caused retinal injuries.31,32,41–43 Brief accidental or inadvertent laser pointer exposures do not cause retinal damage because they are terminated typically in less than 0.25 sec by normal aversion responses to uncomfortable, dazzling light.31,40 A laser pointer injury occurred in an 11-year-old girl who stared into a red laser pointer beam for more than 10 seconds because her classmates wanted to see if her pupil would constrict.41 Prominent foveolar pigment mottling occurred in her affected eye, along with an initial decrease in visual acuity to 20/60. Pigment mottling faded and visual acuity normalized over several months.
Powerful “hand-held laser systems” with dangerous output powers ranging from 20 to 1000 mW (Class 3B or 4) can appear identical to laser pointers and be purchased over the Internet. These devices are photocoagulators, not laser pointers. They have already caused serious photothermal retinal injuries.42,44,45 Lasers in recreational light shows have also caused serious photothermal retinal injuries in bystanders.46
Photochemical effects
Photochemical mechanisms
Accidental photochemical retinal injuries (known as photic retinopathy or retinal phototoxicity) are caused by prolonged intense light exposures that probably would be well tolerated if experienced only momentarily.5 They occur at chorioretinal temperature elevations too low for photothermal damage, at illuminances far exceeding normal environmental levels, in exposures lasting from seconds to minutes. Optical radiation produces highly reactive oxygen radicals that can damage retinal cell membranes, proteins, carbohydrates, and nucleic acids. The extent of a photochemical retinal injury depends on individual defense mechanisms, the location and area of exposed retina, and the duration, intensity, and spectrum of the light exposure.5,10,12,47–49
Photic retinopathy does not occur unless acute cellular damage is so excessive that it acutely overwhelms retinal repair mechanisms.5,37 People safely undergo bright but much lower irradiances in ophthalmic imaging studies and light therapy for seasonal affective disorder.12,37,50
Action spectra characterize how effectively different wavelengths cause a photochemical effect.51 Photic retinopathy can be divided into photosensitizer- and photopigment-mediated phototoxicities which have different action spectra.4,5,52
The hazardousness of photosensitizer-mediated retinal phototoxicity increases rapidly with decreasing wavelength,5,47 similar to the absorption spectrum of lipofuscin in the RPE which is its primary mediator.53,54 Thus, UV radiation is much more hazardous than visible light. In an aphakic eye, UV radiation, violet light (400–440 nm) and blue light (440–500 nm) account for 67%, 18%, and 14% of potential retinal phototoxicity, respectively.55,56 Photosensitizer-mediated phototoxicity is the basis for the international consensus aphakic standard Aλ phototoxicity function used to estimate industrial acute retinal phototoxicity risks.57
In an adult phakic eye, the retina has the additional shielding of crystalline lens attenuation of UV radiation and shorter wavelength visible light. That is why the international consensus phakic standard Bλ phototoxicity function peaks at 440 nm in the blue part of the spectrum. The Bλ function is often termed a “blue light hazard” function, even though blue light has far less retinal phototoxicity than violet light or UV radiation.4,5,57
The hazardousness of photopigment-mediated retinal phototoxicity58 peaks around 500 nm (blue-green), similar to the luminous sensitivity of scotopic vision59 because the photopigment rhodopsin mediates both processes.5 This type of photic retinopathy requires only 1% of the retinal irradiance needed for photosensitizer-mediated phototoxicity,10,52,60,61 but experimental studies were performed with highly light-sensitive nocturnal rodents whose primary photopigment is rhodopsin.5,58,61
Clinical findings are similar in solar and welding arc maculopathies, as they are in operating microscope and endoilluminator injuries.5,37
Photochemical retinal injuries
Solar and welder’s maculopathy
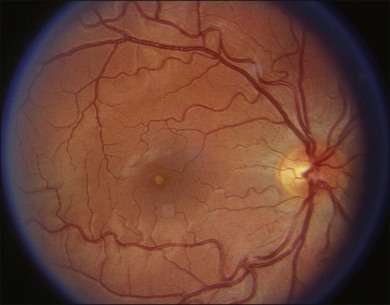
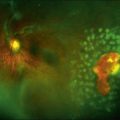
Figure 90.1 shows a typical yellow-white solar maculopathy lesion.62,63 Lesions fade over several weeks, resolving completely or leaving foveolar distortion, pigment mottling or even a macular hole.62 Welding arc injuries produce similar clinical abnormalities.5,64,65 Welder’s maculopathy is extremely rare but it may be underreported because its transient clinical symptoms may be masked by those of associated photokeratitis.5,8,66
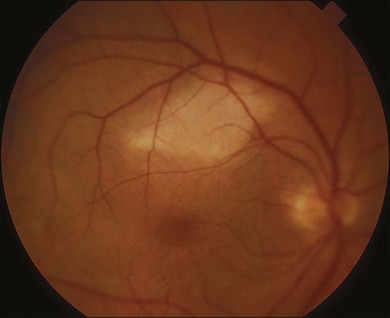
Common visual complaints after acute solar or welding arc injury are blurred vision, central scotoma and erythropsia. Post-injury visual acuity may be normal or decreased to the 20/40 to 20/200 range. Visual acuity usually returns to 20/20 to 20/40 over 6 months.5,62 Fluorescein angiography may be normal but more severe injuries may cause foveal RPE defects.62,67 The characteristic OCT finding is a well-defined outer retinal hyporeflective space primarily involving the photoreceptor inner and outer segment layers, as shown in Fig. 90.2 for a welding arc injury.5,65,68–73 Spectral domain OCT imaging immediately after an injury reveals overlying outer nuclear layer and underlying RPE abnormalities, as shown in Fig. 90.3.
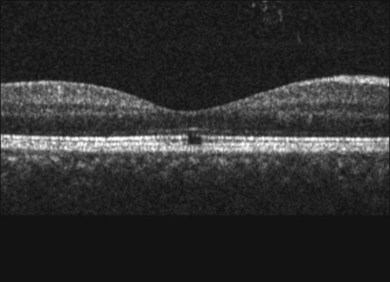
Fig. 90.2 OCT images taken months to years after solar or welding arc injuries usually reveal a well-defined foveolar outer retinal hyporeflective space.5,65,68–73 This 34-year-old male had been welding since 11 years of age and complained of vision loss for 5 months prior to the OCT study. A horizontal scan through his fovea shows largely normal retinal pigment epithelium (RPE) and external limiting membrane reflective bands but interruption of the Verhoeff’s membrane (RPE tight junctions and/or apical processes) as well as the inner/outer-segment junction hyperreflective bands. These OCT findings are consistent with chronic damage to photoreceptor inner- and outer-segment layers.62
(Courtesy of Suman Pilli, MD, Muralidhar Ogoti, MD, and Vishwanath Kalluri, MD.)
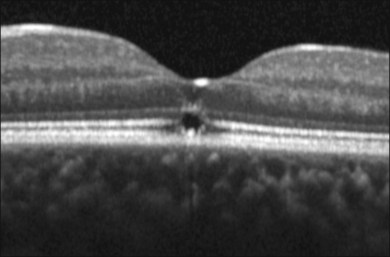
Fig. 90.3 A spectral domain OCT image taken shortly after a solar injury documents the foveolar outer retinal hyporeflective space observed in older lesions and also abnormalities in the overlying outer nuclear layer and underlying retinal pigment epithelium (RPE). These findings are consistent with histopathological evidence of photoreceptor and RPE damage after acute solar maculopathy in human eyes scheduled for enucleation.74,136
(Courtesy of Giovanni Staurenghi, MD, and Marco Pellegrini, MD.)
The histopathology of solar retinopathy has been studied in volunteers who stared at the sun monocularly for 10–60 minutes before enucleation for choroidal melanoma.63,74 Photoreceptor damage included vesiculation and fragmentation of photoreceptor outer segment lamellae, mitochondrial swelling, and nuclear pyknosis.63,74 Cones appeared more damage-resistant than rods, possibly accounting for good visual outcomes after some injuries.74 RPE damage was variable.63,74 Imaging and histopathological data to date do not permit determination of whether solar and welding arc injuries are primarily of RPE and/or photoreceptor origin.
Foveomacular retinitis is a term used to describe foveal abnormalities resembling photic retinopathy. It occurs after blunt ocular trauma and whiplash injury75–77 and in people with no history of mechanical or photic trauma.78,79 Outbreaks were reported in military personnel during World War II and again from 1966 to 1973.80–83 Those incidents were ascribed to solar exposure,82,84 consistent with reports of solar maculopathy in young people who have been sunbathing but not sungazing.85,86
Momentary solar observation is safe or it would be dangerous to look upwards on a bright sunny day. Even at noontime on a clear day, direct solar observation with a 3 mm pupil diameter causes only a 4°C retinal temperature rise, far too low for photocoagulation.87 Thus, typical solar maculopathy is photochemical not photothermal damage. Conversely, solar observation with a dilated 7 mm pupil produces a 22°C retinal temperature increase, well above the 10°C threshold for retinal photocoagulation.34,87 Telescope-assisted solar observation produces even higher retinal temperature increases.87,88
Solar eclipse observation is particularly hazardous because pupillary dilation can occur and increase retinal irradiance.88 There are many indirect methods for safely viewing solar eclipses.88,89 Sungazing injuries have been reported to occur during hypoglycemia, drug abuse, psychosis, and religious rituals.5,71 Prolonged solar observation causes the most damage, especially with pharmacologically dilated pupils.
Photokeratitis is a common welding arc accident but welder’s maculopathy is an unusual event. When welders fail to use appropriate eye protection filters, younger workers are at greater risk for retinal injury because of their clear ocular media. The UV-B window in the crystalline lens discussed previously closes by roughly 30 years of age.6–8 At younger ages, some highly phototoxic UV-B welding arc radiation may be transmitted to the retina, possibly accounting for welding arc maculopathy and welders’ reportedly increased risk of uveal melanoma.6,8 Photic injuries have also been caused by UV-B radiation and/or short-wavelength visible light from a femtosecond laser plasma90 and a short-circuiting high-tension electric circuit.91
Experimental photic retinopathy is enhanced by elevated body temperature,58,92,93 so increased body temperature from a hot day, exercise, or fever could increase the risk of retinal phototoxicity, perhaps accounting for the age-old admonishment to protect children with fevers from bright sunlight.5 Higher chorioretinal pigmentation could increase local chorioretinal temperature rise associated with solar observation, perhaps further increasing the risk of damage. The fovea is at greatest risk for solar damage in direct sungazing, but foveal injuries in young adults who have been sunbathing but not sungazing may occur because of crystalline lens UV-B transmission and/or Henle fiberoptic transmission of short-wavelength photons toward the fovea.94
Operating microscope and endoilluminator injuries
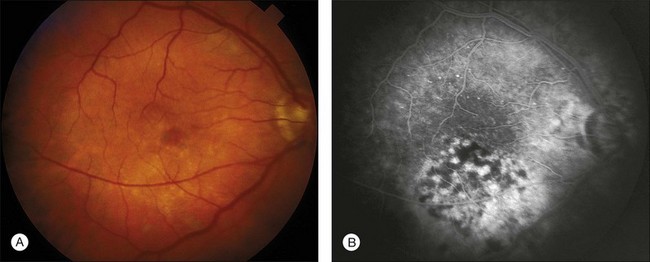
Typical operating microscope injuries are oval 0.5–2 disc diameter lesions.95,96 Damage sites are often inferior to the fovea due to microscope tilt and illumination positioning.97,98 Fiberoptic endoilluminators can cause comparable lesions elsewhere,99 as shown in Fig. 90.4. Injuries have occurred in cornea, cataract, glaucoma, and retinal surgery.95,96,100–102 Photochemical retinal damage is localized primarily to photoreceptors and the RPE as in solar and welder’s maculopathies.103–105
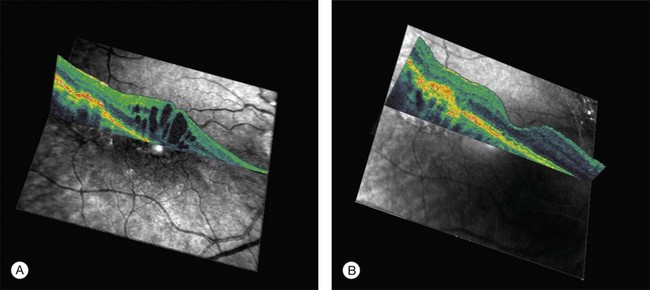
Lesion size, location, and severity determine vision loss from an operating microscope injury. Lesions initially have a yellow-white appearance, sometimes accompanied by a shallow serous neural retinal detachment. This finding resolves rapidly, followed by local RPE atrophy and pigment mottling. Chronic lesions are usually much less apparent ophthalmoscopically than angiographically, as shown in Figs 90.5A and 90.5B, respectively. Figure 90.6A is the OCT image of a 70-year-old female with an operating microscope lesion temporal to her fovea and also central cystoid macular edema (CME) shortly after complicated cataract surgery. The OCT image in Fig. 90.6B taken 3 years later shows chorioretinal scarring at the site of operating microscope illumination,106 consistent with histopathologic findings in human and animal studies.103–105 Imaging and histopathological data to date do not permit determination of whether operating microscope and endoilluminator injuries are primarily of RPE and/or photoreceptor origin.
The risk of operating microscope injury is potentially increased by higher body temperature,92 increased chorioretinal pigmentation (theoretically increasing local light absorption and thermal enhancement of retinal phototoxicity),5 oxygen administration during surgery (hypothetically increasing retinal free radical production),107 and photosensitizing medications such as hydroxychloroquine, hydrochlorothiazide, furosemide, allopurinol, and the benzodiazepines.108–110 Diabetes mellitus and hypertension may also increase retinal phototoxicity risks.111
Faster cataract surgery reduces the risk of operating microscope injury,112 as does minimizing operating microscope intensity and discontinuing photosensitizing medications preoperatively when possible.5,110 Other potentially useful techniques include maintaining patients’ eyes in downgaze, using corneal occluders, and avoiding supplemental oxygen use and/or elevated patient core body temperatures.5,113,114 Operating microscopes produce minimal UV radiation,115–117 so UV-blocking filters are of little value. One study reported that UV-blocking intraocular lenses (IOLs) reduce postoperative cystoid macular edema,118 but three subsequent studies refuted that conclusion.119–121 Reducing operating microscope violet and blue light illumination hypothetically decreases acute retinal phototoxicity risks, but blue light is important for tissue visualization.5
Indocyanine green (ICG) is useful for retinal angiography and staining the inner limiting membrane (ILM) during macular hole surgery. ICG fluorescence can persist for months after ICG-assisted vitreoretinal surgery.122 Visual field defects have been reported after these procedures,123 as has RPE damage possibly of phototoxic origin.124 The potential risks and benefits of ILM staining with ICG and alternative vital dyes continue to be evaluated.125
Ophthalmoscope and fundus camera exposure
Indirect ophthalmoscopes and fundus cameras produce dazzling exposures and transient afterimages, but there is no evidence that they cause photochemical or thermal retinal injury in routine clinical use.12,126–129 A 15 minute indirect ophthalmoscope exposure focused on a single retinal area can cause a localized phototoxic injury in an anesthetized rhesus monkey,92 but that certainly is not a clinical light exposure. No immediate or long-term retinal abnormalities could be detected after similar exposures were attempted in human volunteers with blind eyes or those requiring enucleation.130 These results are consistent with instrumentation data showing that fundus camera, indirect ophthalmoscope, and scanning laser ophthalmoscope retinal irradiances are lower than experimentally determined damage thresholds.12,126,127,129,131 Anterior segment slit-lamp photography has been reported to cause photic retinopathy under unusual circumstances.132
Sixty-second stabilized retinal exposures accelerated local retinal degeneration in dogs with rhodopsin gene mutations, prompting recommendation that retinal photography and clinical light exposure be minimized in patients with retinitis pigmentosa.133 Human and canine retinas differ significantly133 and the experimental exposures were 10 000 times longer than 2 msec clinical fundus camera flashes.129 Light exposure has never been proven to adversely affect the clinical course of retinitis pigmentosa and even light deprivation does not prevent its progression.134 No current scientific evidence suggests the need for retinal disease-specific light exposure safety standards.129 Nonetheless, regardless of a patient’s condition, clinical retinal imaging should be performed only when necessary and patient comfort dictates that clinical procedures always be carried out at the lowest clinically effective retinal illumination levels.12,129
Environmental issues
Light and macular degeneration
There has been speculation for almost a century that environmental light exposure may be a risk factor for age-related macular degeneration (AMD).10,135–138 The retina’s high oxygen and light levels increase its vulnerability to oxidative stress which can cause chronic local inflammation.5 Retinal compensatory internal defenses include photoreceptor disc shedding, and enzymatic and nonenzymatic antioxidants. RPE lipofuscin accumulates with aging because photoreceptor phagocytosis and autophagy of spent intracellular organelles produce undegradable endproducts that are not eliminated effectively.5 Excessive lipofuscin may compromise RPE cell function, promote angiogenic factor expression or act as a photoinducible generator of reactive oxygen species.5 Lipofuscin accumulation, phototoxicity, and AMD all involve direct or indirect oxidative damage,5,54,139 but shared mechanisms do not prove causal relationships.4
The retinal phototoxicity-AMD hypothesis conjectures that repetitive acute photic retinopathy from environmental light exposure is a risk factor for AMD. Twelve major epidemiological studies examined this issue over the past three decades: eight studies were population-based and four were case-controlled.55 Ten of the 12 studies found no link between environmental light and AMD,55 including a larger second study140 by the director of the earlier-positive Waterman study.141 Another critical failure of the phototoxicity-AMD hypothesis is compelling and growing evidence that cataract surgery does not accelerate the development or progression of AMD,142–146 as it should if light were a significant causative factor in AMD.55 More than four decades after the discovery of photic retinopathy, there is no experimental or clinical proof that environmental light exposure or repetitive acute phototoxicity is a significant risk factor in AMD.
Sunglasses
Sunglasses are worn for comfort and fashion.9,147 There is strong evidence that environmental UV exposure plays a significant role in cortical cataractogenesis.148 Thus, UV-blocking sunglasses and other avoidance strategies may decrease the risk of cataract formation. If environmental light exposure plays any role in AMD in susceptible individuals, then groups that might benefit from wearing sunglasses for retinal protection include lightly pigmented younger people in tropical climates, individuals taking photosensitizing drugs for skin or other systemic diseases, and people suffering from malabsorption syndromes or other conditions contributing to malnutrition.5
Sunglass design considers visual comfort and ocular protection.10 There must be sufficient visible light transmission to permit effective perception of fine detail and color contrast,9,149 but enough absorption or reflection of optical radiation to provide comfort and protection from UV radiation. Brimmed hats reduce overhead light exposure, but much of eye’s UV exposure may come from reflective terrain below or viewing the horizontal sky.51 Sunglasses are not safe for sungazing and pupillary dilation potentially associated with their use may increase the risk of solar injury.9,12,89
Safety standards
Safety standards that affect laser users include the voluntary American National Standard Institute (ANSI) Standards “Safe Use of Lasers” (ANSI Z136.1–2007)150,151 and “Safe Use of Lasers in Health Care Facilities” (ANSI Z136.3–2005).152 The ANSI Z136.1 Standard is similar to the International Standard IEC 60825–1.153 ANSI standards are technically “voluntary,” but regulatory groups use them to assess medical laser facility safety and litigants use them to scrutinize purported injuries.154,155
Safety standards that affect laser manufacturers in the United States include the FDA’s regulatory “Laser Performance Standard” (21 CFR1040).156 This standard requires manufacturers to equip laser devices with features such as emission indicators and keyed switches. There are also specific standards for ophthalmic devices such as slit lamps and endoilluminators.
ANSI standards define four laser classes.150,155 Class 1 lasers are considered incapable of causing damaging ocular exposures and do not require control measures. Their maximum power output in the visible spectrum ranges from 0.0004 mW or less for blue or green light to 0.024 mW or less for red light. Class 2, 3a, and 3b lasers produce laser power that is less than 1 mW, between 1 and 5 mW, and between 5 and 500 mW, respectively. Laser pointers are Class 3a devices. Some dangerous Class 3b handheld laser devices are identical in appearance to laser pointers. Class 4 lasers include potentially hazardous industrial, military or medical lasers that generate more than 500 mW of laser power. Safety standards assign control measures to each laser class.150,152
Practical considerations
Control measures are designed to decrease the risk of laser accidents.88,154,155 “Engineering” controls are protective measures built into laser systems such as housings, labels, and interlocks. “Administrative” and “procedural” controls are designed to assure the proper use of potentially hazardous laser systems. They include written protocols for (1) operating, maintaining, and servicing laser systems, (2) assuring proper personnel education and training, and (3) using appropriate protective eyewear.
For an ophthalmic laser facility, the ANSI Z136.3–2005 standard recommends that (1) a “Laser Safety Officer” be given local safety oversight responsibility, (2) a warning sign with the signal word “danger” be displayed on the outside of the closed treatment room door during any laser procedure, (3) personnel wear laser eye protection in the nominal hazard zone where diffuse reflections and stray beams could be hazardous, and (4) operators be trained in the safe use of their laser equipment.152,154 From a practical perspective, the nominal hazard zone is the treatment or operating room in which a surgical laser is located. The laser safety office is responsible for assuring that (1) protective eyewear is available and used regularly, (2) personnel using lasers are trained properly, and (3) laser safety measures are audited regularly.
1 Mainster MA, White TJ, Allen RG. Spectral dependence of retinal damage produced by intense light sources. J Opt Soc Am. 1970;60(6):848–855.
2 Mainster MA. Wavelength selection in macular photocoagulation. Tissue optics, thermal effects, and laser systems. Ophthalmology. 1986;93(7):952–958.
3 Mainster MA. Decreasing retinal photocoagulation damage: principles and techniques. Semin Ophthalmol. 1999;14(4):200–209.
4 Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90(6):784–792.
5 Mainster MA, Boulton M. Retinal phototoxicity. Albert DM, Miller JW, Blodi BA, et al. Principles and practice of ophthalmology, 3rd ed, London: Elsevier, 2008. ch. 174
6 Boettner EA, Wolter JR. Transmission of the ocular media. Invest Ophthalmol. 1962;1(6):776–783.
7 Barker FM, Brainard GC. The direct spectral transmittance of the excised human lens as a function of age, (FDA 785345 0090 RA). Washington, DC: US Food and Drug Administration; 1991.
8 Mainster MA, Turner PL. Ultraviolet-B phototoxicity and hypothetical photomelanomagenesis: intraocular and crystalline lens photoprotection. Am J Ophthalmol. 2010;149(4):543–549.
9 Sliney DH. Eye protective techniques for bright light. Ophthalmology. 1983;90(8):937–944.
10 Mainster MA. Light and macular degeneration: a biophysical and clinical perspective. Eye. 1987;1(Pt 2):304–310.
11 Stamper DA, Lund DJ, Molchany JW, et al. Human pupil and eyelid response to intense laser light: implications for protection. Percept Mot Skills. 2002;95(3 Pt 1):775–782.
12 Mainster MA, Ham WT, Jr., Delori FC. Potential retinal hazards. Instrument and environmental light sources. Ophthalmology. 1983;90(8):927–932.
13 Mainster MA. Finding your way in the photoforest: laser effects for clinicians. Ophthalmology. 1984;91(7):886–888.
14 Marshall J. Structural aspects of laser-induced damage and their functional implications. Health Phys. 1989;56(5):617–624.
15 Mainster MA. Photic retinal injury. In: Ryan SJ, ed. Retina. St Louis: Mosby–Year Book, 1989.
16 Mainster MA. Classification of ophthalmic photosurgery. Lasers Light Ophthalmol. 1994;6(2):65–67.
17 Mainster MA, Ho PC, Mainster KJ. Nd: YAG laser photodisruptors. Ophthalmology. 1983;Suppl:45–47.
18 Gabel VP, Birngruber R, Lorenz B, et al. Clinical observations of six cases of laser injury to the eye. Health Phys. 1989;56(5):705–710.
19 Thach AB, Lopez PF, Snady-McCoy LC, et al. Accidental Nd:YAG laser injuries to the macula. Am J Ophthalmol. 1995;119(6):767–773.
20 Barkana Y, Belkin M. Laser eye injuries. Surv Ophthalmol. 2000;44(6):459–478.
21 Mainster MA. Retinal laser accidents: mechanisms, managment and rehabilitation. J Laser Appl. 2000;12(1):3–9.
22 Harris MD, Lincoln AE, Amoroso PJ, et al. Laser eye injuries in military occupations. Aviat Space Environ Med. 2003;74(9):947–952.
23 Brown J, Jr., Hacker H, Schuschereba ST, et al. Steroidal and nonsteroidal antiinflammatory medications can improve photoreceptor survival after laser retinal photocoagulation. Ophthalmology. 2007;114(10):1876–1883.
24 Shulman S, Belokopytov M, Dubinsky G, et al. Ameliorative effect of PN-277 on laser-induced retinal damage. Graefes Arch Clin Exp Ophthalmol. 2009;247(3):343–348.
25 Ciulla TA, Topping TM. Surgical treatment of a macular hole secondary to accidental laser burn. Arch Ophthalmol.. 1997;115(7):929–930.
26 Newman DK, Flanagan DW. Spontaneous closure of a macular hole secondary to an accidental laser injury. Br J Ophthalmol.. 2000;84(9):1075.
27 Sasahara M, Noami S, Takahashi M, et al. Optical coherence tomographic observations before and after macular hole formation secondary to laser injury. Am J Ophthalmol. 2003;136(6):1167–1170.
28 Nehemy M, Torqueti-Costa L, Magalhaes EP, et al. Choroidal neovascularization after accidental macular damage by laser. Clin Experiment Ophthalmol. 2005;33(3):298–300.
29 Ying HS, Symons RC, Lin KL, et al. Accidental Nd:YAG laser-induced choroidal neovascularization. Lasers Surg Med. 2008;40(4):240–242.
30 Mainster MA, Stuck BE, Brown J, Jr. Assessment of alleged retinal laser injuries. Arch Ophthalmol. 2004;122(8):1210–1217.
31 Mainster MA, Timberlake GT, Warren KA, et al. Pointers on laser pointers. Ophthalmology. 1997;104(8):1213–1214.
32 Mainster MA. Blinded by the light – not!. Arch Ophthalmol. 1999;117(11):1547–1548.
33 Mainster MA, Sliney DH, Marshall J, et al. But is it really light damage? Ophthalmology. 1997;104(2):179–180.
34 Mainster MA, White TJ, Tips JH, et al. Retinal-temperature increases produced by intense light sources. J Opt Soc Am. 1970;60(2):264–270.
35 Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89(1):74–80.
36 Bernstein PS, Steffensmeier A. Optical coherence tomography before and after repair of a macular hole induced by an unintentional argon laser burn. Arch Ophthalmol. 2005;123(3):404–405.
37 Mainster MA, Turner PL. Retinal injuries from light: mechanisms, hazards and prevention. Ryan SJ, Hinton DR, Schachat AP, et al. Retina, 4th ed, London: Elsevier, 2006. vol. 2, ch. 109
38 Mainster MA. Ophthalmic laser surgery: principles, technology, and technique. Trans New Orleans Acad Ophthalmol. 1985;33:81–101.
39 Jenkins DL. Hazard evaluation of the coherent model 900 photocoagulator laser system, non-ionizing radiation protection specialty study no. 25-42-0310-79 (NTIS no. ADA 068713). Aberdeen Proving Ground, MD: US Army Environment Hygiene Agency; 1979.
40 Sliney DH, Dennis JE. Safety concerns about laser pointers. J. Laser Applications. 1994;6:159–164.
41 Sell CH, Bryan JS. Maculopathy from handheld diode laser pointer. Arch Ophthalmol. 1999;117(11):1557–1558.
42 Ziahosseini K, Doris JP, Turner GS. Laser eye injuries. Maculopathy from handheld green diode laser pointer. BMJ. 2010;340:c2982.
43 Fujinami K, Yokoi T, Hiraoka M, et al. Choroidal neovascularization in a child following laser pointer-induced macular injury. Jpn J Ophthalmol. 2010;54(6):631–633.
44 Wyrsch S, Baenninger PB, Schmid MK. Retinal injuries from a handheld laser pointer. N Engl J Med. 2010;363(11):1089–1091.
45 Ueda T, Kurihara I, Koide R. A case of retinal light damage by green laser pointer (Class 3b). Jpn J Ophthalmol. 2011;55(4):428–430.
46 Boosten K, Van Ginderdeuren R, Spileers W, et al. Laser-induced retinal injury following a recreational laser show: two case reports and a clinicopathological study. Bull Soc Belge Ophtalmol. 2011;317:11–16.
47 Ham WT, Jr., Mueller HA, Sliney DH. Retinal sensitivity to damage from short wavelength light. Nature. 1976;260:153–155.
48 Lawwill T. Three major pathologic processes caused by light in the primate retina: a search for mechanisms. Trans Am Ophthalmol Soc. 1982;80:517–579.
49 Sliney DH, Mellerio J, Gabel VP, et al. What is the meaning of threshold in laser injury experiments? Implications for human exposure limits. Health Phys. 2002;82(3):335–347.
50 Gallin PF, Terman M, Reme CE, et al. Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy. Am J Ophthalmol. 1995;119(2):202–210.
51 Sliney DH. How light reaches the eye and its components. Int J Toxicol. 2002;21(6):501–509.
52 van Norren D, Gorgels TG. The action spectrum of photochemical damage to the retina: a review of monochromatic threshold data. Photochem Photobiol. 2011;87(4):747–753.
53 Rozanowska M, Jarvis-Evans J, Korytowski W, et al. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995;70(32):18825–18830.
54 Boulton M, Rozanowska M, Rozanowski B. Retinal photodamage. J Photochem Photobiol B. 2001;64(2–3):144–161.
55 Mainster MA, Turner PL. Blue-blocking IOLs decrease photoreception without providing significant photoprotection. Surv Ophthalmol. 2010;55(3):272–289.
56 Mainster MA. Blue-blocking intraocular lenses and pseudophakic scotopic sensitivity. J Cataract Refract Surg. 2006;32(9):1403–1404.
57 ACGIH. Threshold Limit Values for chemical substances and physical agents: Biological Exposure Indices. Cincinnati: American Conference of Governmental Industrial Hygienists; 1997.
58 Noell WK, Walker VS, Kang BS, et al. Retinal damage by light in rats. Invest Ophthalmol. 1966;5(5):450–473.
59 Griswold MS, Stark WS. Scotopic spectral sensitivity of phakic and aphakic observers extending into the near ultraviolet. Vision Res. 1992;32(9):1739–1743.
60 Kremers JJ, van Norren D. Two classes of photochemical damage of the retina. Lasers Light Ophthalmol. 1988;2:41–52.
61 Mellerio J. Light effects on the retina. In: Albert DM, Jakobiec FA. Principles and practice of ophthalmology. Philadelphia: W.B. Saunders, 1994. vol. 1
62 Gass JDM. Stereoscopic atlas of macular diseases, 3rd ed. St Louis: Mosby–Year Book; 1987.
63 Tso MO, La Piana FG. The human fovea after sungazing. Trans Am Acad Ophthalmol Otolaryngol. 1975;79(6):OP788–OP795.
64 Naidoff MA, Slinkey DH. Retinal injury from a welding arc. Am J Ophthalmol. 1974;77(5):663–668.
65 Lucas RS, Harper CA, McCombe MF, et al. Optical coherence tomography findings in welder’s maculopathy. Retinal Cases Brief Reports. 2007;1:169–171.
66 Magnavita N. Photoretinitis: an underestimated occupational injury? Occup Med (Lond). 2002;52(4):223–225.
67 Kaushik S, Gupta V, Gupta A. Optical coherence tomography findings in solar retinopathy. Ophthalmic Surg Lasers Imaging. 2004;35(1):52–55.
68 Steinkamp PN, Watzke RC, Solomon JD. An unusual case of solar retinopathy. Arch Ophthalmol. 2003;121(12):1798–1799.
69 Charbel Issa P, Fleckenstein M, Scholl HP, et al. Confocal scanning laser ophthalmoscopy findings in chronic solar retinopathy. Ophthalmic Surg Lasers Imaging. 2008;39(6):497–499.
70 Chen RW, Gorczynska I, Srinivasan VJ, et al. High-speed ultrahigh-resolution optical coherence tomography findings in chronic solar retinopathy. Retin Cases Brief Rep. 2008;2(2):103–105.
71 Jain A, Desai RU, Charalel RA, et al. Solar retinopathy: comparison of optical coherence tomography (OCT) and fluorescein angiography (FA). Retina. 2009;29(9):1340–1345.
72 Symons RC, Mainster MA, Goldberg MF. Solar maculopathy in a young child. Br J Ophthalmol. 2010;94(9):1258–1259.
73 Pilli S, Ogoti M, Kalluri V. Fourier-domain optical coherence tomography findings in welder’s maculopathy. Ophthalmic Surg Lasers Imaging. 2010:1–5. Epub ahead of print, doi: 10.3928/15428877-20100215-93
74 Hope-Ross MW, Mahon GJ, Gardiner TA, et al. Ultrastructural findings in solar retinopathy. Eye. 1993;7(Pt 1):29–33.
75 Grey RH. Foveo-macular retinitis, solar retinopathy, and trauma. Br J Ophthalmol. 1978;62(8):543–546.
76 Kelley JS, Hoover RE, George T. Whiplash maculopathy. Arch Ophthalmol. 1978;96(5):834–835.
77 Abebe MT, De Laey JJ. Foveomacular retinitis as a result of ocular contusion. Bull Soc Belge Ophtalmol. 1992;243:171–175.
78 Kuming BS. Foveomacular retinitis. Br J Ophthalmol. 1986;70(11):816–818.
79 Jacobs NA. Foveomacular retinitis. Br J Ophthalmol. 1987;71(7):563.
80 Cordes FC. A type of foveomacular retinitis observed in the U.S. Navy. Am J Ophthalmol. 1944;27:803–816.
81 Kerr LM, Little HL. Foveomacular retinitis. Arch Ophthalmol. 1966;76(4):498–504.
82 Ritchey CL, Ewald RA. Sun gazing as the cause of foveomacular retinitis. Am J Ophthalmol. 1970;70(4):491–497.
83 Marlor RL, Blais BR, Preston FR, et al. Foveomacular retinitis, an important problem in military medicine: epidemiology. Invest Ophthalmol. 1973;12(1):5–16.
84 Wergel FLJ, Brenner EH. Solar retinopathy foveomacular retinitis. Ann Ophthalmol. 1975;7:495–503.
85 Gladstone GJ, Tasman W. Solar retinitis after minimal exposure. Arch Ophthalmol. 1978;96(8):1368–1369.
86 Yannuzzi LA, Fisher YL, Krueger A, et al. Solar retinopathy: a photobiological and geophysical analysis. Trans Am Ophthalmol Soc. 1987;85:120–158.
87 White TJ, Mainster MA, Wilson PW, et al. Chorioretinal temperature increases from solar observation. Bull Math Biophys. 1971;33(1):1–17.
88 Sliney DH, Wolbarsht ML. Safety with lasers and other optical sources: a comprehensive handbook. New York: Plenum Press; 1980. p. 1035
89 Mainster MA. Solar eclipse safety. Ophthalmology. 1998;105(1):9–10.
90 Yang X, Jiang F, Song Y, et al. Accidental macular injury from prolonged viewing of a plasma flash produced by a femtosecond laser. Ophthalmology. 2010;117(5):972–975.
91 Gardner TW, Ai E, Chrobak M, et al. Photic maculopathy secondary to short-circuiting of a high-tension electric current. Ophthalmology. 1982;89(7):865–868.
92 Friedman E, Kuwabara T. The retinal pigment epithelium. IV. The damaging effects of radiant energy. Arch Ophthalmol. 1968;80(2):265–279.
93 Rinkoff J, Machemer R, Hida T, et al. Temperature-dependent light damage to the retina. Am J Ophthalmol. 1986;102(4):452–462.
94 Mainster MA. Henle fibers may direct light toward the center of the fovea. Lasers Light Ophthalmol. 1988;2:79–86.
95 Fishman GA. Light-induced maculopathy from surgical microscopes during cataract surgery. In: Ernest JT, ed. The 1985 year book of ophthalmology. St Louis: Mosby–Year Book, 1985.
96 Michels M, Sternberg P, Jr. Operating microscope-induced retinal phototoxicity: pathophysiology, clinical manifestations and prevention. Surv Ophthalmol. 1990;34(4):237–252.
97 Brod RD, Olsen KR, Ball SF, et al. The site of operating microscope light-induced injury on the human retina. Am J Ophthalmol. 1989;107(4):390–397.
98 Pavilack MA, Brod RD. Site of potential operating microscope light-induced phototoxicity on the human retina during temporal approach eye surgery. Ophthalmology. 2001;108(2):381–385.
99 Michels M, Lewis H, Abrams GW, et al. Macular phototoxicity caused by fiberoptic endoillumination during pars plana vitrectomy. Am J Ophthalmol. 1992;114(3):287–296.
100 Khwarg SG, Geoghegan M, Hanscom TA. Light-induced maculopathy from the operating microscope. Am J Ophthalmol. 1984;98(5):628–630.
101 Robertson DM, Feldman RB. Photic retinopathy from the operating room microscope. Am J Ophthalmol. 1986;101(5):561–569.
102 Mares-Perlman JA, Brady WE, Klein BE, et al. Diet and nuclear lens opacities. Am J Epidemiol. 1995;141(4):322–334.
103 Parver LM, Auker CR, Fine BS. Observations on monkey eyes exposed to light from an operating microscope. Ophthalmology. 1983;90(8):964–972.
104 Irvine AR, Wood I, Morris BW. Retinal damage from the illumination of the operating microscope. An experimental study in pseudophakic monkeys. Arch Ophthalmol. 1984;102(9):1358–1365.
105 Green WR, Robertson DM. Pathologic findings of photic retinopathy in the human eye. Am J Ophthalmol. 1991;112(5):520–527.
106 Mansour AM, Yunis MH, Medawar WA. Ocular coherence tomography of symptomatic phototoxic retinopathy after cataract surgery: a case report. J Med Case Reports. 2011;1:133.
107 Jaffe GJ, Irvine AR, Wood IS, et al. Retinal phototoxicity from the operating microscope. The role of inspired oxygen. Ophthalmology. 1988;95(8):1130–1141.
108 Ferguson J. Photosensitivity due to drugs. Photodermatol Photoimmunol Photomed. 2002;18(5):262–269.
109 Long VW, Woodruff GH. Bilateral retinal phototoxic injury during cataract surgery in a child. J Aapos. 2004;8(3):278–279.
110 Manzouri B, Egan CA, Hykin PG. Phototoxic maculopathy following uneventful cataract surgery in a predisposed patient. Br J Ophthalmol. 2002;86(6):705–706.
111 Khwarg SG, Linstone FA, Daniels SA, et al. Incidence, risk factors, and morphology in operating microscope light retinopathy. Am J Ophthalmol. 1987;103(3 Pt 1):255–263.
112 Kleinmann G, Hoffman P, Schechtman E, et al. Microscope-induced retinal phototoxicity in cataract surgery of short duration. Ophthalmology. 2002;109(2):334–338.
113 O’Brien DP, Francis IC. The corneal quilt: a protective device designed to reduce intraoperative retinal phototoxicity. Ophthalmic Surg. 1994;25(3):191–194.
114 Kraff MC, Lieberman HL, Jampol LM, et al. Effect of a pupillary light occluder on cystoid macular edema. J Cataract Refract Surg. 1989;15(6):658–660.
115 Jampol LM, Kraff MC, Sanders DR, et al. Near-UV radiation from the operating microscope and pseudophakic cystoid macular edema. Arch Ophthalmol. 1985;103(1):28–30.
116 Keates RH, Genstler DE. UV radiation. Ophthalmic Surg. 1982;13(4):327.
117 Sliney DH, Armstrong BC. Radiometric analysis of surgical microscope lights for hazards analyses. Appl Opt. 1986;25(12):1882–1889.
118 Kraff MC, Sanders DR, Jampol LM, et al. Effect of an ultraviolet-filtering intraocular lens on cystoid macular edema. Ophthalmology. 1985;92(3):366–369.
119 Colin J, Ropars YM, Bonissent JF, et al. Cystoid macular oedema and intraocular lenses with ultraviolet filters. J Eur Implant Soc. 1987;4:5–10.
120 Clarke MP, Yap M, Weatherill JR. Do intraocular lenses with ultraviolet absorbing chromophores protect against macular oedema? Acta Ophthalmol (Copenh). 1989;67(5):593–596.
121 Komatsu M, Kanagami S, Shimizu K. Ultraviolet-absorbing intraocular lens versus non-UV-absorbing intraocular lens: comparison of angiographic cystoid macular edema. J Cataract Refract Surg. 1989;15(6):654–657.
122 Ciardella AP, Schiff W, Barile G, et al. Persistent indocyanine green fluorescence after vitrectomy for macular hole. Am J Ophthalmol. 2003;136(1):174–177.
123 Uemura A, Kanda S, Sakamoto Y, et al. Visual field defects after uneventful vitrectomy for epiretinal membrane with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2003;136(2):252–257.
124 Engelbrecht NE, Freeman J, Sternberg P, Jr., et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2002;133(1):89–94.
125 Thompson JT, Haritoglu C, Kampik A, et al. Should indocyanine green be used to facilitate removal of the internal limiting membrane in macular hole surgery. Surv Ophthalmol. 2009;54(1):135–138.
126 Delori FC, Parker JS, Mainster MA. Light levels in fundus photography and fluorescein angiography. Vision Res. 1980;20(12):1099–1104.
127 Delori FC, Pomerantzeff O, Mainster MA. Light levels in ophthalmic diagnostic instruments. Proc Soc Photo Optical Instrum Enginering. 1980;229:154–160.
128 Klingbeil U. Safety aspects of laser scanning ophthalmoscopes. Health Phys. 1986;1(1):81–93.
129 Mainster MA, Turner PL. Retinal examination and photography are safe: is anyone surprised? Ophthalmology. 2010;117(2):197–198.
130 Robertson DM, Erickson GJ. The effect of prolonged indirect ophthalmoscopy on the human eye. Am J Ophthalmol. 1979;87(5):652–661.
131 Delori FC, Webb RH, Sliney DH. Maximum permissible exposures for ocular safety (ANSI 2000), with emphasis on ophthalmic devices. J Opt Soc Am A Opt Image Sci Vis. 2007;24(5):1250–1265.
132 Kohnen S. Light-induced damage of the retina through slit-lamp photography. Graefes Arch Clin Exp Ophthalmol. 2000;238(12):956–959.
133 Cideciyan AV, Jacobson SG, Aleman TS, et al. In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa. Proc Natl Acad Sci U S A. 2005;102(14):5233–5238.
134 Berson EL. Light deprivation and retinitis pigmentosa. Vision Res. 1980;20(12):1179–1184.
135 van der Hoeve J. Eye lesions produced by light rich in ultraviolet rays: senile cataract, senile degeneration of the macula. Am J Ophthalmol. 1920;3:178–194.
136 Ts’o MO, La Piana FG, Appleton B. The human fovea after sungazing. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:OP-677.
137 Mainster MA. Solar retinitis, photic maculopathy and the pseudophakic eye. J Am Intraocul Implant Soc. 1978;4(3):84–86.
138 Young RW. A theory of central retinal disease. In: Sears ML, ed. New directions in ophthalmic research. New Haven, CT: Yale University Press, 1981.
139 Margrain TH, Boulton M, Marshall J, et al. Do blue light filters confer protection against age-related macular degeneration? Prog Retin Eye Res. 2004;23(5):523–531.
140 McCarty CA, Mukesh BN, Fu CL, et al. Risk factors for age-related maculopathy: the Visual Impairment Project. Arch Ophthalmol. 2001;119(10):1455–1462.
141 Taylor HR, West S, Munoz B, et al. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110(1):99–104.
142 Dong LM, Stark WJ, Jefferys JL, et al. Progression of age-related macular degeneration after cataract surgery. Arch Ophthalmol. 2009;127(11):1412–1419.
143 Chew EY, Sperduto RD, Milton RC, et al. Risk of advanced age-related macular degeneration after cataract surgery in the Age-Related Eye Disease Study: AREDS report 25. Ophthalmology. 2009;116(2):297–303.
144 Baatz H, Darawsha R, Ackermann H, et al. Phacoemulsification does not induce neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(3):1079–1083.
145 Sutter FK, Menghini M, Barthelmes D, et al. Is pseudophakia a risk factor for neovascular age-related macular degeneration? Invest Ophthalmol Vis Sci. 2007;48(4):1472–1475.
146 Xu L, Li Y, Zheng Y, et al. Associated factors for age related maculopathy in the adult population in China: the Beijing eye study. Br J Ophthalmol. 2006;90(9):1087–1090.
147 Sliney DH. Photoprotection of the eye – UV radiation and sunglasses. J Photochem Photobiol B. 2001;64(2–3):166–175.
148 Abraham AG, Cox C, West S. The differential effect of ultraviolet light exposure on cataract rate across regions of the lens. Invest Ophthalmol Vis Sci. 2011;51(8):3919–3923.
149 Rabin JC, Wiley RW, Levine RR, et al. U.S. Army sunglasses: issues and solutions. J Am Optom Assoc. 1996;67(4):215–222.
150 American National Standard for the Safe Use of Lasers, ANSI Z136.1-2007. Washington, DC: American National Standards Institute, 2007.
151 Sliney DH, Wolborsht ML. Safety standards and measurement techniques for high intensity light sources. Vision Res. 1980;20(12):1133–1141.
152 American National Standard for the Safe Use of Lasers in Health Care Facilities, ANSI Z136.3-2005. Washington, DC: American National Standards Institute, 2005.
153 Safety of Laser Products–Part 1: Equipment classification, requirements and user’s guide, IEC 60825-1, Ed. 2.0b. Geneva, Switzerland: International Electrotechnical Commission, 2008.
154 Sliney DH, Trokel SL. Medical lasers and their safe use. New York: Springer-Verlag; 1993.
155 Sliney DH, Mainster MA. Ophthalmic laser safety: tissue interactions, hazards and protection. Ophthalmol Clin North Am. 1998;11(2):157–164.
156 Performance Standards for Light-Emitting Products, 21CFR1040. Washington, DC: Center for Devices and Radiological Health, United States Food and Drug Administration, 2011.