CHAPTER 26 Pharmacologic Stress Agents
VASODILATOR AGENTS
Coronary Blood Flow
As heart muscle contracts during systole, the myocardium impedes its own blood supply because most CBF occurs during diastole. This pulsatile flow is conducted through a highly curved, branching vascular system composed of larger epicardial vessels and smaller intramural vessels. While epicardial coronary arteries serve as conductance vessels, intramural arteries distribute and regulate blood flow. This adaptation, termed autoregulation, occurs rapidly in response to changes in myocardial oxygen demands, primarily through functional hyperemia.1 This ability of the coronary tree to alter its resistance and increase CBF in response to myocardial metabolic demands is maintained by a balance between vasoconstrictors, such as endothelin, and vasodilators, such as nitric oxide. Acting in smaller arteries and arterioles, these vasoactive factors are integral in allowing rapid, sustained adaptation of CBF based on the requirements of the myocardium.
Coronary Flow Reserve
As atherosclerosis develops in epicardial coronary arteries, the resulting stenosis produces resistance to blood flow. The physiologic effect of a coronary stenosis depends on the degree to which the resistance to flow can be compensated by dilation of the microcirculation distal to the stenosis. This mechanism, regulated by vasoactive factors, maintains resting CBF at a constant level despite the presence of a growing burden of atherosclerosis. When an epicardial coronary constriction exceeds 80% to 90% of the normal segment diameter, the distal vasodilatory mechanisms are unable to compensate, and resting CBF cannot provide adequate oxygen to meet the resting needs of the myocardium.1,2 When a stenotic vessel is exposed to a vasodilator (e.g., adenosine or dipyridamole) intended to induce hyperemic (or maximal) flow, the relative increase in flow decreases proportionately when a stenosis exceeds 50%, and is generally abolished at a 90% occlusion (Fig. 26-1).
Coronary Blood Flow and Radiotracer Uptake
Vasodilators can increase CBF five times normal levels. This large increase in CBF occurs in myocardium supplied by normal coronary arteries, in contrast to the lack of an increase to myocardium supplied by stenosed arteries as discussed previously; this forms the basis for vasodilator perfusion imaging (Fig. 26-2). If the myocardial uptake of radiotracers such as thallium 201 and Tc 99m were purely linear and based on CBF, a high level of image contrast would theoretically exist between myocardium supplied by normal arteries (i.e., without stenosis) and arteries with hemodynamically significant stenoses. In reality, the extraction fraction of most tracers decreases when CBF increases beyond 2.5 times baseline values. The practical effect of this “roll-off” in the uptake-flow relationship is a physiologic constraint in detecting mild-to-moderate stenoses. The flow increase in response to vasodilators in vessels with a mild-to-moderate stenosis may still be two to three times normal because of coronary flow reserve. This prevents differentiation with a normal coronary artery owing to radiotracer roll-off at a fixed level of hyperemic flow because the normal and mildly stenotic arteries would have identical tracer uptake. Generally with the more common radiotracers, it is difficult to identify noninvasively stenoses that are less than 50% because of the preservation of CBF in the resting and hyperemic states through natural vascular response mechanisms.
Adenosine
Adenosine is a small heterocyclic compound that is endogenously produced in variable amounts as part of a normal cellular metabolism and during ischemia. Through adenosine receptors, it serves as a potent coronary vasodilator (adenosine A2 receptors) and inhibitor of the sinoatrial and atrioventricular (AV) nodes (adenosine A1 receptors).3 Adenosine is produced intracellularly, but interacts on the adenosine receptors on the outer cell membrane (Fig. 26-3). Theophylline and caffeine act at these receptors as competitive blockers, reducing the effects of adenosine.
Clinically, adenosine is infused intravenously and has a very short half-life of less than 2 seconds. As a result, the time-to-peak effect occurs in the first minute of infusion. Adenosine is given typically as a continuous infusion of 140 µg/kg/min for 4 to 6 minutes, with radiotracer injection halfway into the infusion. To avoid an unintended rapid bolus of residual adenosine, radionuclide dose injection and flushing and adenosine infusion should be done through separate intravenous lines or through the use of a dual port.3 Hemodynamically, adenosine causes a modest decrease in systolic blood pressure and increase in heart rate, in total leading to an increase in cardiac output and mild increase in pulmonary capillary wedge pressure.
Side effects are common with adenosine infusion, occurring in more than 75% of patients (Table 26-1). These side effects are transient, usually resolving within 1 to 2 minutes of stopping the infusion, and rarely require reversal with aminophylline. Most side effects are related to stimulation of the adenosine A1 and A3 receptors. Chest pain is common, although it is not specific for the presence of coronary artery disease, occurring in patients with no coronary artery disease at the same frequency as in patients with documented coronary artery disease. Although stimulation of the adenosine A3 receptors can theoretically lead to bronchospasm, most dyspnea occurring with adenosine infusion is likely related to hyperventilation. Caution should be used in patients with a history of asthma, severe chronic obstructive pulmonary disease (COPD), or active wheezing because of the potential for bronchospasm. Changes in AV conduction usually result in transient first-degree or second-degree AV block. Most bradyarrhythmias are transient and resolve despite continued adenosine infusion.
Dipyridamole
Dipyridamole is a pyrimidine base that induces vasodilation by elevating blood and interstitial levels of adenosine. By blocking reuptake of adenosine into cells, dipyridamole increases extracellular levels of adenosine, allowing interaction with adenosine A2 receptors on the outer cell membrane. Activation of the adenosine A2 receptor leads to a complex series of events mediated by G proteins and leading to a decrease in intracellular calcium uptake, culminating in coronary vasodilation (see Fig. 26-3). Dipyridamole also exhibits antiplatelet activity, likely related to phosphodiesterase inhibition, increased adenosine triphosphate (ATP) levels, potentiation of aspirin effects, and increased adenosine.3
Clinically, dipyridamole is infused intravenously and has a biologic half-life of 88 to 136 minutes. It is given as a continuous infusion of 0.57 mg/kg over 4 minutes, with radiotracer injection 3 to 4 minutes after the dipyridamole infusion is completed. This is distinct from adenosine, where tracer injection is during vasodilator infusion (Fig. 26-4). The standard intravenous dose of dipyridamole results in a slight increase in heart rate, a decrease in systolic blood pressure, a modest increase in cardiac output, and a slight increase in pulmonary capillary wedge pressure.
Side effects occur in about 50% of patients and include chest pain, nausea, dizziness, and headache (see Table 26-1). As with adenosine, chest pain is mostly nonanginal and does not correlate with the presence of coronary artery disease. Bronchospasm is rare, but can be precipitated in patients with asthma or severe COPD. Arrhythmias, including AV block, are highly uncommon. Dipyridamole has generally been extensively studied and has an excellent safety record. Most significant side effects can be reversed with slow intravenous administration of theophylline (100 to 300 mg), which is a competitive blocker of adenosine receptors.
INOTROPIC AGENTS
Mechanism of Action
Synthetic inotropic agents act similarly to endogenous catecholamines, such as epinephrine and norepinephrine. Normal exercise results in the release of norepinephrine from sympathetic nerve endings that are abundant on the cardiac ventricles, atria, and conduction system. Norepinephrine stimulates β1 receptors located on the cardiac cell membrane resulting in a complex protein signaling cascade that results in the enhancement of calcium entry into the cell. Calcium potentiates the action between actin and myosin increasing myofibril contraction (Fig. 26-5). The cardiac conduction system also contains β1 receptors allowing for increased AV conduction and increases in heart rate. The actions of specific inotropes depend on the balance of β and α activity, which predominantly mediates peripheral vasoconstriction. Other catecholamines stimulate β2 receptors located in vascular smooth muscle and result in vasodilation.4
Dobutamine
Dobutamine has a short half-life and is given typically as a continuous infusion at a starting dose of 5 µg/kg/min, increasing by 5 to 10 µg/kg/min doses up to 40 µg/kg/min at 3-minute intervals. The patient is instructed to discontinue β blockers before the imaging study at a minimum of 24 to 48 hours to mitigate its negative inotropic and chronotropic effects. Atropine at 0.5- to 1-mg doses can be added in patients who are not reaching target heart rate with higher doses of dobutamine (see Fig. 26-4). Continuous heart rate monitoring and periodic ECG and blood pressure recordings are required. Criteria for termination of the infusion include severe hypotension or hypertension, malignant arrhythmia, severe chest pain, ST segment elevation greater than 1 mm or ST segment depression 2 mm or greater, or intractable noncardiac symptoms.
Coronary hyperemia induced by higher doses of dobutamine depends on increasing myocardial oxygen demand in response to increases in cardiac contractility and heart rate. This effect is less than that of vasodilators such as adenosine, and the latter agent is preferred if nuclear myocardial perfusion imaging is the modality of choice unless contraindicated. Near-equivalent coronary vasodilation can be achieved with the combination of dobutamine and atropine as with dipyridamole in younger patients.5
Side effects with dobutamine are varied, but usually well tolerated (see Table 26-1). Chest pain, palpitations, and nausea are common during prolonged infusions. Ventricular and supraventricular arrhythmias can be seen, but they often resolve on cessation of the infusion or treatment with low-dose intravenous β blockers. Hypotension can occur in some patients, but because of the short half-life of dobutamine, this generally resolves on discontinuation of the infusion.3
UTILITY IN CORONARY ARTERY DISEASE
Commonly used imaging modalities for detection of myocardial ischemia include myocardial perfusion studies and stress echocardiography. As discussed earlier, vasodilators are used primarily in nuclear perfusion studies, whereas dobutamine plays an integral part in detecting physiologically significant coronary artery disease in both. With myocardial perfusion imaging, flow heterogeneity develops when myocardial oxygen demand is increased in the setting of a fixed coronary stenosis with normal coronary vessels compensating by dilating and essentially “stealing” flow from the diseased vessel (see Fig. 26-2); this results in decreased nuclear tracer uptake in the territory of the obstructed vessel relative to the distribution of the disease-free vessels, manifesting as a perfusion defect. With stress echocardiography, ischemia develops in a territory supplied by a coronary vessel with a high-grade stenosis when demand outstrips the ability of the coronary vessel to dilate and is typically reflected in regional wall motion abnormalities. In the ischemia cascade, perfusion abnormalities precede that of wall motion dysfunction, making myocardial perfusion imaging typically more sensitive than stress echocardiography for detection of ischemic heart disease. Stress echocardiography has a relatively higher specificity, however.
Diagnosis and Prognosis in Coronary Artery Disease
The primary utility of pharmacologic stress agents is in the diagnosis of coronary artery disease. In patients with and without known coronary artery disease, pharmacologic myocardial perfusion studies help to localize ischemia and quantify the burden of disease. The prognostic information gained is additive to clinical and historical data. After a normal myocardial perfusion examination, most clinical trials looking at risk have reported cardiac event rates of less than 1%6; this is generally independent of imaging type, radiopharmaceutical, or patient clinical characteristics. In patients undergoing pharmacologic stress, reported cardiac event rates are higher, ranging from 1.3% to 2.7% per year.6 This higher rate is probably secondary to the generally higher clinical risk in patients referred for pharmacologic stress testing (vs. exercise stress), who typically have an inherently higher cardiac event risk rate secondary to their underlying debilitating disease, such as peripheral occlusive arterial disease or diabetes. Numerous studies have confirmed that simply having vasodilator stress testing is associated with the same long-term cardiac event risk as having known coronary artery disease.
In patients with abnormal pharmacologic stress perfusion studies, there is a close relationship between the extent and severity of the perfusion abnormalities and the risk of adverse outcomes. The risk of major cardiac events progressively increases as a function of the myocardial perfusion imaging results, with large defects associated with an incrementally higher risk than small defects; this is true for adenosine and dipyridamole stress testing. With dipyridamole, testing with thallium 201 SPECT imaging yields an average sensitivity of 89% and specificity of 75%.7 For adenosine thallium 201 SPECT myocardial perfusion imaging, the average sensitivity is 88%, and specificity is 85%.7
Walking Adenosine Protocols
For patients able to walk, it is advantageous to combine low-level exercise with adenosine myocardial perfusion imaging. In addition to mitigating the side effects of adenosine, there is generally less hepatic uptake of radiotracer and a higher heart/background ratio, which improves overall imaging quality. In limited studies, this improved imaging quality has translated into improved sensitivity and additional prognostic implications, with an inability to walk associated with greater adverse risk.8
DOBUTAMINE: SPECT IMAGING VERSUS ECHOCARDIOGRAPHY
Dobutamine stress myocardial perfusion imaging generally has a higher sensitivity and lower specificity for detecting significant coronary artery disease compared with dobutamine stress echocardiography (Fig. 26-6). In two clinical trials that directly compared dobutamine stress myocardial perfusion imaging with stress echocardiography, the sensitivities were 86% and 80%, and specificities were 73% and 86%.9,10 This difference reflects the earlier detection of ischemia with perfusion stress testing based on its earlier occurrence in the myocardial ischemic cascade. The sensitivity of detecting coronary artery disease increases incrementally with the extent of disease involvement. Dobutamine stress myocardial perfusion imaging has a sensitivity of 80%, 92%, and 97% for one-vessel, two-vessel, and three-vessel disease, respectfully. Compared with vasodilator stress myocardial perfusion imaging, dobutamine has a slightly lower sensitivity with similar specificity for the diagnosis of coronary artery disease (Fig. 26-7).10
Post-infarct Myocardial Imaging
In the United States, most patients presenting with myocardial infarction undergo early definitive risk stratification with coronary angiography. There is still a role, however, in low-risk myocardial infarction patients for predischarge noninvasive risk stratification in lieu of an early invasive strategy. Pharmacologic stress imaging has numerous advantages over exercise stress testing. Patients do not need to exercise, which has inherent safety advantages after myocardial infarction, where most guidelines advocate a delay in submaximal stress testing of at least 7 days after an event and waiting 3 to 4 weeks after myocardial infarction for full exercise protocols. Dobutamine and vasodilator use early after a myocardial infarction have the potential for adverse hemodynamic events, but have been shown to be safe in multiple cardiac imaging studies 2 days after an acute ischemic event.7,11,12
Viability Assessment with Dobutamine
Patients with ischemic heart disease may have large territories of dysfunctional myocardium that may recover function on revascularization. The detection of this myocardial viability is uniquely suited to dobutamine as a stress agent. Examinations using a protocol of low dose followed by higher doses of dobutamine infusion with the aid of echocardiography may unmask areas of viability. These myocardial segments would show a biphasic response to dobutamine. At low doses, the ventricular myocardium would show an improvement in contractility compared with the resting state, but at higher doses, owing to increasing oxygen demand, would show a decrement in function. Many small studies have shown a survival advantage and an improvement in global left ventricular function in patients with viability who underwent surgical revascularization compared with medical therapy alone. Similar to the detection of coronary artery disease, dobutamine stress myocardial perfusion imaging shows a higher sensitivity and lower specificity for the identification of myocardial viability than echocardiography.13
EXERCISE VERSUS PHARMACOLOGIC STRESS
Generally, exercise stress imaging provides a wealth of information from a physiologic, functional, and prognostic perspective. Exercise stress imaging is the preferred stress modality in patients who can exercise and achieve an adequate workload. When patients are able to complete only a submaximal (<85% target heart rate) exercise protocol, stress imaging becomes less sensitive and can underestimate the degree and extent of ischemia. It has been estimated that at least 25% of outpatients and 50% of hospitalized patients cannot perform adequate exercise to achieve a maximum workload.7 Pharmacologic stress imaging has become the optimal modality of testing in these patients for diagnosis of coronary artery disease, quantification of ischemic burden, and important prognostic determination. As with all types of stress testing, patient selection is crucial, and certain underlying conditions may make pharmacologic stress testing better than exercise testing, even in patients with normal functional capacity. In addition, similar to exercise testing, the physiologic, ECG, and imaging parameters obtained during pharmacologic stress testing have unique implications that can often assist in accurate image interpretation.
Patient Selection
Beyond patients who are unable to exercise, pharmacologic stress imaging, whether with vasodilators or inotropic agents, is often the study of choice. A prime example in which vasodilator stress perfusion imaging is most accurate is in the setting of patients with underlying left bundle branch block (LBBB). Myocardial perfusion imaging in patients with LBBB may show reversible anteroseptal defects with exercise or dobutamine stress in the absence of obstructive coronary artery disease. These defects are a result of asynchronous and delayed systolic septal contraction secondary to the conduction system delay in septal depolarization associated with LBBB. Fifty percent of patients with LBBB undergoing exercise SPECT and 84% to 100% of patients with LBBB undergoing dobutamine SPECT show falsely reversible anteroseptal defects.14 Patients with ventricular paced rhythms show similar false-positive stress perfusion results owing to asynchronous septal depolarization (and a “pseudo”-LBBB), although this is generally less of an issue with exercise testing—unless patients are pacemaker-dependent.15 The best way to avoid this scenario is to use vasodilator stress with either adenosine or dipyridamole in patients with LBBB or in patients who are pacemaker-dependent.16
Another patient characteristic that may influence stress modality is underlying severe reactive airways disease. In patients with active wheezing or a propensity for bronchospasm, it is preferable to avoid adenosine and dipyridamole, both of which have cross-reactivity with the adenosine A3 receptor, which can induce bronchoconstriction. Multiple studies have shown that vasodilators are generally safe in patients with COPD, unless there is a significant reactive airway component or there is an acute exacerbation of the underlying condition. Prophylactic bronchodilator use before vasodilator infusion is also an option, although limited data are available to support routine use with standard protocols. Table 26-2 reviews many of these conditions, most of which have been described in this chapter.
| Condition | Test of Choice |
|---|---|
| If patient can achieve an adequate level of exercise | Use exercise |
| If patient has exercise limitations | Use adenosine, dipyridamole, or dobutamine |
| If patient has bronchospasm | Use dobutamine |
| If patient has LBBB/pacemaker | Use adenosine, dipyridamole, or dobutamine |
| If patient is <72 hr after acute MI | Use adenosine or dipyridamole |
| If patient is <24 hr after PCI | Use adenosine or dipyridamole |
LBBB, left bundle branch block; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Adapted from Zaret BL, Beller GA. Clinical Nuclear Cardiology: State of the Art and Future Directions, 3rd ed. Philadelphia, Mosby, 2005.
Electrocardiographic Changes
Monitoring of the ECG during exercise or pharmacologic stress imaging is crucial to accurate test assessment, and often provides important additional information to help with image interpretation. During exercise, ST segment depression has been associated with higher clinical risk. With pharmacologic stressors, ST segment changes have also been correlated with future adverse outcomes. With vasodilator stress perfusion imaging, ST segment depression is infrequent because of minimal change in heart rate and blood pressure. When it occurs, ST segment depression during vasodilator infusion has been shown to be a significant predictor of adverse outcomes and is independent of and incremental to the subsequent perfusion data.17,18 There has been a significant association with severe, multivessel coronary artery disease and higher cardiac event rates in patients with vasodilator-induced ST segment depression even in the setting of normal SPECT images.
With dobutamine, ST segment changes have also been shown to be additive to imaging information, although to a lesser extent. Patients undergoing dobutamine stress imaging show improved positive predictive value for significant coronary artery disease with a positive stress ECG in addition to the stress images.19 Generally, ECG abnormalities with vasodilator or inotropic stress are additive to the stress images obtained. They often provide information independent of SPECT or echocardiographic data to assist in better risk stratification.20,21
Other Perfusion Imaging Indices
The ability to incorporate ECG data and SPECT images is pivotal in the interpretation of myocardial perfusion studies. Beyond stress/rest mismatch, nuclear stress imaging uses other indices to delineate higher risk studies. Transient ischemic dilation (TID) occurs as a result of subendocardial ischemia during stress, resulting in the appearance of cavity dilation in the stress images compared with rest. TID is a very specific indicator of high cardiac risk, often occurring in the setting of severe, multivessel coronary artery disease. Multiple studies have shown the same diagnostic and prognostic implications of TID with exercise compared with pharmacologic stress, although the threshold ratio of stress to rest cavity size is larger with vasodilators and inotropes.3
Similarly, increased lung uptake of thallium in SPECT studies is also an indicator of elevated pulmonary capillary wedge pressure (or left atrial pressure), delineating a higher risk of subsequent cardiac events and severe coronary artery disease. As with TID, the diagnostic and prognostic information gained from increased lung uptake of thallium is similar when using exercise or pharmacologic stress.3 With myocardial perfusion imaging, TID and increased thallium lung uptake with pharmacologic stress provide incremental diagnostic and prognostic indices comparable to exercise studies.
CONCLUSION
KEY POINTS
 Pharmacologic stress imaging is an effective method to evaluate coronary artery disease in patients with comorbid conditions that preclude exercise.
Pharmacologic stress imaging is an effective method to evaluate coronary artery disease in patients with comorbid conditions that preclude exercise. Vasodilators such as adenosine and dipyridamole and inotropes such as dobutamine are safe and tolerated in most clinical conditions.
Vasodilators such as adenosine and dipyridamole and inotropes such as dobutamine are safe and tolerated in most clinical conditions.Hendel RC, Jamil T, Glover DK. Pharmacologic stress testing: new methods and new agents. J Nucl Cardiol. 2003;10:197-204.
Iskandrian AE, Verani MS. Nuclear Cardiac Imaging: Principles and Applications. New York: Oxford University Press; 2003.
Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol. 2003;42:1318-1333.
Braunwald’s Heart Disease. Libby P, Bonow RO, Zipes DP, et al, editors. 8th ed, 2008. Saunders. Philadelphia.
Miyamoto MI, Vernotico SL, Majmundar H, et al. Pharmacologic stress myocardial perfusion imaging: a practical approach. J Nucl Cardiol. 2007;14:250-255.
Orlandi C. Pharmacology of coronary vasodilation: a brief review. J Nucl Cardiol. 1996;3:S27-S30.
Zaret B, Beller G. Clinical Nuclear Cardiology: State of the Art and Future Directions, 3rd ed. Philadelphia: Mosby; 2005.
1 Lim M, Kern M. Coronary pathophysiology in the cardiac catheterization laboratory. Curr Prob Cardiol. 2006;31:497-504.
2 Zipes DP, Lippy P, Bonow RO, et al, editors. Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine, 7th ed, Vol 2. Philadelphia: Saunders, 2005.
3 Zaret B, Beller G. Clinical Nuclear Cardiology: State of the Art and Future Directions, 3rd ed. Philadelphia: Mosby; 2005.
4 Frishman WH, Sonnenblick EH. Cardiovascular Pharmacotherapeutics. New York: McGraw-Hill; 1997.
5 Tadamura E, Iida H, Matsumoto K, et al. Comparison of myocardial blood flow during dobutamine-atropine infusion with that after dipyridamole administration in normal men. J Am Coll Cardiol. 2001;37:130-136.
6 Heller GV, Herman SD, Travin MI, et al. Independent prognostic value of intravenous dipyridamole with technetium-99m sestamibi tomographic imaging in predicting cardiac events and cardiac-related hospital admissions. J Am Coll Cardiol. 1995;26:1202-1208.
7 Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol. 2003;42:1318-1333.
8 Thomas GS, Prill NV, Majmundar H, et al. Treadmill exercise during adenosine infusion is safe, results in fewer adverse reactions, and improves myocardial perfusion image quality. J Nucl Cardiol. 2000;7:439-446.
9 Calnon DA, McGrath PD, Doss AL, et al. Prognostic value of dobutamine stress technetium-99m sestamibi single-photon emission computed tomography myocardial perfusion imaging: stratification of a high-risk population. J Am Coll Cardiol. 2001;38:1511-1517.
10 Elhendy A, Bax JJ, Poldermans D. Dobutamine stress myocardial perfusion imaging in coronary artery disease. J Nucl Med. 2002;43:1634-1636.
11 Smart SC, Knickelbine T, Stoiber TR, et al. Safety and accuracy of dobutamine-atropine stress echocardiography for the detection of residual stenosis of the infarct-related artery and multivessel disease during the first week after acute myocardial infarction. Circulation. 1997;95:1394-1401.
12 Carlos ME, Smart SC, Wynsen JC, et al. Dobutamine stress echocardiography for risk stratification after myocardial infarction. Circulation. 1997;95:1402-1410.
13 Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation. 2008;117:103-114.
14 O’Keefe JHJr, Bateman TM, Barnhart CS. Adenosine thallium-201 is superior to exercise thallium-201 for detecting coronary artery disease in patients with left bundle branch block. J Am Coll Cardiol. 1993;21:1332-1338.
15 Tse HF, Yu C, Wong KK, et al. Functional abnormalities in patients with permanent right ventricular pacing: the effect of the sites of electrical stimulation. J Am Coll Cardiol. 2002;40:1451-1458.
16 Skalidis EI, Kochiadakis GE, Koukaraki SI, et al. Myocardial perfusion in patients with permanent ventricular pacing and normal coronary arteries. J Am Coll Cardiol. 2001;37:124-129.
17 Hachamovitch R, Berman DS, Kiat H, et al. Value of stress myocardial perfusion single photon emission computed tomography in patients with normal resting electrocardiograms: an evaluation of incremental prognostic value and cost-effectiveness. Circulation. 2002;105:823-829.
18 Klodas E, Miller TD, Christian TF, et al. Prognostic significance of ischemic electrocardiographic changes during vasodilator stress testing in patients with normal SPECT images. J Nucl Cardiol. 2003;10:4-8.
19 Shaheen LJ, Klutstein MW, Rosenmann D, et al. Diganostic value of 12 lead electrocardiogram during dobutamine echocardiographic studies. Am Heart J. 1998;136:1061-1064.
20 Kim C, Kwok YS, Heagerty P, et al. Pharmacologic stress testing for coronary artery disease diagnosis: a meta-analysis. Am Heart J. 2001;142:934-944.
21 Levine MG, Ahlberg AW, Mann A, et al. Comparison of exercise, dipyridamole, adenosine and dobutamine stress with the use of Tc-99m tetrofosmin tomographic imaging. J Nucl Cardiol. 1999;6:389-396.

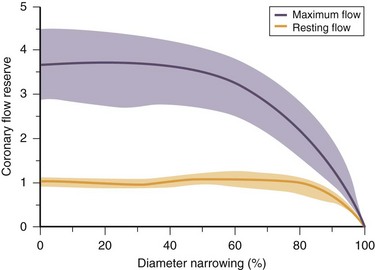
 FIGURE 26-1
FIGURE 26-1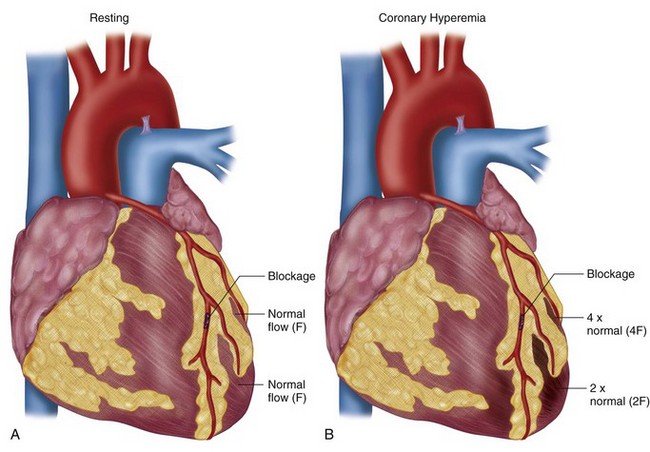
 FIGURE 26-2
FIGURE 26-2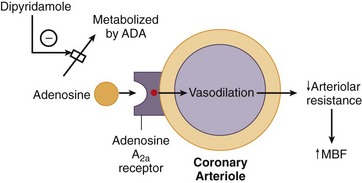
 FIGURE 26-3
FIGURE 26-3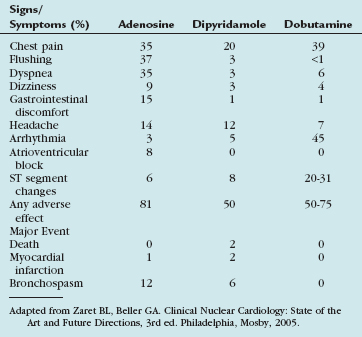
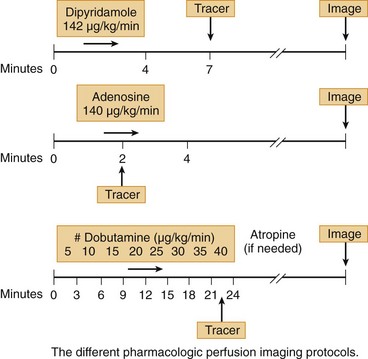
 FIGURE 26-4
FIGURE 26-4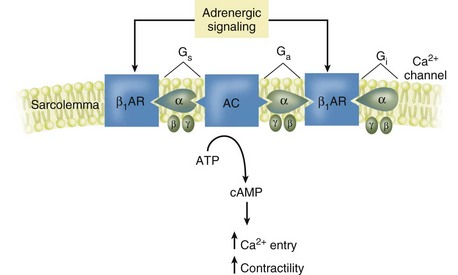
 FIGURE 26-5
FIGURE 26-5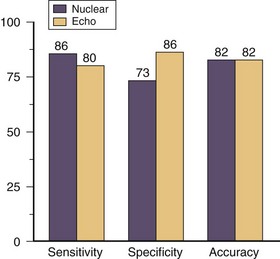
 FIGURE 26-6
FIGURE 26-6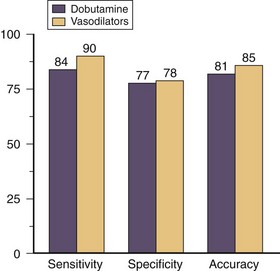
 FIGURE 26-7
FIGURE 26-7

