Chapter 33 PHAs and Bionic Acids: Next Generation Hydroxy Acids
INTRODUCTION
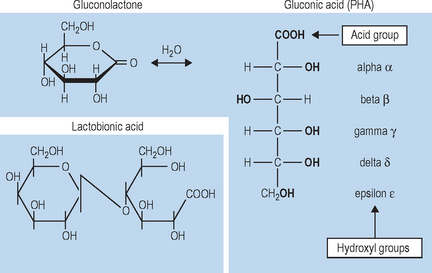
• The PHA and bionic structure
Polyhydroxy acids and bionic acids are organic carboxylic acids, which possess two or more hydroxyl groups on an aliphatic or alicyclic molecular structure. When one of the hydroxyl groups occurs in the alpha position, the PHA is a polyhydroxy AHA Fig. 33.1; when an additional sugar is attached to the PHA structure, the molecule is a bionic acid (Fig. 33.1). Because they share the common AHA structure, PHAs and bionics have the ability to provide skin effects similar to traditional AHAs, such as glycolic acid.
GLUCONOLACTONE: A REPRESENTATIVE PHA
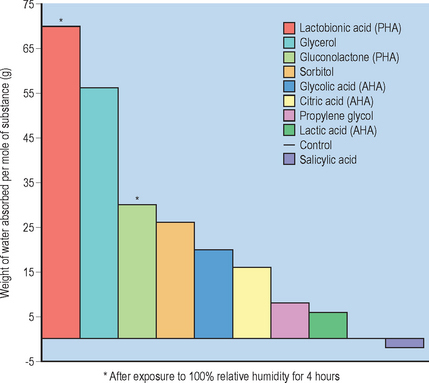
Gluconolactone (gluconic acid delta lactone) is a nontoxic, naturally occurring component of the skin. The molecule’s somewhat larger size (molecular weight 178 vs. 76 for glycolic acid) facilitates a gradual penetration into skin, thus minimizing irritation. The smaller molecule of glycolic acid penetrates the skin more rapidly, often causing stinging and burning. Gluconolactone’s potential for increased hydration is attributed to humectant properties of the multiple hydroxyl groups, which can attract and hydrogen bond water Fig. 33.2.
• Antioxidant and free radical scavenging effects of gluconolactone
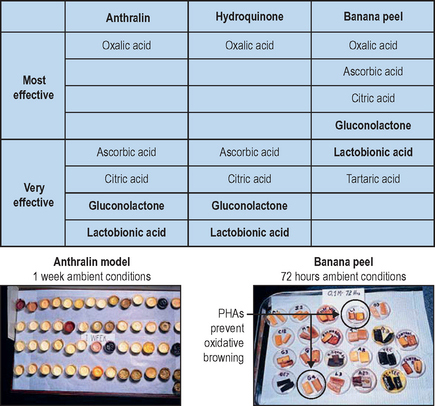
The antioxidant properties of gluconolactone are evident in food and drug substances, in which gluconolactone has been shown to inhibit oxidation and help maintain product integrity Fig. 33.3.
• Clinical effects of gluconolactone
In vivo clinical studies of gluconolactone-containing formulations have demonstrated measurable benefits including antiaging and skin-firming effects that are comparable to commonly used AHAs (e.g. glycolic acid), with reduced irritation potential. In addition, significant antiaging benefits including reduced pigmentation and improved skin texture have been observed over a range of darker Fitzpatrick skin types (IV–VI) represented by a study population of African–Americans, Asians, and Hispanics.
Gluconolactone is well suited for adjunctive use with topical medications. It enhances epidermal cell turnover, which may in part help to explain the adjunctive benefits of PHAs in the treatment of acne. A vehicle-controlled, double-blind clinical study on mild to moderate acne demonstrated antiacne effects with less irritation of a 14% gluconolactone solution in comparison to a 5% benzoyl peroxide lotion. In addition, PHAs can be used to hydrate and condition skin during treatment with drying or irritating medications including topical retinoids, benzoyl peroxide, and azelaic acid. This benefit was demonstrated in a clinical study designed to evaluate the effects of standardized adjunctive use of a PHA cleanser plus PHA moisturizer in combination with 15% azelaic acid gel for the treatment of rosacea. Results indicated significantly greater improvements in global assessment and erythema relative to the azelaic acid treatment group that had not been standardized to adjunctive PHAs (P < 0.05). Compatibility of a 15% gluconolactone-containing cream (pH 3.3) in combination with daily use of tretinoin gel 0.1% has also been demonstrated.
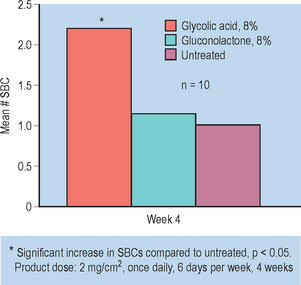
Skin barrier strengthening effects of PHAs were evaluated in a vehicle-controlled, double-blind clinical study. Unlike AHAs (glycolic acid and lactic acid), antioxidant PHAs (i.e. gluconolactone) were shown to strengthen stratum corneum barrier function by reducing transepidermal water loss and erythema assessed via colorimetry following a chemical challenge. Clinically, this finding may help to explain data that revealed a lack of increased sun sensitivity with PHA use. The PHAs gluconolactone and glucoheptonolactone did not cause an increase in the formation of sunburn cells following exposure to UVB in the testing model employed by the Personal Care Products Council (formerly the Cosmetic, Toiletry, and Fragrance Association) and the Food and Drug Administration (FDA) Fig. 33.4.
LACTOBIONIC ACID: A POLYHYDROXY BIONIC ACID
Lactobionic acid (molecular weight 358, pKa 3.8) is a polyhydroxy bionic acid formed by the oxidation of lactose (milk sugar). It is composed of one molecule of the sugar D-galactose and one molecule of D-gluconic acid (i.e. gluconolactone, a PHA). Lactobionic acid is termed a bionic acid due to the attachment of an additional sugar unit to the traditional PHA (gluconolactone) structure (see Fig. 33.1).
One component of the lactobionic acid molecule is gluconic acid, a PHA with multiple beneficial effects on skin as described above. Galactose, the second component of lactobionic acid, is a naturally occurring sugar that is utilized by human skin during dermal proteoglycan and procollagen synthesis. In addition, in vitro wound healing studies indicate that galactose may play a role in wound healing.
• Antioxidant/chelation effects of lactobionic acid
The antioxidant properties of lactobionic acid have also been studied in food and drug substances, demonstrating the capacity to inhibit the oxidation of readily oxidizable drugs including anthralin and hydroquinone, as well as banana peels (see Fig. 33.3). Since oxidation and UV-induced free radicals are a cause of skin aging, the potent antioxidant benefits of lactobionic acid may play an important role in its antiaging effects.
• Clinical effects of lactobionic acid
Lactobionic acid functions similarly to AHAs in providing antiaging and enhanced cell turnover effects. Lactobionic acid provides benefits to skin beyond traditional AHAs as a result of its unique chemical structure and molecular composition. Due to its multiple hydroxyl groups, lactobionic acid is a strong humectant, with the ability to attract and retain water better than common humectants including glycerin and sorbitol (see Fig. 33.2). It also forms a unique gel matrix during the drydown process as a result of its strong water retention properties.
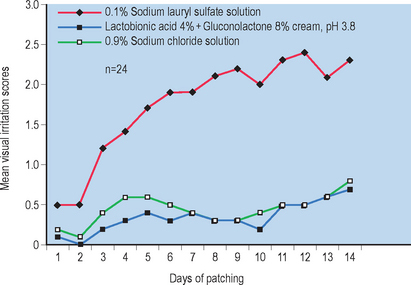
PHA formulations of lactobionic acid in combination with gluconolactone are nonirritating Fig. 33.5. PHA/bionic combinations have also exhibited strong antiaging effects including marked improvements in skin clarity (260%, P < 0.05, n = 26) and notable skin plumping effects (9.7%, P < 0.05, n = 26).
A second independent study of lactobionic acid formulated in a simple cream base (8%, pH 3.8) was conducted to evaluate antiaging effects over a 12-week period in comparison to: (1) baseline condition on the face, and (2) an untreated control for forearm skin thickness measurements. Thirty-one female participants completed the study; statistically significant effects were evident at both 6 and 12 weeks (P < 0.05). Notably, skin clarity was improved by 100% and appearance of fine lines was reduced by 37%. Skin firmness and elasticity via the pinch recoil model increased by 14.5% at 12 weeks. Forearm skin thickness increased 7%, demonstrating a statistically significant response relative to baseline and the untreated control forearm. Histology specimens revealed an increase in viable epidermal thickness and dermal glycosaminoglycans, as well as reduced staining of MMP9 enzymes, demonstrating both preventive and corrective antiaging effects.
MALTOBIONIC ACID: A PLANT-DERIVED BIONIC ACID
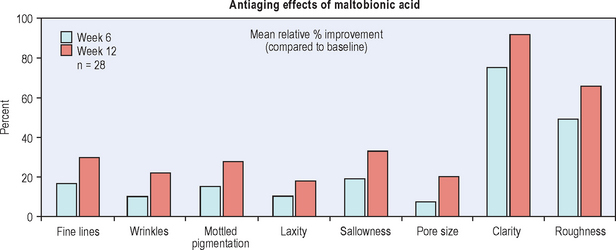
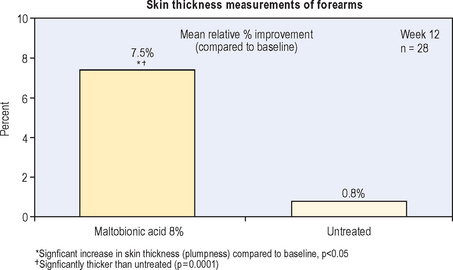
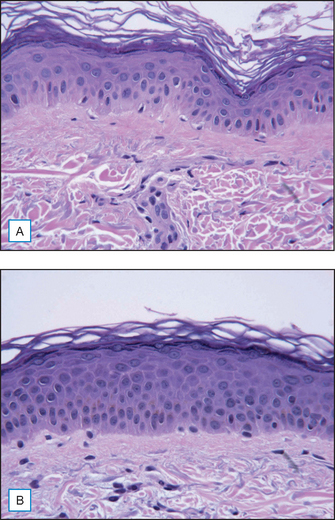
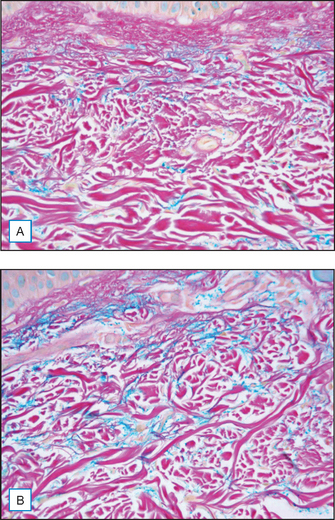
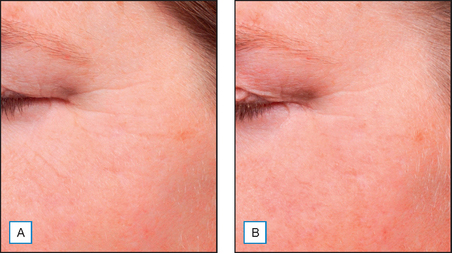
A cream containing 8% maltobionic acid, pH 3.8, was evaluated for antiaging effects over a 12-week period in comparison to: (1) baseline skin condition on the face, and (2) an untreated control for forearm skin thickness measurements. Twenty-eight female participants completed the study; statistically significant effects were evident at both 6 and 12 weeks (P < 0.05), including a 92% improvement in clarity, 30% improvement in fine lines, and 66% improvement in skin roughness Fig. 33.6. Skin firmness and elasticity via the pinch recoil model increased by 14.0% at 12 weeks. Forearm skin thickness increased 7.5%, demonstrating a statistically significant response relative to baseline and the untreated control forearm Fig. 33.7. Histology specimens revealed benefits to skin including increases in viable epidermal thickness Fig. 33.8 and dermal glycosaminoglycans (GAGs) Fig. 33.9. As dermal GAGs increase in content, the water-binding capacity of the skin increases and the skin becomes plumper, building volume in skin resulting in a smoother appearance. Clinical photographs revealed younger-looking skin with reduced periocular fine lines and smoother skin texture Fig. 33.10.
Berardesca E, Distante F, Vignoli GP, Oresajo C, Green B. Alpha hydroxy acids modulate stratum corneum barrier function. British Journal of Dermatology. 1997;137:934–938.
Bernstein EF, Brown DB, Schwartz MD, Kaidbey K, Ksenzenko SM. The polyhydroxy acid gluconolactone protects against ultraviolet radiation in an in vitro model of cutaneous photoaging. Dermatologic Surgery. 2004;30:1–8.
Briden E, Jacobsen E, Johnson C. Combining superficial glycolic acid (AHA) peels with microdermabrasion to maximize treatment results and patient satisfaction. Cutis. 2007;79(Suppl 1[i]):13–16.
Charloux C, Paul M, Loisance D, Astier A. Inhibition of hydroxyl radical production by lactobionate, adenine, and tempol. Free Radicals in Biology and Medicine. 1995;19:699–704.
Draelos ZD, Green BA, Edison BL. An evaluation of a polyhydroxy acid skin care regimen in combination with azelaic acid 15% gel in rosacea patients. Journal of Cosmetic Dermatology. 2006;5:23–29.
Edison BL, Green BA, Wildnauer RH, Sigler ML. A polyhydroxy acid skin care regimen provides antiaging effects comparable to an alpha-hydroxy acid regimen. Cutis. 2004;73(Suppl 2):14–17.
Effron C, Briden ME, Green BA. Enhancing cosmetic outcomes by combining superficial glycolic acid (AHA) peels with nonablative lasers, intense pulsed light, and trichloroacetic acid peels. Cutis. 2007;79(Suppl 1[i]):4–8.
Green BA, Edison BL, Wildnauer RH, Sigler ML. Lactobionic acid and gluconolactone: PHAs for photoaged skin. Cosmetic Dermatology. 2001;14:24–28.
Green BA, Edison BL, Sigler ML. Antiaging effects of topical lactobionic acid: results of a controlted usage study. Cosmetic Dermatology. 2008;21(Suppl 2):76–82.
Grimes PE, Green BA, Wildnauer RH, Edison BL. The use of polyhydroxy acids (PHAs) in photoaged skin. Cutis. 2004;73(Suppl 2):3–13.
Hunt MJ, Barnetson R StC. A comparative study of gluconolactone versus benzoyl peroxide in the treatment of acne. Australian Journal of Dermatology. 1992;33:131–134.
Kossi J, Peltonen J, Ekfors T, Niinikoski J, Laato M. Effects of hexose sugars: glucose, fructose, galactose and mannose on wound healing in the rat. European Surgical Research. 1999;31:74–82.
Rendon MI, Effron C, Edison BL. The use of fillers and botulinum toxin type A in combination with superficial glycolic acid (AHA) peels: optimizing injection therapy with the skin-smoothing properties of peels. Cutis. 2007;79(Suppl 1[i]):9–12.
Thibodeau A. Metalloproteinase inhibitors. Cosmetics and Toiletries. 2000;115:75–76.
Upadhya GA, Strasberg SM. Glutathione, lactobionate, and histidine: cryptic inhibitors of matrix metalloproteinases contained in University of Wisconsin and histidine/tryptophan/ketoglutarate liver preservation solutions. Hepatology. 2000;31:1115–1122.
Van Scott EJ, Yu RJ. Hydroxy acids: past, present, future. In: Moy R, Luftman D, Kakita L, editors. Glycolic acid peels. New York: Marcel Dekker; 2002:1–14.
Wilhelmi BJ, Blackwell SJ, Mancoll JS, Phillips LG. Creep vs. stretch: a review of the viscoelastic properties of skin. Annals of Plastic Surgery. 1998;41:215–219.
Yu RJ, Van Scott EJ. α-Hydroxyacids, polyhydroxy acids, aldobionic acids and their topical actions. In: Baran R, Maibach HI, editors. Textbook of cosmetic dermatology. 3rd edn. New York: Taylor & Francis; 2005:77–93.