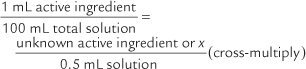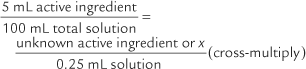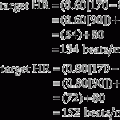9 Pharmacology
Note 1: This book is written to cover every item listed as testable on the Entry Level Examination (ELE), Written Registry Examination (WRE), and Clinical Simulation Examination (CSE).
The listed code for each item is taken from the National Board for Respiratory Care’s (NBRC) Summary Content Outline for CRT (Certified Respiratory Therapist) and Written RRT (Registered Respiratory Therapist) Examinations (http://evolve.elsevier.com/Sills/resptherapist/). For example, if an item is testable on both the ELE and the WRE, it will simply be shown as: (Code: …). If an item is only testable on the ELE, it will be shown as: (ELE code: …). If an item is only testable on the WRE, it will be shown as: (WRE code: …).
MODULE B
1. Administer medications
2. Bronchodilators
a. Inhaled adrenergic (sympathomimetic) agents
1. Recommend their use (ELE code: IIIG4a) [Difficulty: ELE: R, Ap, An]
2. Administer the prescribed medication (Code: IIIC3, IIID5a, IIID5b) [Difficulty: ELE: R, Ap; WRE: An]
a. Acute bronchospasm with severe shortness of breath.
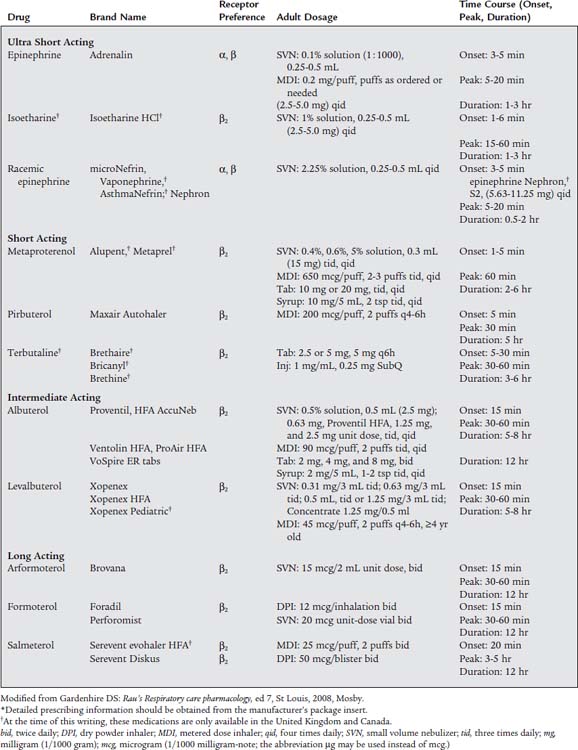
This patient needs rapid relief. Recommend a fast-acting medication such as albuterol. The current asthma-management guidelines list albuterol and similar medications as “rescue” agents. Because these drugs tend to have a shorter duration of action they can be referred to as short-acting beta-agonist (SABA) medications. Table 9-1 lists the peak onset times and duration for the various medications. Avoid drugs with unnecessary α1 and β1 effects or long onset and peak times.
b. Chronic but stable bronchospasm with moderate shortness of breath.
These patients need a dependable medication of longer duration such as salmeterol or formoterol. They are considered to be “controller” agents with a long onset time and a duration of up to 12 hours. Because of their longer duration they can be called long-acting beta-agonist (LABA) medications. Several newer medications also come in both oral and aerosol preparations. The oral forms are especially helpful when taken in the evening to help the patient get a good night’s sleep. It is very important that the patient also have a prescription for a fast-acting drug in case of sudden bronchospasm. Table 9-1 lists information on the administration method, strength, and dosages.
c. Laryngeal edema or bleeding from a bronchoscopy biopsy site.
The laryngeal edema problem requires the administration of a medication that reduces the swelling of the mucous membrane of the larynx and epiglottis. Laryngeal edema can result from a direct injury or irritation of the upper airway, such as postextubation edema or laryngotracheobronchitis (croup). In addition, if the patient has anaphylaxis from an allergic reaction, laryngeal edema and hypotension are often present. If bleeding results from a biopsy during a bronchoscopy, the cut blood vessels must be made to constrict and to form clots. In cases of laryngeal edema or biopsy bleeding, nebulized racemic epinephrine (microNefrin) is given because it stimulates α1-receptors. This results in vasoconstriction of the mucosal and deeper blood vessels. Therefore the laryngeal edema swelling is reduced, and biopsy bleeding stops. In the case of anaphylaxis with hypotension and laryngeal edema, intravenous epinephrine is needed to treat both life-threatening problems. See Table 9-1 for information on specific medications.
Most of the medications listed in this section are chemically derived from adrenaline. They are somewhat different in their structures so that the desired effects and side (unwanted) effects vary. Box 9-1 lists the side effects of the sympathomimetic bronchodilators. Clinically, the most dangerous of these side effects are palpitations, tachycardia, and hypertension.
BOX 9-1 Clinically Observed Side Effects of Sympathomimetic Aerosolized Bronchodilators
(from the Most Commonly Seen to the Least Commonly Seen)
b. Inhaled anticholinergic (parasympatholytic) agents
2. Administer the prescribed medication (Code: IIIC3, IIID5a, IIID5b) [Difficulty: ELE: R, Ap; WRE: An]
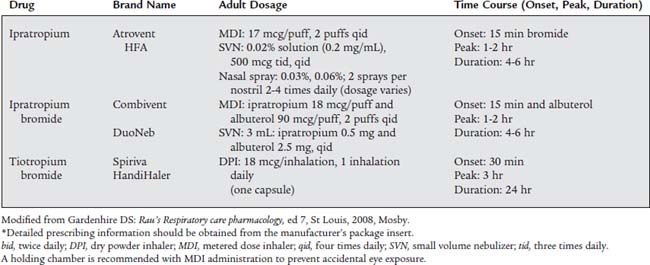
Many patients with COPD and asthma will be treated with nebulized medications from both the sympathomimetic and the parasympatholytic groups. Combivent and DuoNeb combine a sympathomimetic and a parasympatholytic medication. The newest drug in this group, tiotropium bromide (Spiriva), provides the patient with 24 hours of the medication’s benefits. See Table 9-2 for information on the parasympatholytic medications.
3. Antiinflammatory agents
a. Inhaled corticosteroids
1. Recommend use of corticosteroids (ELE code: IIIG4b) [Difficulty: ELE: R, Ap, An]
Corticosteroids affect the respiratory system in two ways: they potentiate the effects of the sympathomimetic agents, and they stop the inflammatory response seen in the airways of asthmatic patients after exposure to an allergen. This prevents mucosal edema from developing. The patient with chronic airflow obstruction, such as asthma or asthmatic bronchitis, should be given inhaled corticosteroids. When they are used as directed, relatively little systemic (bodily) absorption occurs. However, it is best to monitor the patient, especially small children taking inhaled corticosteroids for an extended period, for any side effects. Current guidelines for asthma management classify corticosteroids as “controller” medications that are taken to prevent an asthma attack.
2. Administer the prescribed medication (Code: IIIC3, IIID5a, IIID5b) [Difficulty: ELE: R, Ap; WRE: An]
Table 9-3 shows specific strength and dosage information for the inhaled corticosteroids.
| Drug | Brand Name | Formulation and Dosage |
|---|---|---|
| Beclomethasone dipropionate HFA | QVAR |
bid, twice daily; qid, four times daily; tid, three times daily.
* Detailed prescribing information should be obtained from the manufacturer’s package insert.
† Recommended starting dose if on bronchodilators alone.
‡ Recommended starting dose if on inhaled corticosteroids previously.
§ Recommended starting dose if on oral corticosteroids previously.
Modified from Gardenhire DS: Rau’s Respiratory care pharmacology, ed 7, St Louis, 2008, Mosby.
b. Cromolyn sodium
1. Recommend the use of cromolyn sodium (ELE code: IIIG4b) [Difficulty: ELE: R, Ap, An]
Cromolyn sodium (Intal) and nedrocromil sodium (Tilade) are indicated to prevent an asthma attack. They accomplish this by coating the mast cells found in the airways so that they do not degranulate and rupture. Without pretreatment, the mast cells of asthmatic patients would rupture when exposed to immunoglobulin E (IgE) from their allergen(s). This mast cell rupture would result in the spilling of leukotriene agents, histamine, and other chemical mediators that cause the bronchospasm, airway edema, and increased airway secretions of an asthma attack.
2. Administer the prescribed medication (Code: IIIC3, IIID5a, IIID5b) [Difficulty: ELE: R, Ap; WRE: An]
When cromolyn sodium, or nedrocromil sodium, is inhaled at least 1 week before exposure to the allergen, the asthmatic reaction is prevented or reduced. Cromolyn was first made available through a dry powder inhaler and is now only available by metered dose inhaler or small volume nebulizer (SVN). Nedrocromil sodium is similar to cromolyn in its use and effects. It is available in a metered dose inhaler. See Table 9-4 for detailed information on both medications.
| Drug | Brand Name | Formulation and Dosage |
|---|---|---|
| CROMOLYN-LIKE (MAST CELL STABILIZERS) | ||
| Cromolyn sodium† | Intal | MDI: 800 mcg/actuation |
| Adults and children ≥5 yr: 2 inhalations 4 times daily | ||
| SVN: 20 mg/amp or 20 mg/vial | ||
| Adults and children ≥2 yr: 20 mg inhaled 4 times daily | ||
| Nasalcrom | Spray: 40 mg/mL (4%), gives 5.2 mg of drug | |
| Adults and children ≥2 yr: 1 spray each nostril, 3-6 times daily every 4-6 hr | ||
| Nedocromil sodium | Tilade | MDI: 1.75 mg/actuation |
| Adults and children ≥6 yr: 2 inhalations 4 times daily | ||
| ANTILEUKOTRIENES | ||
| Montelukast | Singulair | Tablets: 10 mg, 4 mg, and 5 mg cherry-flavored chewable; 4 mg packet of granules |
| Adults and children ≥15 yr: one 10 mg tablet daily in evening | ||
| Children 6-14 yr: one 5 mg chewable tablet daily | ||
| Children 2-5 years: one 4 mg chewable tablet or one 4 mg packet of granules daily | ||
| Children 6-23 months: one 4 mg packet of granules daily | ||
| Zafirlukast | Accolate | Tablets: 10 and 20 mg |
| Adults and children ≥12 yr: 20 mg (1 tablet) twice daily, without food | ||
| Children 5-11 yr: 10 mg twice daily | ||
| Zileuton | Zyflo | Tablets: 600 mg |
| Zyflo CR | Adults and children ≥12 yr: one 600 mg tablet 4 times a day | |
| MONOCLONAL ANTIBODY | ||
| Omalizumab | Xolair | Adults and children ≥12 yr: subcutaneous injection every 4 weeks; dose dependent on patient’s weight and serum IgE level |
* Detailed prescribing information should be obtained from the manufacturer’s package insert.
† Note: Cromolyn sodium is also available in an oral concentrate giving 100 mg in 5 mL (Gastrocrom) for treatment of systemic mastocytosis, and as an ophthalmic 4% solution (Opticrom, 40 mg/mL) for treatment of vernal keratoconjunctivitis.
Modified from Gardenhire DS: Rail’s Respiratory care pharmacology, ed 7, St Louis 2008, Mosby.
c. Recommend the use of leukotriene modifiers (ELE code: IIIG4b) [Difficulty: ELE: R, Ap, An]
Leukotrienes are chemicals found in mast cells that are released during an asthma attack. These released leukotriene chemicals stimulate bronchospasm, airway edema, and increased airway secretions. The leukotriene-modifier (also called leukotriene antagonists) medications work to reduce the number of leukotriene chemicals that are released or to block the effect of leukotrienes on the airways. By accomplishing this, the patient’s asthma symptoms are reduced or eliminated. See Table 9-4 for detailed information on all four medications in this group.
4. Mucolytics or proteolytic agents
a. Acetylcysteine
2. Administer the prescribed medication (Code: IIIC3, IIID5a) [Difficulty: ELE: R, Ap; WRE: An]
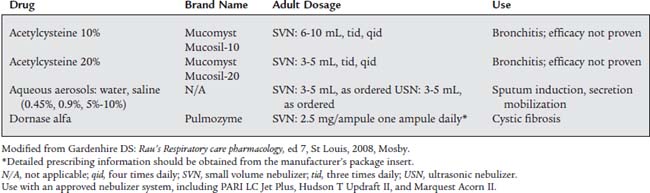
Mucomyst is usually administered with a small volume nebulizer (SVN) or with intermittent positive-pressure breathing (IPPB). Most adult patients are given up to 3 to 5 mL of the 20% solution or up to 6 to 10 mL of the 10% solution. The 20% solution is often diluted with an equal volume of normal saline solution. Direct instillation of 1 to 2 mL of the drug into the trachea also helps to liquefy secretions. The manufacturer recommends that all medication in a vial be used within 96 hours or discarded. It should be stored in the refrigerator. A slightly purple color is commonly seen after the vial has been opened, but it can still be used safely. See Table 9-5 for specific information.
b. RhDNAse
2. Administer the prescribed medication (Code: IIIC3, IIID5a) [Difficulty: ELE: R, Ap; WRE: An]
Usually a single daily dose of 2.5 mL of solution (containing 2.5 mg of dornase alfa) is inhaled by SVN. Store the drug in a refrigerator, and protect it from strong light. It has no serious side effects. See Table 9-5 for detailed information.
c. Hypertonic saline
1. Recommend the use of hypertonic saline (ELE code: IIIG4c) [Difficulty: ELE: R, Ap, An]
The various saline solutions (and sterile water) are known collectively as “bland” aerosols, because they have no direct pharmacologic effect on the lungs and airways. When they are inhaled as aerosols, however, a vagal nerve–mediated reflex causes the bronchial/submucosal glands to release more watery secretions. Therefore a saline aerosol is commonly used to help liquefy secretions and induce a patient to expel sputum. Recent clinical experience has shown hypertonic saline to be an effective mucolytic in cystic fibrosis patients.
2. Administer the prescribed medication (Code: IIIC3, IIID5a) [Difficulty: ELE: R, Ap; WRE: An]
The various saline solutions are given through an SVN or IPPB treatment or, more commonly, mixed with a bronchodilator medication. See Table 9-5 and Box 9-2 for information on the various saline solutions.
BOX 9-2 Saline Solutions Used as Mucolytics
HYPOTONIC SALINE SOLUTION, 0.45% SALINE
HYPERTONIC SALINE SOLUTION, 1.8%-10% SALINE
d. Observe the patient for signs of changes in sputum characteristics (ELE code: IIIE5a) [Difficulty: ELE: R, Ap, An]
The respiratory therapist should objectively evaluate the patient’s cough and sputum production before and after a mucolytic has been administered. This will provide evidence of the medication’s effectiveness. See Chapter 1 for further discussion and Table 1-13 for sputum characteristics.
5. Diuretics
a. Recommend the adjustment of fluid balance (Code: IIIG1d) [Difficulty: ELE: R, Ap; WRE: An]
Treatment of the patient will include fluid restriction and diuresis to rapidly decrease the patient’s fluid volume. Review Box 1-3 for normal fluid intake and output values.
b. Recommend use of diuretics (Code: IIIG4i) [Difficulty: ELE: R, Ap; WRE: An]
These medications have a large molecular weight and, through osmosis, “pull” fluid from the brain into the bloodstream. Therefore they are sometimes called osmotic diuretics. The medication, after crossing into the kidney, prevents the reabsorption of water and increases urine output.
c. Recommend blood tests to measure the potassium level (Code: IIIE8) [Difficulty: ELE: R, Ap; WRE: An]
If a patient with fluid overload has been give a diuretic such as Lasix, all electrolytes, especially the serum potassium level, should be check regularly. As stated previously, Lasix and related diuretic medications cause the kidneys to excrete potassium in addition to sodium and water. The so-called potassium-sparing electrolytes (e.g., spironolactone [Aldactone], amiloride [Midamor], triamterene [Dyrenium]) can lead to an increased potassium level. (Review Table 1-2 for the normal electrolyte values.)
d. Recommend the adjustment of electrolyte therapy (Code: IIIG1e) [Difficulty: ELE: R, Ap; WRE: An]
There will usually be at least one question that regards the use of a diuretic in a patient who is fluid overloaded, and the side effects of using a diuretic. A diuretic drug such as furosemide (Lasix) tends to cause the loss of potassium through the kidneys. Know to check the serum potassium level. Remember that the normal potassium level is 3.5 to 5.5 mEq/L. If the patient has the signs of dangerous hypokalemia (see Chapter 1), know to recommend that replacement potassium be given.
6. Recommend the use of sedatives (Code: IIIG4f, IIIG1c) [Difficulty: ELE: R, Ap; WRE: An]
8. Recommend the use of neuromuscular blocking agents (Code: IIIG4h, IIIG1c) [Difficulty: ELE: R, Ap; WRE: An]
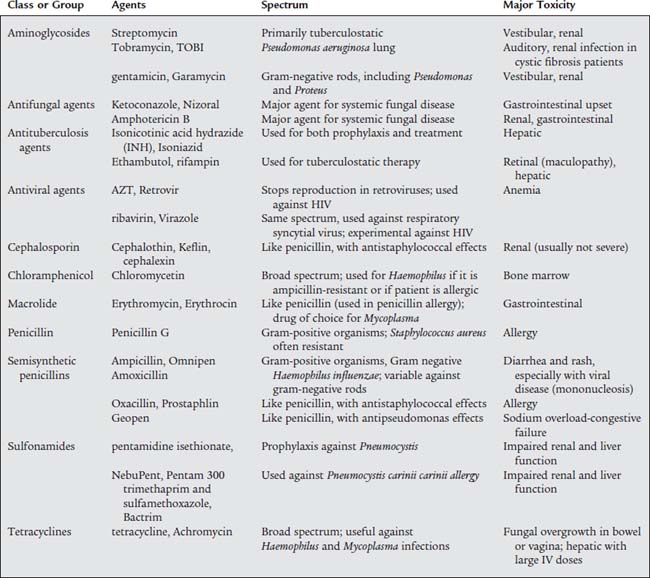
10. Antimicrobials
a. Recommend the use of antimicrobials (e.g., antibiotics) (Code: IIIG4e) [Difficulty: ELE: R, Ap; WRE: An]
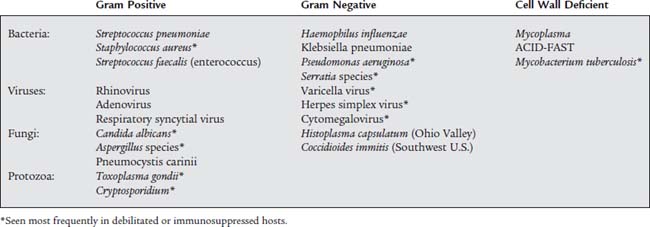
The terms antimicrobial, antiinfective, and antibiotic refer to natural or synthetic chemicals that are toxic to bacteria and other microorganisms. Table 9-6 lists the most commonly found respiratory tract pathogens. See Table 9-7 for a summary of the most common respiratory tract pathogens and the antimicrobial agents used against them. Table 9-8 lists specific information on the four currently approved aerosolized antimicrobial agents given by respiratory therapists and likely to be tested by the NBRC. They are discussed below. Be aware that other systemic antimicrobial medications have been given by aerosol despite not having FDA approval.
b. Administer the prescribed medication (Code: IIIC3, IIID5a, IIID5b) [Difficulty: ELE: R, Ap; WRE: An]
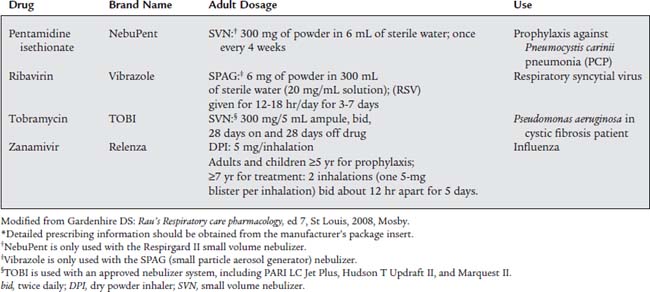
2. Antiviral agent: zanamivir and ribavirin
Zanamivir (Relenza) is approved for the treatment of influenza in adults and children older than 5 years of age. To be effective, Relenza must be started within the first 2 days of symptoms and continued for 5 days. If taken early enough in the course of the infection, the patient’s symptoms should be reduced and the course of the infection shortened. Relenza is available in a multidose dry powder inhaler (DPI) packet. The medication packet is loaded into the Diskhaler delivery device for inhalation (see Figure 8-18).
3. Antiprotozoal agent: pentamidine isethionate
Pentamidine isethionate (NebuPent) has been approved for the prophylactic treatment of the fungal organism Pneumocystis carinii. Patients with impaired immune systems, such as those with acquired immunodeficiency syndrome (AIDS), are most likely to suffer from Pneumocystis carinii pneumonia (PCP). (Note: This may also be called Pneumocystis jiroveci pneumonia [PJP].) Currently, these patients are given a single 300-mg dose of NebuPent mixed with 6 mL of sterile water once every 4 weeks through the Respirgard II small volume nebulizer. This unit also features an expiratory scavenging filter to prevent any droplets from entering the room air (see Figure 8-15). (The AeroTech II unit may also be used to deliver NebuPent.)
11. Recommend the use of vaccines (e.g., Pneumovax, influenza) (Code: IIIG4k) [Difficulty: ELE: R; WRE: Ap]
See Chapter 2 for the discussion on vaccination to prevent avian flu, severe acute respiratory syndrome (SARS) from the coronavirus, and Streptococcus pneumoniae.
MODULE C
The problems are easier to solve by remembering the following:
100x = 0.5 mL (Divide both sides of the equation by 100.)x = 0.005 mL = 0.005 g = 5 mg of active ingredient
100x = 1.25 mL (Divide both sides of the equation by 100.)x = 0.0125 mL = 0.0125 g = 12.5 mg of active ingredient
1000 x = 500 mL (Divide both sides of the equation by 1000.)x = 0.5 mL of Proventil should be given.
MODULE D
1. Analyze the available information to determine the patient’s pathophysiologic state (Code: IIIH1) [Difficulty: ELE: R, Ap; WRE: An]
The respiratory therapist should be able to determine when the patient is having cardiopulmonary problems that could require an inhaled medication. Review, if needed, information presented in Chapters 1, 3, 4, and 5 that deals with bedside assessment, blood gases, pulmonary function tests, and advanced cardiopulmonary monitoring.
2. Determine the appropriateness of the prescribed respiratory care plan and recommend modifications when indicated
a. Determine the appropriateness of the prescribed therapy and goals for the identified pathophysiologic state (Code: IIIH3) [Difficulty: ELE: R, Ap; WRE: An]
b. Review the planned therapy to establish the therapeutic plan (Code: IIIH2a) [Difficulty: ELE: R, Ap; WRE: An]
c. Recommend changes in the therapeutic plan when indicated (Code: IIIH4) [Difficulty: ELE: R, Ap; WRE: An]
e. Terminate the treatment or procedure based on the patient’s response to therapy (Code: IIIF1) [Difficulty: ELE: R, Ap; WRE: An]
As mentioned earlier, Box 9-1 lists the side effects most often seen with adrenergic (sympathomimetic) bronchodilators. The most serious problem is tachycardia. In some cases, patients may have cardiac arrhythmias. A common clinical guideline requests the treatment be discontinued if the patient’s heart rate increases by more than 20% during the treatment. The patient should be monitored to confirm that the heart rate slows. Chart the information, and notify the nurse.
f. Recommend discontinuing the treatment or procedure based on the patient’s response (Code: IIIG1i) [Difficulty: ELE: R, Ap; WRE: An]
Second, the patient has a serious adverse reaction to the medication. As discussed earlier, Box 9-1 lists the adverse reactions to the adrenergic (sympathomimetic) bronchodilators. Repeated episodes of tachycardia or cardiac arrhythmias, or both, could lead to the physician discontinuing their use.
3. Make a recommendation to change the dosage or concentration of an aerosolized medication (Code: IIIG2b) [Difficulty: ELE: R, Ap; WRE: An]
a. Bronchodilators
Make a recommendation to increase the amount of medication if the patient’s bronchospasm is not reversed and no adverse side effects are present. Bedside spirometry should be performed regularly to evaluate the patient’s peak flow. An increase of at least 15% to 20% is clinically significant. Review the discussion on peak flow, percentage improvement in before and after bronchodilator therapy, and asthma zones that is found in Chapter 4.
4. Change the dilution of a medication used in aerosol therapy (ELE code: IIIF2c3) [Difficulty: EL: R, Ap, An]
Saline-only aerosol treatments, as for induced sputum, are more effective at higher concentrations of saline. See Box 9-2 for complete information on the saline solutions.
5. Respiratory care protocols
a. Develop the outcomes of respiratory care pharmacology protocols (Code: IIIH6b) [Difficulty: ELE: R, Ap; WRE: An]
b. Apply respiratory care pharmacology protocols to patient care situations (Code: IIIH8) [Difficulty: EL: R, Ap; WRE: An]
c. Explain planned therapy and goals to the patient in understandable (nonmedical) terms to achieve the best results from the treatment or procedure (Code: IIIA6) [Difficulty: ELE: R, Ap; WRE: An]
d. Monitor the outcomes of respiratory care pharmacology protocols (Code: IIIH7b) [Difficulty: ELE: R, Ap; WRE: An]
The following are common goals of therapy for the COPD patient:
6. Record and evaluate the patient’s response to the treatment(s) or procedure(s), including the following:
a. Record and interpret the following: heart rate and rhythm, respiratory rate, blood pressure, body temperature, and pain level (Code: IIIA1b4) [Difficulty: ELE: R, Ap; WRE: An]
b. Record and interpret the patient’s breath sounds (Code: IIIA1b3) [Difficulty: ELE: R, Ap; WRE: An]
Listen for a reduction in wheezing after the administration of an aerosolized bronchodilator as proof of an effectively reduced bronchospasm. If the patient was given an aerosolized bronchodilator for bronchospasm, the patient’s spirometry results would be expected to change toward more normal values as the bronchospasm is reduced. The two most important bedside spirometry values to monitor are the peak flow and forced expiratory volume in 1 second. Often, a 15% to 20% improvement in either one or both of these parameters after the inhalation of an aerosolized bronchodilator is used as a clinical indication that the medication works, and the patient has reversible bronchospasm. (See Chapter 4 for more specific guidelines.) A patient with stable asthma may be given a drug such as cromolyn sodium (Intal) or nedocromil sodium (Tilade) by inhalation to prevent a future asthma attack. Remember that these drugs are not to be used during an asthma attack.
c. Record and interpret the type of cough the patient has and the nature of the sputum (Code: IIIA1b) [Difficulty: ELE: R, Ap; WRE: An]
AARC Clinical Practice Guideline. Surfactant replacement therapy. Respir Care. 1994;39(8):824.
Au JP, Ziment I. Drug therapy and dosage adjustment in asthma. Respir Care. 1986;31:415.
Bills GW, Soderberg RC. Principles of pharmacology for respiratory care, ed 2. Albany, NY: Delmar, 1998.
Canadian Asthma Consensus Guidelines, 2003 and Canadian Pediatric Asthma Concensus Guidelines, 2003. CMAJ. 2005;173(6 Suppl):S1-S56.
Carter C, Solberg C. Respiratory pharmacology. In: Hess DR, MacIntyre NR, Mishoe SC, et al, editors. Respiratory care: principles and practices. Philadelphia: WB Saunders, 2002.
Colbert BJ, Mason BJ. Cardiopulmonary drug guide. Upper Saddle River, NJ: Prentice Hall, 2003.
Colbert BJ, Mason BJ. Integrated cardiopulmonary pharmacology, ed 2. Upper Saddle River, NJ: Prentice Hall, 2008.
Colice GL. New drugs for asthma. Respir Care. 2008;53(6):688.
Cottrell GP, Surkin HB. Pharmacology for respiratory care practitioners. Philadelphia: FA Davis, 1995.
Donohue JF. Safety and efficacy of B agonists. Respir Care. 2008;53(5):618.
Gardenhire DS. Rau’s Respiratory care pharmacology, ed 7. St Louis: Mosby, 2008.
Gardenhire DS. Airway pharmacology. In Wilkins RL, Stoller JK, Kacmarek RM, editors: Egan’s fundamentals of respiratory care, ed 9, St Louis: Mosby, 2009.
Geller DE. Aerosol antibiotics in cystic fibrosis. Respir Care. 2009;54(5):658.
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Workshop report: global strategy for the diagnosis, management, and prevention of COPD. Bethesda, MD: National Heart, Lung, and Blood Institute and the World Health Organization, 2000.
Global strategy for asthma management and prevention (updated 2006): global initiative for asthma (GINA). http:www.ginasthma.org.
Hill F. Delmar’s respiratory care drug reference. Albany, NY: Delmar, 1999.
Howder CL. Cardiopulmonary pharmacology: a handbook for respiratory practitioners and other allied health personnel, ed 2. Baltimore: Williams & Wilkins, 1996.
Levine SR, McLaughlin AJ. Pharmacology in respiratory care. New York: McGraw-Hill, 2001.
Malmeister M. Pharmacology associated with respiratory care. In: Fink JB, Hunt GE, editors. Clinical practice in respiratory care. Philadelphia: Lippincott-Raven, 1999.
Myers TR. Guidelines for asthma management: a review and comparison of 5 current guidelines. Respir Care. 2008;53(6):751.
McLaughlin AJ, Levine SR. Respiratory care drug reference. Gaithersburg, Md: Aspen, 1997.
National Asthma Education and Prevention Program, Expert Panel Report II. Guidelines for the diagnosis and management of asthma. Bethesda, Md: National Institutes of Health, 1997.
National Asthma Education and Prevention Program, Expert Panel Report II. Guidelines for the diagnosis and management of asthma—update on selected topics 2002. Bethesda, Md: National Institutes of Health, June 2003. Pub. No. 02-5074, Bethesda, Md, 1997, NIH.
National Institutes of Health. Practical guide for the diagnosis and management of asthma. Bethesda, Md: NIH, 1997. Pub. No. 97-4053
Op’t Hold TB. Inhaled beta agonists. Respir Care. 2007;52(7):820.
Physicians’ desk reference. ed 60, 2003; Thomson PDR: Montvale, NJ.
Restropo RD. Use of inhaled anticholinergic agents in obstructive lung disease. Respir Care. 2007;52(7):833.
Rubin BK. Mucolytics, expectorants, and mucokinetic medications. Respir Care. 2007;52(7):859.
Sobande PO, Kercsmar CM. Inhaled cortiosteroids in asthma management. Respir Care. 2008;53(5):625.
Tashkin DP. Dosing strategies for bronchodilator aerosol delivery. Respir Care. 1991;36:977.
Witek TJ, Schachter EN. Pharmacology and therapeutics in respiratory care. Philadelphia: WB Saunders, 1994.
Ziment I. Drugs used in respiratory care. In Burton GG, Hodgkin JE, Ward JJ, editors: Respiratory care: a guide to clinical practice, ed 4, Philadelphia: Lippincott-Raven, 1997.
SELF-STUDY QUESTIONS FOR THE ENTRY LEVEL EXAM See page 590 for answers
SELF-STUDY QUESTIONS FOR THE WRITTEN REGISTRY EXAM See page 615 for answers