Chapter 7 Pharmacology of Anesthetic Drugs
VOLATILE AGENTS
Acute Effects
Myocardial Function
The influence of volatile anesthetics on contractile function has been investigated extensively, and it is now widely agreed that volatile agents cause dose-dependent depression of contractile function (Box 7-1). Moreover, different volatile agents are not identical in this regard and the preponderance of information indicates that halothane and enflurane exert equal but more potent myocardial depression than do isoflurane, desflurane, or sevoflurane.1 This reflects in part reflex sympathetic activation with the latter agents. It is also widely accepted that in the setting of preexisting myocardial depression, volatile agents have a greater effect than in normal myocardium. At the cellular level, volatile anesthetics exert their negative inotropic effects mainly by modulating sarcolemmal (SL) L-type Ca2+ channels, the sarcoplasmic reticulum (SR), and the contractile proteins. However, the mechanisms whereby anesthetic agents modify ion channels are not completely understood.
BOX 7-1 Volatile Anesthetic Agents
Coronary Vasoregulation
The effect of isoflurane on coronary vessels was controversial and dominated much of the literature in this area in the 1980s and early 1990s. The current assessments of the effects of isoflurane have been succinctly detailed by Tanaka and associates.2 Several reports had indicated that it caused direct coronary arteriolar vasodilatation in vessels of 100 μm or less and that isoflurane could cause “coronary steal” in patients with “steal-prone” coronary anatomy. Several studies in which potential confounding variables were controlled indicated clearly that isoflurane did not cause coronary steal. Studies of sevoflurane and desflurane showed similar results and are consistent with a mild direct coronary vasodilator effect of these agents.
Baroreceptor Reflex
All volatile agents attenuate the baroreceptor reflex. Baroreceptor reflex inhibition by halothane and enflurane is more potent than that observed with isoflurane, desflurane, or sevoflurane, each of which has a similar effect. Each component of the baroreceptor reflex arc (afferent nerve activity, central processing, efferent nerve activity) is inhibited by volatile agents.
Delayed Effects
Reversible Myocardial Ischemia
Prolonged ischemia results in irreversible myocardial damage and necrosis (Box 7-2). Shorter durations of myocardial ischemia can, depending on the duration and sequence of ischemic insults, lead to either preconditioning or myocardial stunning (Fig. 7-1). Stunning, first described in 1975, occurs after brief ischemia and is characterized by myocardial dysfunction in the setting of normal restored blood flow and by an absence of myocardial necrosis. Ischemic preconditioning (IPC) was first described by Murray and colleagues in 1986 and is characterized by an attenuation in infarct size after sustained ischemia, if this period of sustained ischemia is preceded by a period of brief ischemia. Moreover, this effect is independent of collateral flow. Thus, short periods of ischemia followed by reperfusion can lead to either stunning or preconditioning with a reduction in infarct size (Fig. 7-2).3
BOX 7-2 Volatile Agents and Myocardial Ischemia
Anesthetic Preconditioning
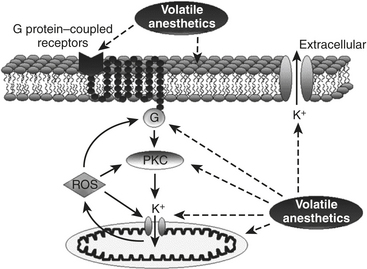
Volatile agents can elicit delayed (late), as well as classic (early), preconditioning. Moreover, APC is dose dependent, exhibits synergy with ischemia in affording protection, and, perhaps not surprisingly in view of differential uptake and distribution of volatile agents, has been demonstrated to require different time intervals between exposure and the maintenance of a subsequent benefit that is agent dependent. Volatile agents that exhibit APC activate mitochondrial K+ATP channels, and this effect is blocked by specific mitochondrial K+ATP channel antagonists. However, the precise relative contributions of SL versus mitochondrial K+ATP channel activation to APC remain to be elucidated (Fig. 7-3).
INTRAVENOUS INDUCTION AGENTS
Acute Cardiac Effects
Myocardial Contractility
The effect of drugs such as propofol may also be markedly affected by the underlying myocardial pathology. For instance, Sprung and coworkers determined the direct effects of propofol on the contractility of human nonfailing atrial and failing atrial and ventricular muscles obtained from the failing human hearts of transplant patients or from nonfailing hearts of patients undergoing coronary artery bypass graft (CABG) surgery.4 They concluded that propofol exerts a direct negative inotropic effect in nonfailing and failing human myocardium but only at concentrations larger than typical clinical concentrations. Negative inotropic effects are reversible with β-adrenergic stimulation, suggesting that propofol does not alter the contractile reserve but may shift the dose responsiveness to adrenergic stimulation.
Vasculature
As with the heart, the cumulative physiologic effects in the vasculature represent a summation of the effects of the agents on the central autonomic nervous system, as well as the direct effects of these agents on the vascular smooth muscle, and the modulating effects on the underlying endothelium. It is now well established that propofol decreases SVR in humans. This was demonstrated in a patient with an artificial heart in whom the CO remained fixed. The effect is predominantly mediated by alterations in sympathetic tone; however, in isolated arteries, propofol decreases vascular tone and agonist-induced contraction. The mechanism by which propofol mediates these effects has been attributed in part to inhibition of Ca2+ influx through voltage or receptor-gated Ca2+ channels, as well as inhibition of Ca2+ release from intracellular Ca2+ stores.
INDIVIDUAL AGENTS
Thiopental
Thiopental has survived the test of time as an intravenous anesthetic drug (Box 7-3). Since Lundy introduced it in 1934, thiopental was the most widely used induction agent because of the rapid hypnotic effect (one arm-to-brain circulation time), highly predictable effect, lack of vascular irritation, and general overall safety. The induction dose of thiopental is lower for older than for younger healthy patients. Pharmacokinetic analyses confirm that the awakening from thiopental is due to rapid redistribution. Thiopental has a distribution half-life (t½α) of 2.5 to 8.5 minutes, and the total body clearance varies, according to sampling times and techniques, from 0.15 to 0.26 L/kg/hr. The elimination half-life (t½β) varies from 5 to 12 hours. Barbiturates and propofol have increased volumes of distribution (Vd) when used during cardiopulmonary bypass (CPB).
BOX 7-3 Intravenous Anesthetics
Midazolam
There are only small hemodynamic changes after the intravenous administration of midazolam.
Cardiovascular Effects
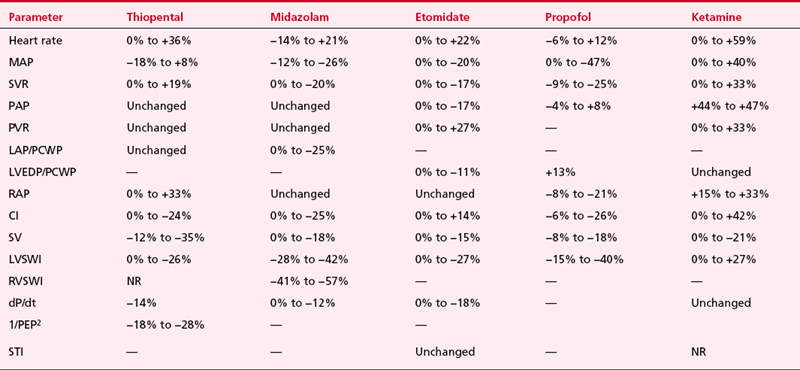
The hemodynamic changes produced by thiopental have been studied in normal patients and in patients with cardiac disease (Table 7-1). The principal effect is a decrease in contractility, which results from reduced availability of calcium to the myofibrils. There is also an increase in HR. The cardiac index (CI) is unchanged or reduced, and the mean aortic pressure (MAP) is maintained or slightly reduced. In the dose range studied, no relationship between plasma thiopental and hemodynamic effect has been found.
Mechanisms for the decrease in CO include (1) direct negative inotropic action, (2) decreased ventricular filling, resulting from increased venous capacitance, and (3) transiently decreased sympathetic outflow from the central nervous system. The increase in HR (10% to 36%) that accompanies thiopental administration probably results from the baroreceptor-mediated sympathetic reflex stimulation of the heart. Thiopental produces dose-related negative inotropic effects that appear to result from a decrease in calcium influx into the cells with a resultant diminished amount of calcium at sarcolemma sites. Patients who had compensated heart disease and received 4 mg/kg of thiopental had a greater (18%) BP drop than did other patients. The increase in HR (11% to 36%) encountered in patients with coronary artery disease (CAD), anesthetized with thiopental (1 to 4 mg/kg), is potentially deleterious because of the obligatory increase in myocardial oxygen consumption (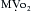 ).
).
Uses in Cardiac Anesthesia
An additional use for thiopental infusion is cerebral protection during CPB in patients undergoing selected cardiac operations. However, the cerebral protective effect of thiopental during CPB has been challenged by Zaidan and associates,5 who demonstrated no differences in outcome between thiopental and control patients undergoing hypothermic CPB for CABG. Although the administration of a barbiturate during CPB may result in myocardial depression, necessitating additional inotropic support, a study by Ito and colleagues suggested beneficial effects of a thiopental infusion during CPB in maintaining peripheral perfusion, which allowed more uniform warming, decreased base deficit, and decreased requirements for postoperative pressor support.
Midazolam
Cardiovascular Effects
The hemodynamic effects of midazolam have been investigated in normal subjects, in ASA Class III patients, and in patients who have ischemic and valvular heart disease (VHD). Table 7-1 summarizes the hemodynamic changes after induction of anesthesia with midazolam. In general, there are only small hemodynamic changes after the intravenous administration of midazolam (0.2 mg/kg) in premedicated patients who have coronary artery disease (CAD). Changes of potential importance include a decrease in MAP of 20% and an increase in HR of 15%. The CI is maintained. Filling pressures are either unchanged or decreased in patients who have normal ventricular function but are significantly decreased in patients who have an elevated PCWP (18 mm Hg or higher). As in patients with ischemic heart disease, the induction of anesthesia in patients with VHD is associated with minimal changes in CI, HR, and MAP after midazolam. When intubation follows anesthesia induction with midazolam, significant increases in HR and BP occur, because midazolam is not an analgesic. Adjuvant analgesic drugs are required to block the response to noxious stimuli.
Midazolam (0.15 mg/kg) and ketamine (1.5 mg/kg) have proved to be a safe and useful combination for a rapid-sequence induction for emergency surgery. This combination was superior to thiopental alone, because it caused less cardiovascular depression, more amnesia, and less postoperative somnolence. If midazolam is given to patients who have received fentanyl, significant hypotension may occur, as seen with diazepam and fentanyl. However, midazolam is routinely combined with fentanyl for induction and maintenance of general anesthesia during cardiac surgery without adverse hemodynamic sequelae.6,7
Etomidate
Cardiovascular Effects
Etomidate (0.3 mg/kg IV), used to induce general anesthesia in patients with acute myocardial infarction undergoing percutaneous coronary angioplasty, did not alter HR, MAP, and rate-pressure product (RPP), demonstrating the remarkable hemodynamic stability of this agent.8 However, the presence of VHD may influence the hemodynamic responses to etomidate. Whereas most patients can maintain their BP, patients with both aortic and mitral VHD had significant decreases of 17% to 19% in systolic and diastolic BP and decreases of 11% and 17% in PAP and PCWP, respectively. CI in patients who had VHD and received 0.3 mg/kg either remained unchanged or decreased 13%. There was no difference in response to etomidate between patients who had aortic valve disease and those who had mitral valve disease.
Ketamine
Cardiovascular Effects
The hemodynamic effects of ketamine have been examined in noncardiac patients, critically ill patients, geriatric patients, and patients who have a variety of heart diseases. Table 7-1 contains the range of hemodynamic responses to ketamine. One unique feature of ketamine is stimulation of the cardiovascular system. The most prominent hemodynamic changes are significant increases in HR, CI, SVR, PAP, and MAP. These circulatory changes cause an increase in 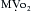 with an apparently appropriate increase in coronary blood flow (CBE). A second dose of ketamine produces hemodynamic effects opposite to those of the first. Thus, the cardiovascular stimulation seen after ketamine induction of anesthesia (2 mg/kg) in a patient who has VHD is not observed with the second administration, which is accompanied instead by decreases in the BP, PCWP, and CI.
with an apparently appropriate increase in coronary blood flow (CBE). A second dose of ketamine produces hemodynamic effects opposite to those of the first. Thus, the cardiovascular stimulation seen after ketamine induction of anesthesia (2 mg/kg) in a patient who has VHD is not observed with the second administration, which is accompanied instead by decreases in the BP, PCWP, and CI.
Propofol
Cardiovascular Effects
The hemodynamic effects of propofol have been investigated in healthy ASA Class I and II patients, elderly patients, patients with CAD and good left ventricular function, and in patients with impaired left ventricular function (see Table 7-1). Numerous studies have also compared the cardiovascular effects of propofol with other commonly used induction drugs, including the thiobarbiturates and etomidate. It is clear that with propofol, systolic arterial pressure falls 15% to 40% after intravenous induction with 2 mg/kg and maintenance infusion with 100 μg/kg/min. Similar changes are seen in both diastolic arterial pressure and MAP.
The effect of propofol on HR is variable. The majority of studies have demonstrated significant reductions in SVR (9% to 30%), CI, SV, and left ventricular stroke work index (LVSWI) after propofol. Although controversial, the evidence points to a dose-dependent decrease in myocardial contractility.9,10
OPIOIDS IN CARDIAC ANESTHESIA
Opioid Receptors
The existence of separate opioid receptors was shown by correlating analgesic activity to the chemical structure of many opioid compounds (Box 7-4). The idea of multiple opioid receptors is an accepted concept, and a number of subtypes for each class of opioid receptors have been identified. Through biochemical and pharmacologic methods, the μ-, δ-, and κ-receptors have been characterized. Pharmacologically, it is well known that δ-opioid receptors consist of two subtypes: δ1 and δ2.11
BOX 7-4 Opioids
Opioid receptors involved in regulating the cardiovascular system have been localized centrally to the cardiovascular and respiratory centers of the hypothalamus and brainstem and peripherally to cardiac myocytes, blood vessels, nerve terminals, and the adrenal medulla. It is generally accepted that opioid receptors are differentially distributed between atria and ventricles. The highest specific receptor density for binding of κ-agonists is in the right atrium and least in the left ventricle. As with the κ-opioid receptor, the distribution of the δ-opioid receptor favors atrial tissue and the right side of the heart more than the left.
Cardiac Effects of Opioids
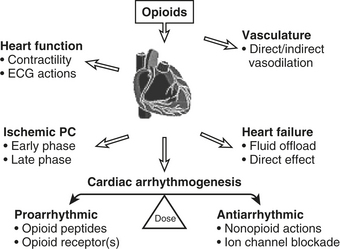
At clinically relevant doses, the cardiovascular actions of opioids are limited. The actions opioids exhibit are mediated both by opioid receptors located centrally in specific areas of the brain and nuclei that regulate the control of cardiovascular function and peripherally by tissue-associated opioid receptors. The opioids in general exhibit a variety of complex pharmacologic actions on the cardiovascular system (Fig. 7-4).12
Hypotension can occur after even small doses of morphine and is primarily related to decreases in SVR. The most important mechanism responsible for these changes is probably histamine release. The amount of histamine release is reduced by slow administration (<10 mg/min). Pretreatment with an H1 or H2 antagonist does not block these reactions, but they are significantly attenuated by combined H1 and H2 antagonist pretreatment. Opioids may also have a direct action on vascular smooth muscle, independent of histamine release.13
Cardioprotective Effects of Exogenous Opioid Agonists
In 1996, Schultz and colleagues were the first to demonstrate that an opioid could attenuate ischemia-reperfusion damage in the heart. Morphine at the dose of 300 μg/kg was given before left anterior descending coronary artery occlusion for 30 minutes in rats in vivo. Infarct area/area at risk was diminished from 54% to 12% by this treatment.14 The infarct-reducing effect of morphine has been shown in hearts in situ, isolated hearts, and cardiomyocytes. Morphine also improved postischemic contractility. It is now well accepted that morphine provides protection against ischemia-reperfusion injury.15 Fentanyl has been studied in a limited fashion and has had mixed results as far as its ability to protect the myocardium.16 This may be due to differences in species studied and/or fentanyl concentrations.
Opioids in Cardiac Anesthesia
A major advantage of fentanyl and its analogs for patients undergoing cardiac surgery is their lack of cardiovascular depression.17 This is of particular importance during the induction of anesthesia, when episodes of hypotension can be critical. Cardiovascular stability may be less evident during surgery; in particular, the period of sternotomy, pericardiectomy, and aortic root dissection may be associated with significant hypertension and tachycardia. During and after sternotomy, arterial hypertension, increases in SVR, and decreases in CO frequently occur. The variability in the hemodynamic responses to surgical stimulation, even with similar doses of fentanyl, is probably a reflection of differences in the patient populations studied by different authors. One factor is the influence of β-blocking agents. In patients undergoing CABG anesthetized with fentanyl, 86% of those not taking β-adrenergic blockers became hypertensive during sternal spread versus only 33% of those who were taking these agents.
EFFECTS OF CARDIOPULMONARY BYPASS ON PHARMACOKINETICS AND PHARMACODYNAMICS
The institution of cardiopulmonary bypass has profound effects on the plasma concentration, distribution, and elimination of administered drugs. The major factors responsible for this are hemodilution and altered plasma protein binding, hypotension, hypothermia, pulsatile versus nonpulsatile flow, isolation of the lungs from the circulation, and uptake of anesthetic drugs by the bypass circuit. These changes result in altered blood concentrations, which are also dependent on particular pharmacokinetics of the drug in question.18
Sequestration
Many drugs bind to components of the CPB circuit, and their distribution may be affected by changes in circuit design, for example, the use of membrane versus bubble oxygenators. In vitro, various oxygenators bind lipophilic agents such as volatile anesthetic agents, propofol, opioids, and barbiturates.19,20
SUMMARY
1. Harkin C.P., Pagel P.S., Kersten J.R., et al. Direct inotropic and lusitropic effects of sevoflurane. Anesthesiology. 1994;72:659.
2. Tanaka K., Ludwig L.M., Kersten J.R., et al. Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology. 2004;100:707.
3. Kloner R.A., Jennings R.B. Consequences of brief ischemia: Stunning, preconditioning, and their clinical implications: I. Circulation. 2001;104:2981.
4. Sprung J., Ogletree-Hughes M.L., McConnell B.K., et al. The effects of propofol on the contractility of failing and nonfailing human heart muscle. Anesth Analg. 2001;93:550.
5. Zaidan J., Klochany A., Martin W., et al. Effect of thiopental on neurologic outcome following coronary artery bypass grafting. Anesthesiology. 1991;74:406.
6. Newman M., Reves J. Pro: Midazolam is the sedative of choice to supplement narcotic anesthesia. J Cardiothorac Vasc Anesth. 1993;7:615.
7. Theil D., Stanley T., White W., et al. Midazolam and fentanyl continuous infusion anesthesia for cardiac surgery: A comparison of computer-assisted versus manual infusion systems. J Cardiothorac Vasc Anesth. 1993;7:300.
8. Kates R., Stack R., Hill R., et al. General anesthesia for patients undergoing percutaneous transluminal coronary angioplasty during acute myocardial infarction. Anesth Analg. 1986;65:815.
9. de Hert S., Vermeyen K., Adriensen H. Influence of thiopental, etomidate and propofol on regional myocardial function in the normal and acute ischemic heart segments. Anesth Analg. 1990;70:600.
10. Mulier J., Wouters P., van Aken H., et al. Cardiodynamic effects of propofol in comparison with thiopental: assessment with a transesophageal echocardiographic approach. Anesth Analg. 1991;72:28.
11. McDonald J., Lambert D. Opioid receptors. Cont Educ. Anaesth Crit Care Pain. 2005;5:1.
12. Barron B.A. Opioid peptides and heart. Cardiovasc Res. 1999;43:13.
13. White D.A., Reitan J.A., Kien N.D., et al. Decrease in vascular resistance in the isolated canine hindlimb after graded doses of alfentanil, fentanyl, and sufentanil. Anesth Analg. 1990;71:29.
14. Schultz J.J., Hsu A.K., Gross G.J. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibenclamide-sensitive mechanism in the rat heart. Circ Res. 1996;78:1100.
15. Benedict P.E., Benedict M.B., Su T.P., et al. Opiate drugs and delta-receptor–mediated myocardial protection. Circulation. 1999;100(19 suppl):II-357.
16. Kato R., Ross S., Foëx P. Fentanyl protects the heart against ischemic injury via opioid receptors, adenosine A1 receptors and KATP channel linked mechanism in rats. Br J Anaesth. 2000;84:204.
17. Howie M.B., Cheng D., Newman M.F., et al. A randomized double-blinded multicenter comparison of remifentanil versus fentanyl when combined with isoflurane/propofol for early extubation in coronary artery bypass graft surgery. Anesth Analg. 2001;92:1084.
18. Wood M. Pharmacokinetics and principles of drug infusions in cardiac patients. In: Kaplan J.A., editor. Cardiac Anesthesia. 4th ed. Philadelphia: WB Saunders; 1999:657-685.
19. Hickey S., Goylor J.D., Kenny G.N. In vitro uptake and elimination of isoflurane by different membrane oxygenators. J Cardiothorac Vasc Anesth. 1996;10:352.
20. Rosen D.A., Rosen K.R. Elimination of drugs and toxins during cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:337.



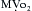 with an appropriate increase in coronary blood flow.
with an appropriate increase in coronary blood flow.